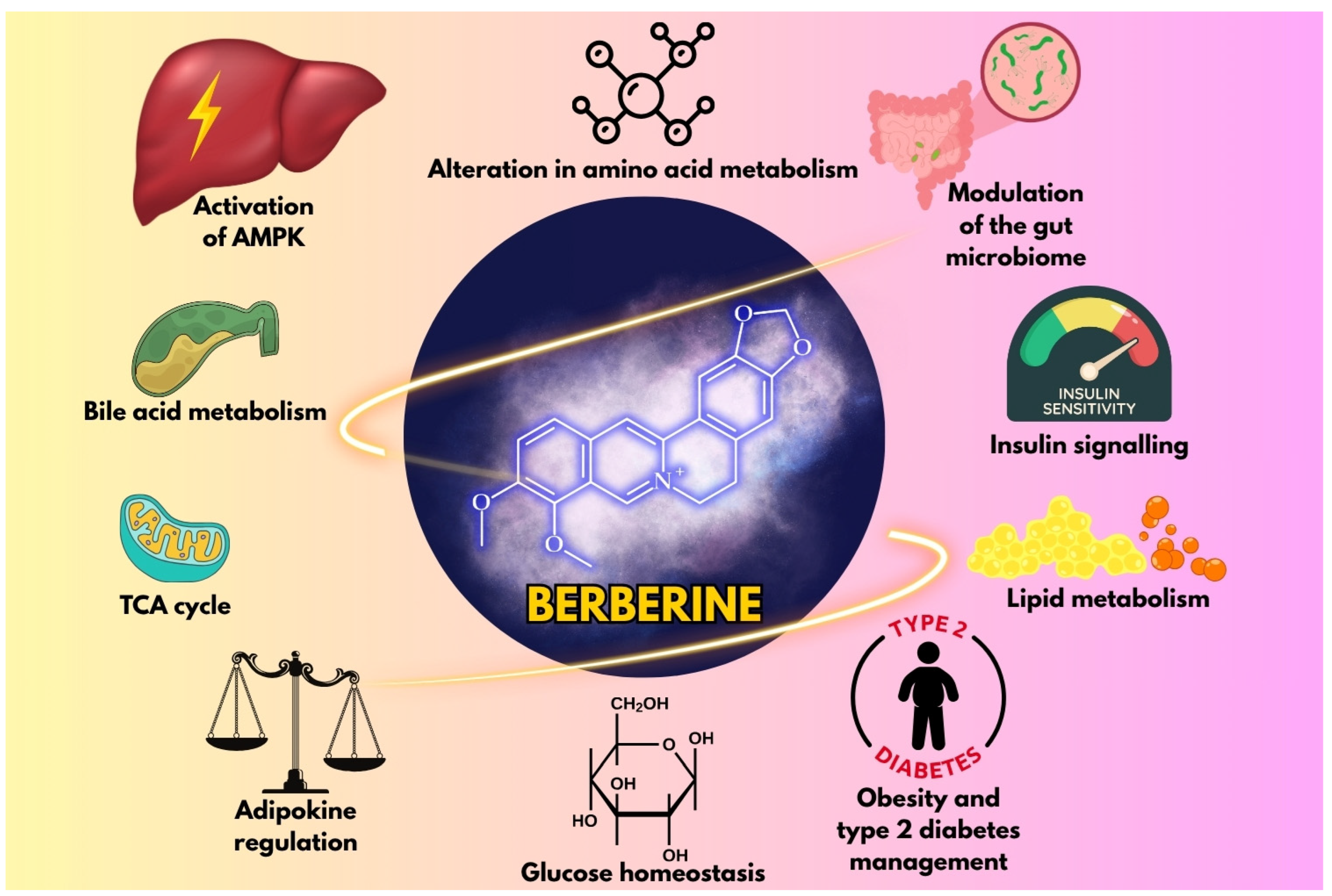
Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management
Abstract
1. Introduction
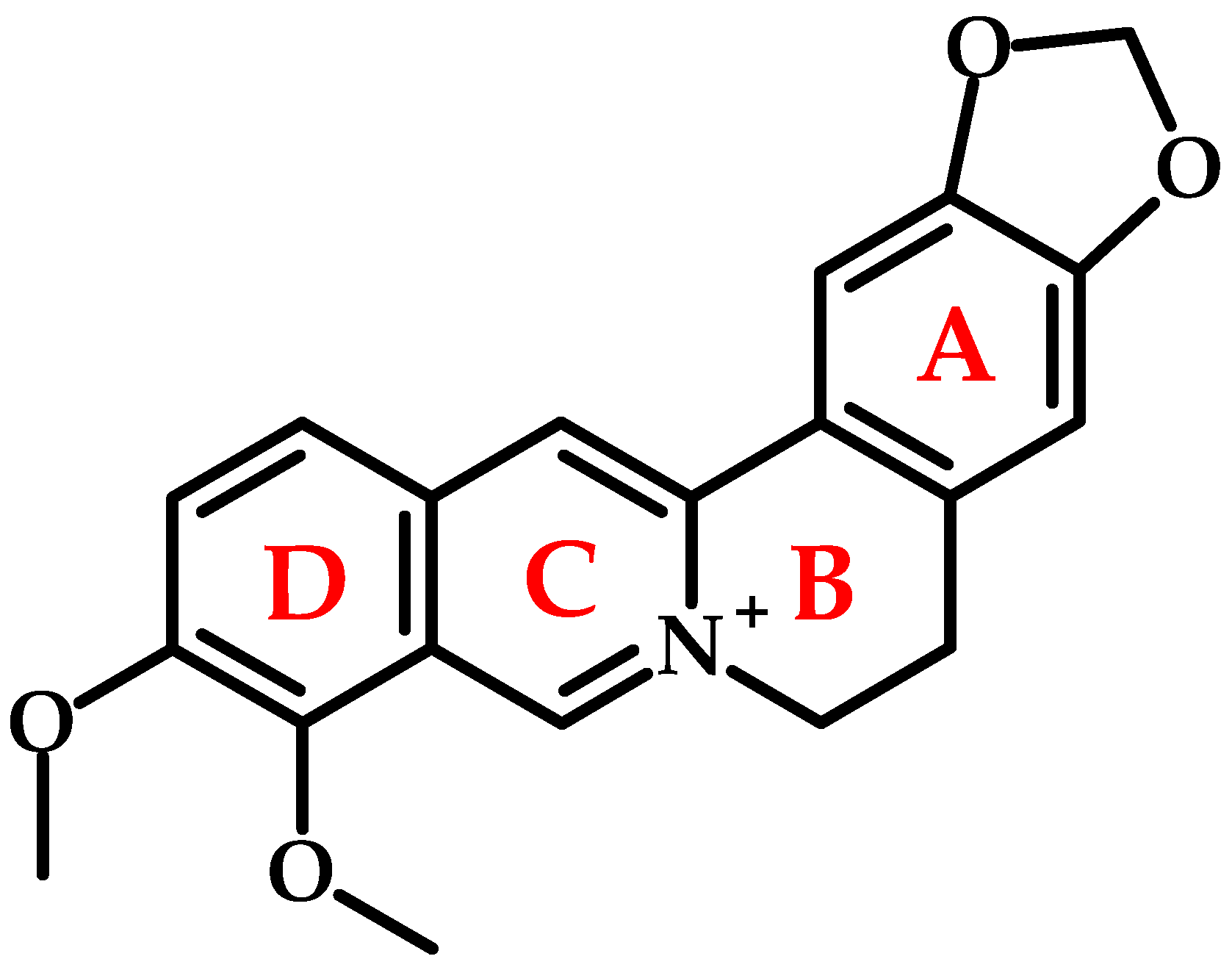
2. Chemical Structure and Physicochemical Properties
3. Natural Occurrence and Sources
4. Pharmacological Mechanisms in Obesity and Diabetes
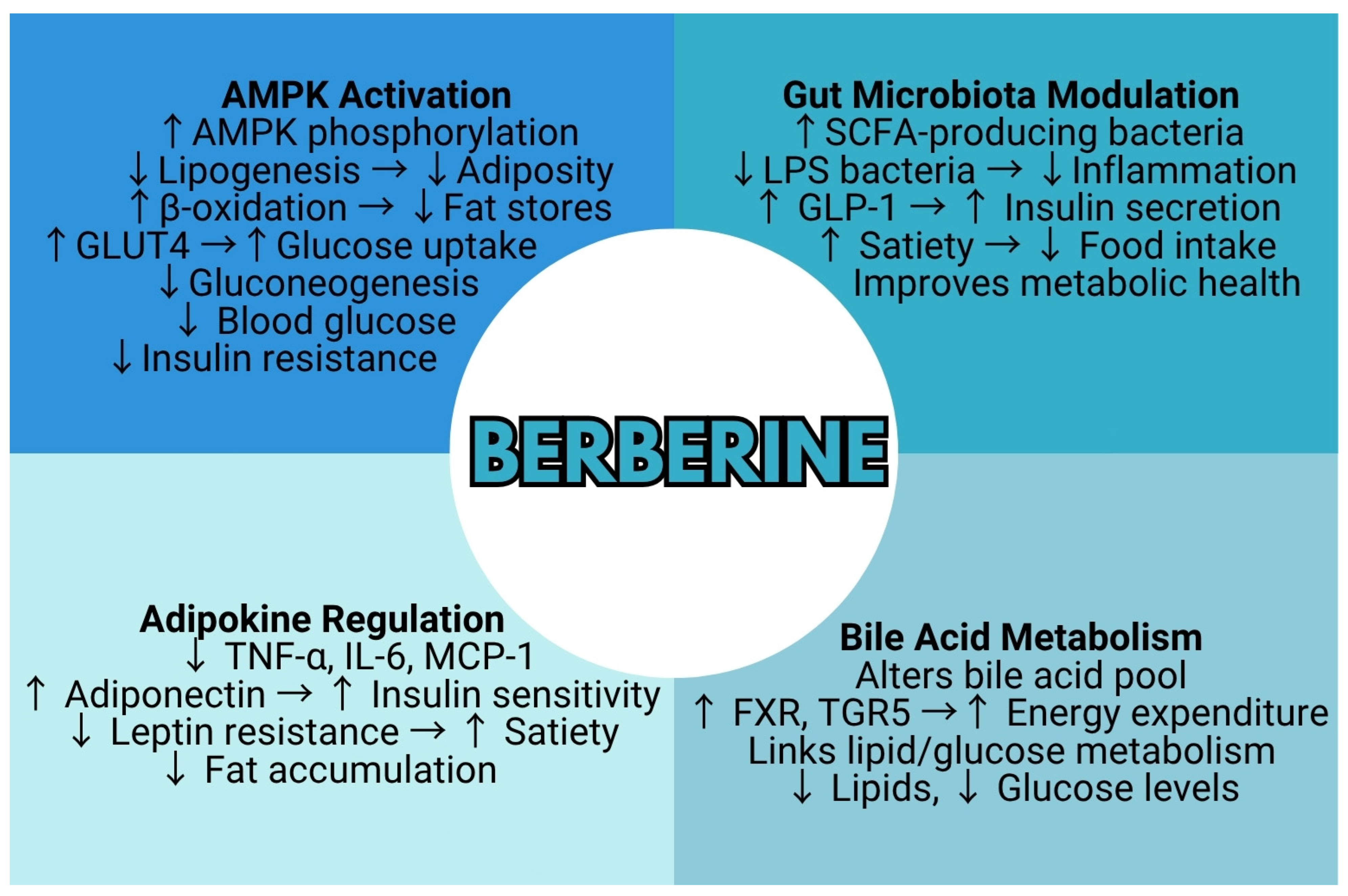
4.1. Impact on Obesity
4.1.1. Lipid Metabolism
4.1.2. Gut Microbiota
4.1.3. Adipokine Regulation
4.2. Role in Diabetes and Insulin Resistance
4.2.1. Glucose Homeostasis
4.2.2. Insulin Signaling
4.2.3. Mitochondrial Function
5. Metabolomic Insights into Berberine’s Therapeutic Effects
5.1. Obesity and the Anti-Obesity Effects of Berberine
5.1.1. Lipid Profile Modulation
5.1.2. Bile Acid Metabolism
5.2. Diabetes: Shifts in Amino Acids (e.g., Branched-Chain Amino Acids), TCA Cycle Intermediates, and Gut Microbiota-Derived Metabolites
5.2.1. Alterations in Amino Acid Metabolism
5.2.2. Disruptions in TCA Cycle Intermediates
5.2.3. Gut Microbiota-Derived Metabolites and Their Impact
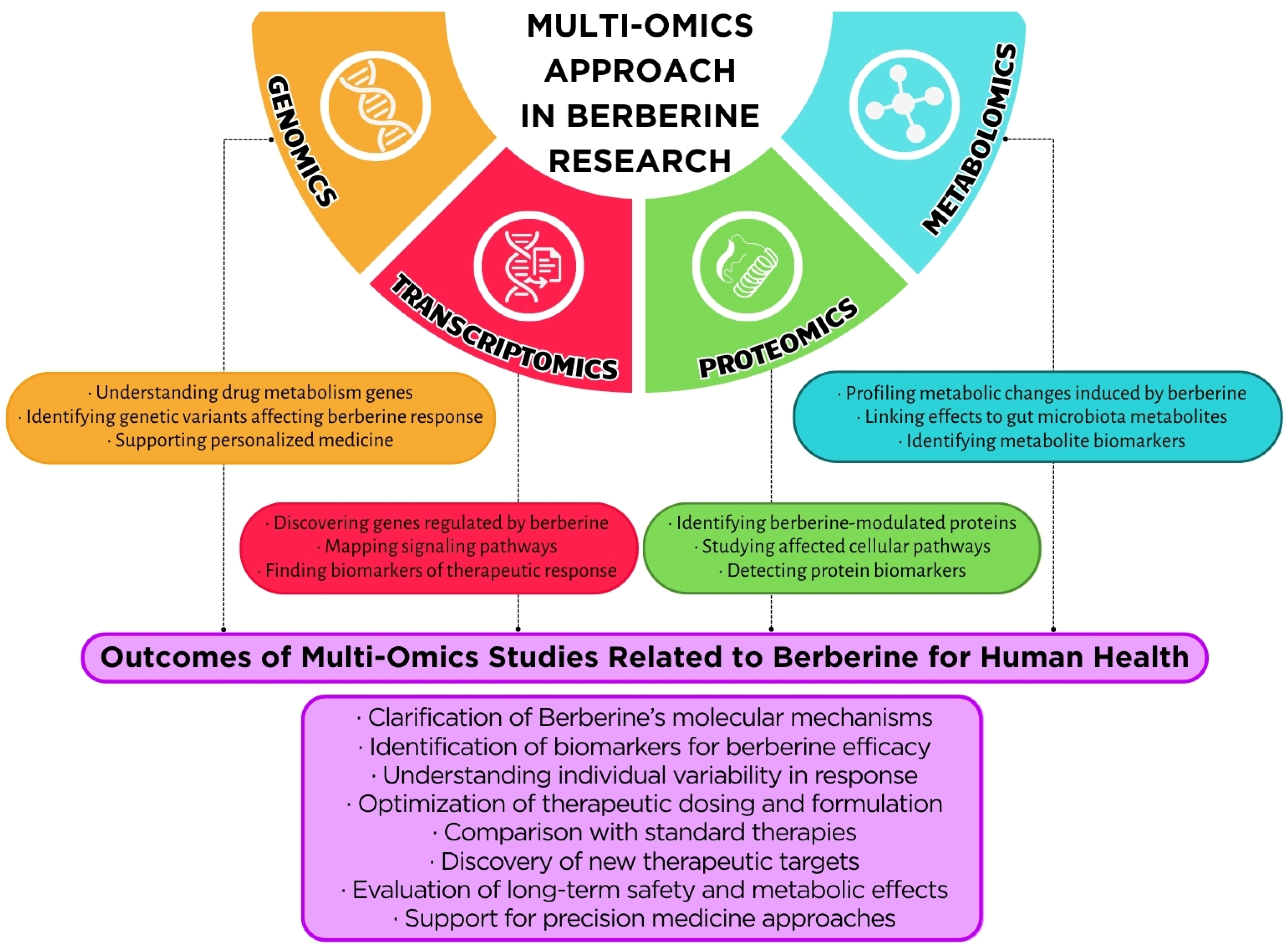
5.3. Multi-Omics Integration: Linkages Between Metabolomics, Transcriptomics, and Proteomics Data
5.3.1. Holistic Insights Through Multi-Omics Platforms
5.3.2. Berberine in Inflammatory Bowel Disease: A Multi-Omics Paradigm
5.3.3. Transcriptome–Proteome Interplay in Energy Metabolism
5.3.4. MAPK and Inflammatory Pathways: Multilayered Regulation
6. Safety, Bioavailability, and Future Directions
6.1. Pharmacokinetics, Toxicity, and Side Effects
6.2. Future Research and Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| AOM | Anti-obesity medication |
| ASE | Accelerated solvent extraction |
| ATP | Adenosine triphosphate |
| BA | Bile acid |
| BBR | Berberine |
| BCAA | Branched-chain amino acid |
| BSEP | Bile salt export pump |
| C/EBP-α | CCAAT/enhancer-binding protein alpha |
| CI | Color Index |
| CPT1A | Carnitine palmitoyltransferase 1A |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| CYP2C9 | Cytochrome P450 2C9 enzyme |
| CYP2D6 | Cytochrome P450 2D6 enzyme |
| CYP3A4 | Cytochrome P450 3A4 enzyme |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| DES-UA-MSPD | Deep eutectic solvent ultrasound-assisted matrix solid-phase dispersion |
| ERK | Extracellular signal-regulated kinase (subgroup of MAPK) |
| FASN | Fatty acid synthase |
| FXR | Farnesoid X receptor |
| G6Pase | Glucose-6-phosphatase |
| GC-MS | Gas chromatography-mass spectrometry |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLUT4 | Glucose transporter type 4 |
| GLP-1 | Glucagon-like peptide-1 |
| HbA1c | Glycated hemoglobin |
| HFD | High-fat diet |
| HO-1 | Heme oxygenase-1 |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| HPLC-DAD | High-performance liquid chromatography with a diode array detector |
| HPLC-ESI-Q-TOF-MS/MS | High-performance liquid chromatography–electrospray ionization–quadrupole time-of-flight tandem mass spectrometry |
| HPTLC | High-performance thin-layer chromatography |
| IBD | Inflammatory bowel disease |
| IKK-β | Inhibitor of nuclear factor kappa-B kinase subunit beta |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IRS-1 | Insulin receptor substrate 1 |
| JNK | c-Jun N-terminal kinase (subgroup of MAPK) |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | Low-density lipoprotein receptor |
| LPS | Lipopolysaccharide |
| LXRα | Liver X receptor alpha |
| MAE | Microwave-assisted extraction |
| MAPK | Mitogen-activated protein kinase |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis (inflammatory form of MASLD) |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDR | Multidrug resistance |
| mTOR | Mechanistic target of rapamycin |
| MW | Molecular weight |
| NADES | Natural deep eutectic solvent |
| NAFLD | Non-alcoholic fatty liver disease |
| NCD | Non-communicable disease |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHANES | National Health and Nutrition Examination Survey |
| NO | Nitric oxide |
| NRF-1 | Nuclear respiratory factor 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Opa1 | Optic atrophy 1 (mitochondrial fusion protein) |
| OTU | Operational taxonomic unit |
| p38 | p38 mitogen-activated protein kinase (subgroup of MAPK) |
| P-gp | P-glycoprotein |
| PDA | Photo diode array |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PHB2 | Prohibitin 2 (mitophagy receptor) |
| PI3K | Phosphatidylinositol 3-kinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PINK1 | PTEN induced kinase 1 (mitophagy regulator) |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| PPAR-δ | Peroxisome proliferator-activated receptor delta |
| PYY | Peptide YY |
| ROS | Reactive oxygen species |
| SCFA | Short-chain fatty acid |
| SHP | Small heterodimer partner |
| SIRT1 | Sirtuin 1 (NAD+-dependent deacetylase) |
| SREBP-1c | Sterol regulatory element-binding protein-1c |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TCA | Tricarboxylic acid cycle (Krebs cycle) |
| TC | Total cholesterol |
| TFAM | Mitochondrial transcription factor A |
| TG | Triglycerides |
| TGR5 | Takeda G protein-coupled receptor 5 |
| TMAO | Trimethylamine N-oxide |
| TNF-α | Tumor necrosis factor alpha |
| TORC2 | Target of rapamycin complex 2 |
| UAE | Ultrasound-assisted extraction |
| UCP1 | Uncoupling protein 1 |
| UPLC-Q-TOF-MS/MS | Ultra-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry |
| WHO | World Health Organization |
| ZO-1 | Zonula occludens-1 (tight-junction protein) |
References
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The Obesity Transition: Stages of the Global Epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 8 June 2025).
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017-March 2020 Prepandemic Data Files-Development of Files and Prevalence Estimates for Selected Health Outcomes. Natl. Health Stat. Rep. 2021, 158, 10–15620. [Google Scholar] [CrossRef]
- Available online: https://iris.who.int/bitstream/handle/10665/353747/9789289057738-eng.pdf?sequence=1 (accessed on 8 June 2025).
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Polonsky, H.M. The Psychosocial Burden of Obesity. Endocrinol. Metab. Clin. N. Am. 2016, 45, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Ali Ahmadi, M.; Hefner, M.; Kudchadkar, G.; Najam, W.; Nateqi, M.; Siddik, M.A.B.; Booe, H.; Dhurandhar, N.V. An Algorithm for the Use of Anti-Obesity Medications. Nutr. Diabetes 2024, 14, 20. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Hao, H.-P.; Xie, H.-G.; Lai, L.; Wang, Q.; Liu, C.-X.; Wang, G.-J. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current Knowledge and Pharmacological Profile of Berberine: An Update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Patel, P. A Bird’s Eye View on a Therapeutically ‘Wonder Molecule’: Berberine. Phytomedicine Plus 2021, 1, 100070. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The Effect of Berberine on Weight Loss in Order to Prevent Obesity: A Systematic Review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Cheng, X.-X.; Lui, Y.; Hu, Y.-J.; Liu, Y.; Li, L.-W.; Di, Y.-Y.; Xiao, X.-H. Thermal Behavior and Thermodynamic Properties of Berberine Hydrochloride. J. Therm. Anal. Calorim. 2013, 114, 1401–1407. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical Characterization of Berberine Chloride: A Perspective in the Development of a Solution Dosage Form for Oral Delivery. AAPS PharmSciTech 2010, 11, 1466–1475. [Google Scholar] [CrossRef]
- Available online: https://www.sigmaaldrich.com/PL/pl/product/sigma/b3251 (accessed on 9 June 2025).
- Ai, X.; Yu, P.; Peng, L.; Luo, L.; Liu, J.; Li, S.; Lai, X.; Luan, F.; Meng, X. Berberine: A Review of Its Pharmacokinetics Properties and Therapeutic Potentials in Diverse Vascular Diseases. Front. Pharmacol. 2021, 12, 762654. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, Y.; Liu, G.; Shen, L. Study on Microwave Extraction of Berberine Coloring Compound and Extracts Stability: Process Optimization by Response Surface Method (RSM). Dye. Pigment. 2025, 237, 112609. [Google Scholar] [CrossRef]
- Hazra, K.; Kumar, D.; Debnath, S.; Mondal, S.; Batule, M.; Dutta, S.; Singh, A.; Singh, R.; Mangal, A.K. Dynamicity and Extractability of Hydro-Alcoholic Solvents for Tinospora Cordifolia Stem: An Investigation for Target-Oriented Traditional Drug Discovery Based on Biologically Active Phytocompounds. Vegetos 2024, 38, 931–941. [Google Scholar] [CrossRef]
- Singh, S.M.; Tripathy, S.; Srivastav, P.P. Bioactive Compound Extraction from Giloy Leaves and Steam Using Ultrasound: Bioactivity, Antimicrobial, and LC-MS/MS Study. Food Sci. Biotechnol. 2025, 34, 1835–1847. [Google Scholar] [CrossRef]
- Chacón-Fuentes, M.; Burgos-Díaz, C.; Opazo-Navarrete, M.; Mercado, A.; Westermeyer, F. Berberine and Palmatine Distribution across Plant Organs in Berberis darwinii: Basis for Selecting Superior-Producing Accessions. Molecules 2025, 30, 1849. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.V.; Le, N.T.; Dang, V.T.T.; Koshovyi, O.; Raal, A.; Nguyen, H.T. Alkaloid Extraction from Coptis chinensis Franch. Using Ultrasound-Assisted Aqueous Solutions of Surfactants, Organic Acids, Deep Eutectic Solvents, and Supramolecular Deep Eutectic Solvents. Molecules 2025, 30, 1418. [Google Scholar] [CrossRef]
- Wei, L.; Ma, W.; Liu, S.; Mi, S.; Shen, Q.; Lu, Q.; Liu, Z. Mahonia bealei (Fort.) Carr. Leaf Extract Modulates the TLR2/MyD88/NF-κB Signaling Pathway to Inhibit PGN-Induced Inflammation in RAW264.7 Cells. J. Ethnopharmacol. 2025, 344, 119510. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, X.; Huang, X.; Zhai, L.; Li, Z.; Sun, G.; Jiang, R.; Sun, L. Coptis Cream Ethanol Extract Regulates Degranulation Caused by Allergic Reactions through MGPRB3/PLC/TRPV1 Signaling Pathway. J. Ethnopharmacol. 2025, 343, 119473. [Google Scholar] [CrossRef]
- Satija, S.; Vyas, M.; Pandey, P.; Garg, M. Taguchi L9 Optimization of Microwave-Assisted Solid-Liquid Duo Heating for Berberine Extraction with HPTLC Analysis. Biomed. Chromatogr. 2025, 39, e70008. [Google Scholar] [CrossRef]
- Moravcová, P.; Schröterová, L.; Zíka, J.; Hošťálková, A.; Švec, F.; Šatínský, D. A UHPLC-DAD Method for Quantification of Berberine and Protoberberine Alkaloids in Herbal Food Supplements Based on Berberis aristata Extract and Evaluation of Their Biological Activity. J. Food Compos. Anal. 2025, 139, 107150. [Google Scholar] [CrossRef]
- Sarma, L.; Patra, F.; Borah, P.K.; Meena, S.; Duary, R.K. Comparative Modeling of Microwave and Ultrasound Assisted Extraction of Phenolics and Berberine from Coptis teeta Wall. Rhizomes. Sustain. Food Technol. 2025, 3, 570–581. [Google Scholar] [CrossRef]
- Didar, Z.; Afshar, A.S.; Nia, M.M.Z. Evaluation of Chemical and Biological Characteristics of Subcritical Water Extract from Helicteres isora L. Fruit and Its Incorporation into Chia Seed Mucilage Film. Iran. J. Chem. Chem. Eng. 2025, 44, 479–491. [Google Scholar] [CrossRef]
- Singh, B.; Singh, L.; Bhatt, I.D.; Kandpal, N.D. Tailored NADES Solvents for the Extraction Optimization of Benzylisoquinoline Alkaloids from Thalictrum foliolosum DC.—A Potential Phyto-Nutraceutical Source. Food Chem. 2025, 463, 141016. [Google Scholar] [CrossRef] [PubMed]
- Marcinčáková, D.; Hudáková, N.; Miłek, M.; Kolesárová, M.; Dżugan, M.; Cizkova, D.; Legáth, J. Evaluation of the Antioxidant Properties and Biological Effects of a Novel Combined Barberry Root-Propolis Extract on HEK293T Cells. Pharmaceuticals 2024, 18, 27. [Google Scholar] [CrossRef]
- Le, N.T.; Le, T.T.; Ho, D.V.; Nguyen, K.V.; Nguyen, H.T. Extraction and Recovery of Bioactive Alkaloids from Phellodendron Amurense Rupr. Using Ultrasound-Assisted Green Solvents and Macroporous Resins. Sustain. Chem. Pharm. 2024, 42, 101809. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.-Q.; Zhang, Y.-C.; Wu, Y.-Q.; Yang, Z.; Zheng, Y.-Z.; Lu, J.-H.; Tu, P.-F.; Zeng, K.-W. Cayratia albifolia C.L.Li Exerts Anti-Rheumatoid Arthritis Effect by Inhibiting Macrophage Activation and Neutrophil Extracellular Traps (NETs). Chin. Med. 2024, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Dżugan, M.; Pieńkowska, N.; Galiniak, S.; Mołoń, M.; Litwińczuk, W. Ornamental Barberry Twigs as an Underexploited Source of Berberine-Rich Extracts-Preliminary Research. Curr. Issues Mol. Biol. 2024, 46, 13193–13208. [Google Scholar] [CrossRef]
- Liu, C.; Gong, F.; Xiong, Z.; Wang, C.; Ran, X.; Ran, J.; Li, R.; Ou, Y.; Xia, Q.; Wei, P.; et al. An Extraction Process Based on the Collaborative Extraction of Coptis chinensis Franch. Phytoconstituents Using a Deep Eutectic Solvent and an Organic Solvent. Separations 2024, 11, 249. [Google Scholar] [CrossRef]
- Han, M.; Du, K.; He, X.; Li, H.; Li, J.; Li, X.; Chang, Y. Advancing Green Extraction of Bioactive Compounds Using Deep Eutectic Solvent-Based Ultrasound-Assisted Matrix Solid-Phase Dispersion: Application to UHPLC-PAD Analysis of Alkaloids and Organic Acids in Coptidis rhizoma. Talanta 2024, 274, 125983. [Google Scholar] [CrossRef]
- Ivan, I.M.; Olaru, O.T.; Popovici, V.; Chițescu, C.L.; Popescu, L.; Luță, E.A.; Ilie, E.I.; Brașoveanu, L.I.; Hotnog, C.M.; Nițulescu, G.M.; et al. Antioxidant and Cytotoxic Properties of Berberis vulgaris (L.) Stem Bark Dry Extract. Molecules 2024, 29, 2053. [Google Scholar] [CrossRef]
- Nakonieczna, S.; Susniak, K.; Bozhadze, A.; Grabarska, A.; Głowniak-Lipa, A.; Głowniak, K.; Kukula-Koch, W. Optimized Centrifugal Partition Chromatography (CPC) Protocol for Isolation of Urease Inhibitors: Magnoflorine and Berberine from Berberis vulgaris Extracts. Separations 2024, 11, 94. [Google Scholar] [CrossRef]
- Trong Le, N.; Huyen Thi Chau, N.; Quynh Dinh Nguyen, P.; Thuy Thi Tran, L.; Thanh Phung, H.; Thi Nguyen, H. Green Extraction of Berberine from Coscinium fenestratum (Gaertn.) Colebr. Using Ultrasound-Assisted Aqueous Solutions of Organic Acids, Polyalcohols, and Deep Eutectic Solvents. Sep. Purif. Technol. 2024, 330, 125541. [Google Scholar] [CrossRef]
- Erhunse, N.; Kumari, S.; Anmol; Singh, P.; Omoregie, E.S.; Singh, A.P.; Sharma, U.; Sahal, D. Annickia affinis (Exell) Versteegh & Sosef Methanol Stem Bark Extract, Potent Fractions and Isolated Berberine Alkaloid Target Both Blood and Liver Stages of Malaria Parasites. J. Ethnopharmacol. 2024, 319, 117269. [Google Scholar] [CrossRef]
- Li, R.; Wu, X.; Jiao, X.; Zhang, X.; Wang, C.; Han, L.; Song, M.; Zhang, Y.; Pan, G.; Zhang, Z. Chemical Profiles, Differentiation, and Quality Evaluation of Radix et Rhizoma Thalictri Foliolosi Based on LC-MS. J. Pharm. Biomed. Anal. 2024, 237, 115747. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Gong, Z. Berberine Inhibits 3T3-L1 Adipocyte Differentiation through the PPAR-γ Pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.-M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase with Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Brusq, J.-M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of Lipid Synthesis through Activation of AMP Kinase: An Additional Mechanism for the Hypolipidemic Effects of Berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, Y.S.; Cha, S.H.; Jeong, H.W.; Choe, S.S.; Lee, M.-R.; Oh, G.T.; Park, H.-S.; Lee, K.-U.; Lane, M.D.; et al. Berberine Improves Lipid Dysregulation in Obesity by Controlling Central and Peripheral AMPK Activity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E812–E819. [Google Scholar] [CrossRef]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine Is a Novel Cholesterol-Lowering Drug Working through a Unique Mechanism Distinct from Statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, Y.; Zhao, L.; Lu, F. The Effects of Berberine on Blood Lipids: A Systemic Review and Meta-Analysis of Randomised Controlled Trials. Planta Medica 2013, 79, 437–446. [Google Scholar]
- Gu, S.; Cao, B.; Sun, R.; Tang, Y.; Paletta, J.L.; Wu, X.; Liu, L.; Zha, W.; Zhao, C.; Li, Y.; et al. A Metabolomic and Pharmacokinetic Study on the Mechanism Underlying the Lipid-Lowering Effect of Orally Administered Berberine. Mol. Biosyst. 2015, 11, 463–474. [Google Scholar] [CrossRef]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine Improves Glucose Metabolism through Induction of Glycolysis. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E148–E156. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight-Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cells. J. Nutr. 2009, 139, 1619–1625. [Google Scholar]
- Pham, N.H.T.; Joglekar, M.V.; Wong, W.K.M.; Nassif, N.T.; Simpson, A.M.; Hardikar, A.A. Short-Chain Fatty Acids and Insulin Sensitivity: A Systematic Review and Meta-Analysis. Nutr. Rev. 2024, 82, 193–209. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C. Changes in Gut Microbiota Control Metabolic Endotoxaemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of Gut Microbiota by Berberine and Metformin during the Treatment of High-Fat Diet-Induced Obesity in Rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural Changes of Gut Microbiota during Berberine-Mediated Prevention of Obesity and Insulin Resistance in High-Fat Diet-Fed Rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Ni, J.; Zhang, C.; Jia, J.; Wu, G.; Sun, H.; Wang, S. Berberine Relieves Metabolic Syndrome in Mice by Inhibiting Liver Inflammation Caused by a High-Fat Diet and Potential Association with Gut Microbiota. Front. Microbiol. 2021, 12, 752512. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, Y.; Zhang, Y. Gut Microbiota Specifically Mediates the Anti-Hypercholesterolemic Effect of Berberine (BBR) and Facilitates to Predict BBR’s Cholesterol-Decreasing Therapeutic in Patients. J. Adv. Res. 2022, 37, 197–208. [Google Scholar] [PubMed]
- Yang, Y.-N.; Wang, Q.-C.; Xu, W.; Yu, J.; Zhang, H.; Wu, C. The Berberine-Enriched Gut Commensal Blautia Producta Ameliorates High-Fat Diet (HFD)-Induced Hyperlipidemia and Stimulates Liver LDLR Expression. Biomed. Pharmacother. 2022, 155, 113749. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, D.; Yuan, X.; Li, Z.; Zhang, J.; Ming, X.; Shaw, P.C.; Zhang, C.; Kong, A.P.S.; Zuo, Z. Effects of Combination Treatment with Metformin and Berberine on Hypoglycemic Activity and Gut Microbiota Modulation in Db/Db Mice. Phytomedicine 2022, 101, 154099. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.-I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. 1999. Biochem. Biophys. Res. Commun. 2012, 425, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Wang, M.; Xu, R.; Liu, X.; Zhang, L.; Qiu, S.; Lu, Y.; Zhang, P.; Yan, M.; Zhu, J. A Co-Crystal of Berberine and Ibuprofen Improves Obesity by Inhibiting the Protein Kinase TBK1 and IKKε. Commun. Biol. 2022, 5, 807. [Google Scholar]
- Rey, V.; Tamargo-Gómez, I. From Kinases to Diseases: Investigating the Role of AMPK in Human Pathologies. Kinases Phosphatases 2023, 1, 181–205. [Google Scholar]
- Zhao, J.V.; Yeung, W.F.; Chan, Y.H.; Vackova, D.; Leung, J.Y.; Ip, D.K.; Zhao, J.; Ho, W.K.; Tse, H.; Schooling, C.M. Effect of Berberine on Cardiovascular-Risk Factors: A Mechanistic Randomised Controlled Trial. Nutrients 2021, 13, 2550. [Google Scholar]
- Qiu, Y.; Li, M.; Zhang, Y.; Liu, Y.; Zhao, Y.; Zhang, J.; Jia, Q.; Li, J. Berberine Treatment for Weight Gain in Patients with Schizophrenia by Regulating Leptin Rather than Adiponectin. Asian J. Psychiatr. 2022, 67, 102896. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin Signalling and GLUT4 Trafficking in Insulin Resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, J.; Wu, R.; Kong, Y.; Sun, C. Recent Advances of Long Non-Coding RNAs in Control of Hepatic Gluconeogenesis. Front. Endocrinol. 2023, 14, 1167592. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, X.; Yan, G.; Yu, Q.; Huang, Z.; Lin, G.; Li, M.; Guan, F.; Su, Z.; Yan, F.; et al. High Fructose-Induced Skeletal Muscle Insulin Resistance Could Be Alleviated by Berberine via AMPD1 and ADSL. Food Chem. Toxicol. 2023, 175, 113731. [Google Scholar] [CrossRef]
- Xu, X.-H.; Hu, Q.; Zhou, L.-S.; Xu, L.-J.; Zou, X.; Lu, F.-E.; Yi, P. Berberine Inhibits Gluconeogenesis in Skeletal Muscles and Adipose Tissues in Streptozotocin-Induced Diabetic Rats via LKB1-AMPK-TORC2 Signaling Pathway. Curr. Med. Sci. 2020, 40, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, Y.; Jiang, Y. Berberine Inhibits Gluconeogenesis in Spontaneous Diabetic Rats by Regulating the AKT/MAPK/NO/cGMP/PKG Signalling Pathway. Mol. Cell. Biochem. 2023, 478, 2013–2027. [Google Scholar]
- Ma, J.L.; Ma, J. Berberine Ursodeoxycholate for the Treatment of Type 2 Diabetes: A Randomised Clinical Trial. JAMA Netw. Open 2025, 8, e2462185. [Google Scholar]
- Li, Y.; Chen, X.; Chen, Y.; Yu, D.; Jiang, R.; Kou, X.; Sheng, L.; Liu, Y.; Song, Y. Berberine Improves TNF-α-Induced Hepatic Insulin Resistance by Targeting MEKK1/MEK Pathway. Inflammation 2022, 45, 2016–2026. [Google Scholar] [CrossRef]
- Su, X.; Yang, D.; Hu, Y.; Yuan, Y.; Song, L. Berberine Suppressed Sarcopenia Insulin Resistance through SIRT1-Mediated Mitophagy. Open Life Sci. 2023, 18, 20220648. [Google Scholar] [CrossRef]
- Amssayef, A.; Eddouks, M. Alkaloids as Promising Agents for the Management of Insulin Resistance: A Review. Curr. Pharm. Des. 2023, 29, 3123–3136. [Google Scholar] [CrossRef]
- Sun, A.; Yang, H.; Li, T.; Luo, J.; Zhou, L.; Chen, R.; Han, L.; Lin, Y. Molecular Mechanisms, Targets and Clinical Potential of Berberine in Regulating Metabolism: A Review Focussing on Databases and Molecular Docking Studies. Front. Pharmacol. 2024, 15, 1368950. [Google Scholar] [CrossRef]
- Georgiev, A.; Granata, C.; Roden, M. The Role of Mitochondria in the Pathophysiology and Treatment of Common Metabolic Diseases in Humans. Am. J. Physiol. Cell Physiol. 2022, 322, C1248–C1259. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Yu, Z.; Guan, Y.; Lv, Y.; Zhang, M.; Zhang, M.; Chen, L.; Lv, X.; Guan, F. Berberine Mitigates Hepatic Insulin Resistance by Enhancing Mitochondrial Architecture via the SIRT1/Opa1 Signalling Pathway. Acta Biochim. Biophys. Sin. 2022, 54, 1464–1475. [Google Scholar] [CrossRef]
- Um, J.H.; Lee, K.M.; Kim, Y.Y.; Lee, D.Y.; Kim, E. Berberine Induces Mitophagy through AMPK and Ameliorates Mitochondrial Dysfunction in PINK1-Knockout Mouse Embryonic Fibroblasts. Int. J. Mol. Sci. 2023, 25, 219. [Google Scholar]
- Hu, Y.; Chen, X.; Zhao, Q.; Li, G.; Zhang, H.; Ma, Z.; Yu, H.; Zeng, Q.; Zhang, H.; Xu, D. Berberine Improves Cardiac Insufficiency through AMPK/PGC-1α Signalling-Mediated Mitochondrial Homeostasis and Apoptosis in HFpEF Mice. Int. Immunopharmacol. 2025, 155, 114613. [Google Scholar]
- Chen, B.; Zhang, J.-P. Bcl-xL Is Required for the Protective Effects of Low-Dose Berberine against Doxorubicin-Induced Cardiotoxicity through Blocking Apoptosis and Activating Mitophagy-Mediated ROS Elimination. Phytomedicine 2022, 101, 154130. [Google Scholar] [CrossRef]
- Wang, L.; Tang, X.-Q.; Shi, Y.; Li, H.-M.; Meng, Z.-Y.; Chen, H.; Li, X.-H.; Chen, Y.-C.; Liu, H.; Hong, Y.; et al. Tetrahydroberberrubine Retards Heart Aging in Mice by Promoting PHB2-Mediated Mitophagy. Acta Pharmacol. Sin. 2023, 44, 332–344. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Li, M.; Yu, M.; Ping, F.; Zheng, J.; Wang, T. Berberine Moderates Glucose and Lipid Metabolism through Multi-Target Regulation and Promotes Gut Microbiota Profile in Obesity. Sci. Rep. 2020, 10, 924851. [Google Scholar]
- Yue, K.; Haokun, Y.; Rong, N.; Xuxiang, Z.; Hongtao, Z.; Xin, N. Progress of the Anti-Obesity of Berberine. arXiv 2025, arXiv:2501.02282. [Google Scholar]
- Zhang, Y.; Wang, X.; Wang, Y. Berberine Reduces Lipid Accumulation in Obesity via Mediating PPARδ-Dependent Pathway. Biomed. Pharmacother. 2023, 161, 11600. [Google Scholar]
- Nie, X.; Lu, Q.; Yin, Y.; He, Z.; Bai, Y.; Zhu, C. Microbiome and Metabolome Analyses Reveal Significant Alterations of Gut Microbiota and Bile Acid Metabolism in ETEC-Challenged Weaned Piglets by Dietary Berberine Supplementation. Front. Microbiol. 2024, 15, 1428287. [Google Scholar] [CrossRef]
- Sun, R.; Yang, N.; Kong, B.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Huang, J.; Shen, J.; Wang, P.; et al. Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol. Pharmacol. 2017, 91, 110–122. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Hu, Y.-R.; Ma, H.; Feng, M.; Li, X.-G.; Ye, X.-L. Epiberberine Reduces Serum Cholesterol in Diet-Induced Dyslipidemia Syrian Golden Hamsters via Network Pathways Involving Cholesterol Metabolism. Eur. J. Pharmacol. 2016, 774, 1–9. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic β-Cells in Type 1 and Type 2 Diabetes Mellitus: Different Pathways to Failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Sjögren, R.J.O.; Rizo-Roca, D.; Chibalin, A.V.; Chorell, E.; Furrer, R.; Katayama, S.; Harada, J.; Karlsson, H.K.R.; Handschin, C.; Moritz, T.; et al. Branched-Chain Amino Acid Metabolism Is Regulated by ERRα in Primary Human Myotubes and Is Further Impaired by Glucose Loading in Type 2 Diabetes. Diabetologia 2021, 64, 2077–2091. [Google Scholar] [CrossRef]
- Kövamees, O.; Shemyakin, A.; Checa, A.; Wheelock, C.E.; Lundberg, J.O.; Östenson, C.-G.; Pernow, J. Arginase Inhibition Improves Microvascular Endothelial Function in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the Gut Microbiota and Its Metabolites for Type 2 Diabetes Mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, S.; Li, A.; Zhang, P.; Peng, X.; Lu, X.; Fan, X. Ginsenoside Rb1 and Berberine Synergistically Protect against Type 2 Diabetes Mellitus via GDF15/HAMP Pathway throughout the Liver Lobules: Insights from Spatial Transcriptomics Analysis. Pharmacol. Res. 2025, 215, 107711. [Google Scholar] [CrossRef]
- Di, S.; Han, L.; An, X.; Kong, R.; Gao, Z.; Yang, Y.; Wang, X.; Zhang, P.; Ding, Q.; Wu, H.; et al. In Silico Network Pharmacology and in Vivo Analysis of Berberine-Related Mechanisms against Type 2 Diabetes Mellitus and Its Complications. J. Ethnopharmacol. 2021, 276, 114180. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ghosh, M.; Rangra, N.K. Herbal Approaches to Diabetes Management: Pharmacological Mechanisms and Omics-Driven Discoveries. Phytother. Res. 2024. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, Y.; Liu, C.; Xie, Z.; Liu, Q.; Yang, S.; Tian, Q.; Song, C.; Chen, S. Multi-Omics Reveals the Alleviating Effect of Berberine on Ulcerative Colitis through Modulating the Gut Microbiome and Bile Acid Metabolism in the Gut-Liver Axis. Front. Pharmacol. 2024, 15, 1494210. [Google Scholar] [CrossRef]
- Yang, T.; Qin, N.; Liu, F.; Zhao, Y.; Liu, W.; Fan, D. Berberine Regulates Intestinal Microbiome and Metabolism Homeostasis to Treat Ulcerative Colitis. Life Sci. 2024, 338, 122385. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Guo, R.; Jing, W.; Lu, H. Cell Metabolomics Reveals Berberine-Inhibited Pancreatic Cancer Cell Viability and Metastasis by Regulating Citrate Metabolism. J. Proteome Res. 2020, 19, 3825–3836. [Google Scholar] [CrossRef]
- Li, Y.-H.; Sun, W.; Zhou, B.-J.; Rosenstein, A.; Zhao, J.; Wang, J.; Bian, Z.-X. iTRAQ-Based Pharmacoproteomics Reveals Potential Targets of Berberine, a Promising Therapy for Ulcerative Colitis. Eur. J. Pharmacol. 2019, 850, 167–179. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Koppen, L.M.; Whitaker, A.; Rosene, A.; Beckett, R.D. Efficacy of Berberine Alone and in Combination for the Treatment of Hyperlipidemia: A Systematic Review. J. Evid. Based Complement. Altern. Med. 2017, 22, 956–968. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.-Q.; Fan, D.-J.; Yang, S.-S.; Lin, X.; Meng, L.-K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of Berberine and Its Main Metabolites in Conventional and Pseudo Germ-Free Rats Determined by Liquid Chromatography/Ion Trap Mass Spectrometry. Drug Metab. Dispos. 2006, 34, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front. Pharmacol. 2020, 11, 594852. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Berberine, a Necessary Procedure to Understand the Mechanisms of Berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming Berberine into Its Intestine-Absorbable Form by the Gut Microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Dehau, T.; Cherlet, M.; Croubels, S.; Van De Vliet, M.; Goossens, E.; Van Immerseel, F. Berberine-Microbiota Interplay: Orchestrating Gut Health through Modulation of the Gut Microbiota and Metabolic Transformation into Bioactive Metabolites. Front. Pharmacol. 2023, 14, 1281090. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Bonaventura, A.; Bianchi, L.; Romano, D.; Maffioli, P. Effects of Berberine on Lipid Profile in Subjects with Low Cardiovascular Risk. Expert Opin. Biol. Ther. 2013, 13, 475–482. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-Analysis of the Effect and Safety of Berberine in the Treatment of Type 2 Diabetes Mellitus, Hyperlipemia and Hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Zhao, L. The Drug Interaction Potential of Berberine Hydrochloride When Co-Administered with Simvastatin, Fenofibrate, Gemfibrozil, Metformin, Glimepiride, Nateglinide, Pioglitazone and Sitagliptin in Beagles. Arab. J. Chem. 2022, 15, 103562. [Google Scholar] [CrossRef]
- Chan, E. Displacement of Bilirubin from Albumin by Berberine. Neonatology 1993, 63, 201–208. [Google Scholar] [CrossRef]
- Javed Iqbal, M.; Quispe, C.; Javed, Z.; Sadia, H.; Qadri, Q.R.; Raza, S.; Salehi, B.; Cruz-Martins, N.; Abdulwanis Mohamed, Z.; Sani Jaafaru, M.; et al. Nanotechnology-Based Strategies for Berberine Delivery System in Cancer Treatment: Pulling Strings to Keep Berberine in Power. Front. Mol. Biosci. 2020, 7, 624494. [Google Scholar] [CrossRef]
- Raju, M.; Kulkarni, Y.A.; Wairkar, S. Therapeutic Potential and Recent Delivery Systems of Berberine: A Wonder Molecule. J. Funct. Foods 2019, 61, 103517. [Google Scholar] [CrossRef]
- Behl, T.; Singh, S.; Sharma, N.; Zahoor, I.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules 2022, 27, 3705. [Google Scholar] [CrossRef]
- Demet, E.D.Ö.; Baş, K.U.; Alpaslan, A.; Aslan, D.T.; Reçber, T.; Gülsün, T.; Çelebier, M.; Göktaş, Z. Exploring the Potential of Liposomal Delivery of Naringenin and Berberine for Browning Adipose Tissue and Obesity Management. Eur. J. Lipid Sci. Technol. 2025, e70033. [Google Scholar] [CrossRef]
- Habtemariam, S. The Quest to Enhance the Efficacy of Berberine for Type-2 Diabetes and Associated Diseases: Physicochemical Modification Approaches. Biomedicines 2020, 8, 90. [Google Scholar] [CrossRef]
| Property | Value/Description |
|---|---|
| Molecular formula | C20H17NO4HCl·2H2O |
| Molecular weight | 407.65 g/mol |
| Appearance | Yellow crystalline solid |
| Melting point | 145.1–146.7 °C |
| Solubility | pH-dependent: 9.69 mM (pH 7.0, 37 °C); insoluble in nonpolar solvents [22] |
| Stability | <5% degradation (6 months, pH 1.2–9.0, 40 °C) [21] |
| Fluorescence | Absorption: 350 nm; emission: 530 nm [19] |
| Plant Taxa | Plant Family | Plant Part Used | Geographical Origin | Concentration (Reported Value) | Analytical Method for Quantification | Extraction Method | Reference |
|---|---|---|---|---|---|---|---|
| Phellodendron amurense Rupr. | Rutaceae | Bark | Dujiangyan, Sichuan, China | 0.243 mg/g of extract | UV-Vis spectrophotometry (λmax = 354 nm); UPLC-Q-TOF-MS/MS | Microwave-assisted extraction (ethanol solvent; 350 W; 75 s; material–liquid ratio 1:25 g/mL) | [23] |
| Tinospora cordifolia (Willd.) Miers | Menispermaceae | Stems | Kolkata, West Bengal, India | Not reported | HPLC-DAD | hydro–alcoholic (ethanol–water, 60:40) | [24] |
| T. cordifolia | Menispermaceae | Stems | Kharagpur, West Bengal, India | Not reported | LC-MS/MS | UAE (22.5:1 solvent-to-solid ratio, 40 min sonication time, 75% ethanol) | [25] |
| Berberis darwinii Hook. | Berberidaceae | Roots | Temuco, Chile | Up to 26,482.20 µg/g dry weight; mean: ~1437–4427 µg/g across locations | HPLC-DAD | Freeze-drying of plant tissues, acid extraction, liquid–liquid extraction with chloroform | [26] |
| B. darwinii | Stems | Temuco, Chile | Up to 6639.58 µg/g dry weight; mean: ~828–2035 µg/g across locations | ||||
| B. darwinii | Seeds | Valdivia, Chile | Up to 1181.75 µg/g dry weight; mean: ~89–467 µg/g across locations | ||||
| B. darwinii | Leaves | Nueva Imperial, Chile | Up to 511.02 µg/g dry weight; mean: ~24–118 µg/g across locations | ||||
| Coptis chinensis Franch. | Ranunculaceae | Rhizomes | Hue, Vietnam | 15.2–46.2 mg/g dry weight | HPLC | UAE (solvents: 96% lactic acid, 40% malic acid, or 88% pyruvic acid (w/w), liquid–solid ratio: 30 mL/g, temperature: 60 °C (lactic acid), 80 °C (malic acid), 75 °C (pyruvic acid), time: 30 min | [27] |
| Mahonia bealei (Fort.) Carr. | Berberidaceae | Leaves | Sichuan Province, China | Not reported | UPLC-Q-TOF-MS/MS | Ultrasonic synergistic high-speed homogeneous extraction (50% ethanol, 1:10 solid–liquid ratio, 20,000 r/min homogenization + 300 W ultrasonication for 30 min) | [28] |
| C. chinensis Franch. (as a part of a multi-herb formula) | Ranunculaceae | Not specified | China | Not reported | HPLC (UV detector) | Reflux with 75% ethanol (1:8 ratio) | [29] |
| T. cordifolia | Menispermaceae | Stems | Not specified | 2.49% (w/w) | HPTLC | MAE (500 W, 3 min, 1:15 ratio, 50 °C, methanol) | [30] |
| B. aristata DC. | Berberidaceae | Not specified | Not specified | Not specified | UHPLC-DAD | Ultrasonic bath (10 min, 25 °C, methanol) | [31] |
| C. teeta Wall. | Ranunculaceae | Rhizomes | Arunachal Pradesh, India | 212.18 ppm for MAE; 162.96 ppm for UAE | HPLC (UV detector) | MAE (65% solvent concentration, 310 W power, 30 min extraction time, and 1:39 g/mL solid–liquid ratio); UAE (36% solvent concentration, 160 W ultrasound power, 10 min extraction time, and 1:78 g/mL solid–liquid ratio) | [32] |
| Helicteres isora L. | Malvaceae | Fruits | Neyshabur, Iran | 21.37% of the total area | GC-MS | Subcritical water extraction (175 °C, 4 mL/min, 120 min, 4 g sample) | [33] |
| Thalictrum foliolosum DC. | Ranunculaceae | Roots | Almora district, Uttarakhand, India | 13.14 mg/g dry weight | HPLC-PDA | UAE using NADES (tartaric acid–glycerol, 1:1 molar ratio with 30% water content, duty cycle 80%, liquid-to-solid ratio of 30 mL/g, time: 14 min) | [34] |
| B. vulgaris | Berberidaceae | Roots | Poland | 111.06 mg/g | HPTLC | 70% aqueous ethanol, sonicated (2 cycles of 20 min each, 700 W, 50 °C) | [35] |
| Phellodendron amurense Rupr. | Rutaceae | Bark | Thua Thien Hue, Vietnam | 50.88 mg/g | HPLC | UAE (46% aqueous malic acid, 80 °C, 33.5 min, 26.8 mL/g solvent-to-solid ratio) | [36] |
| Cayratia albifolia C.L.Li | Vitaceae | Roots | Hunan Province, China | Not specified | LC-MS/MS | Boiled in 95% ethanol for 1 h | [37] |
| B. koreana Palib. | Berberidaceae | Twigs | Stobierna, Poland | 0.042–0.067% | HPTLC | Ultrasonic bath (70% ethanol, 50 °C, 2 × 20 min) | [38] |
| Berberis × ottawensis “Superba” | 0.103% | ||||||
| B. thunbergii DC. | 0.364–0.676% | ||||||
| C. chinensis Franch. | Ranunculaceae | Roots | China | Up to 10% | HPLC | DES-based UAE (choline chloride–urea (1:2 molar ratio) in 50% aqueous DES, 150 W, 60 °C, 15 min) | [39] |
| C. chinensis | Ranunculaceae | Rhizomes | Sichuan, China | 39.57–77.12 mg/g dry weight | UHPLC-PDA | DES-UA-MSPD (betaine–acrylic acid (1:4) with 50% water, silica gel as a sorbent in a 1:1 ratio with the sample, 1:62 g/mL solid-to-DES ratio, 200 W of ultrasonication for 6 min) | [40] |
| B. vulgaris L. | Berberidaceae | Stem bark | Oratia, Buzau County, Romania | 78.95 µg/g dry extract | HPLC-DAD | Hydro–ethanolic (50% ethanol) reflux extraction | [41] |
| B. vulgaris L. | Berberidaceae | Roots | Poland (commercial) | Not reported | HPLC-ESI-Q-TOF-MS/MS | ASE with methanol (90 °C, 96 bar, 4 cycles) | [42] |
| B. vulgaris L. | Berberidaceae | Stems | Tbilisi, Georgia | ||||
| Coscinium fenestratum (Gaertn.) Colebr. | Menispermaceae | Stems and Roots | Thua Thien Hue, Vietnam | 38.23 mg/g | HPLC | 60% lactic acid (w/w), liquid–solid ratio of 17.25 mL/g, 66 °C, 20 min | [43] |
| Annickia affinis (Exell) Versteegh & Sosef | Annonaceae | Stem bark | Ovia area, Benin City, Edo State, Nigeria | ~0.02% w/w | LC-ESI-MS/MS | Direct methanol extraction (pulverized stem bark shaken in methanol, 120 rpm, 72 h, 25 °C) | [44] |
| Thalictrum spp. (57 batches) | Ranunculaceae | Stems and roots | Yunnan and Xizang Provinces, China | 0.01–12.44 mg/g | LC-MS/MS | 75% methanol, solid-to-liquid ratio of 1:100, 60 min of sonication | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieniuk, B.; Pawełkowicz, M. Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management. Metabolites 2025, 15, 467. https://doi.org/10.3390/metabo15070467
Zieniuk B, Pawełkowicz M. Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management. Metabolites. 2025; 15(7):467. https://doi.org/10.3390/metabo15070467
Chicago/Turabian StyleZieniuk, Bartłomiej, and Magdalena Pawełkowicz. 2025. "Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management" Metabolites 15, no. 7: 467. https://doi.org/10.3390/metabo15070467
APA StyleZieniuk, B., & Pawełkowicz, M. (2025). Berberine as a Bioactive Alkaloid: Multi-Omics Perspectives on Its Role in Obesity Management. Metabolites, 15(7), 467. https://doi.org/10.3390/metabo15070467







