The Adjuvant Effect of Hyperbaric Oxygenation for Loxosceles rufescens Bite: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
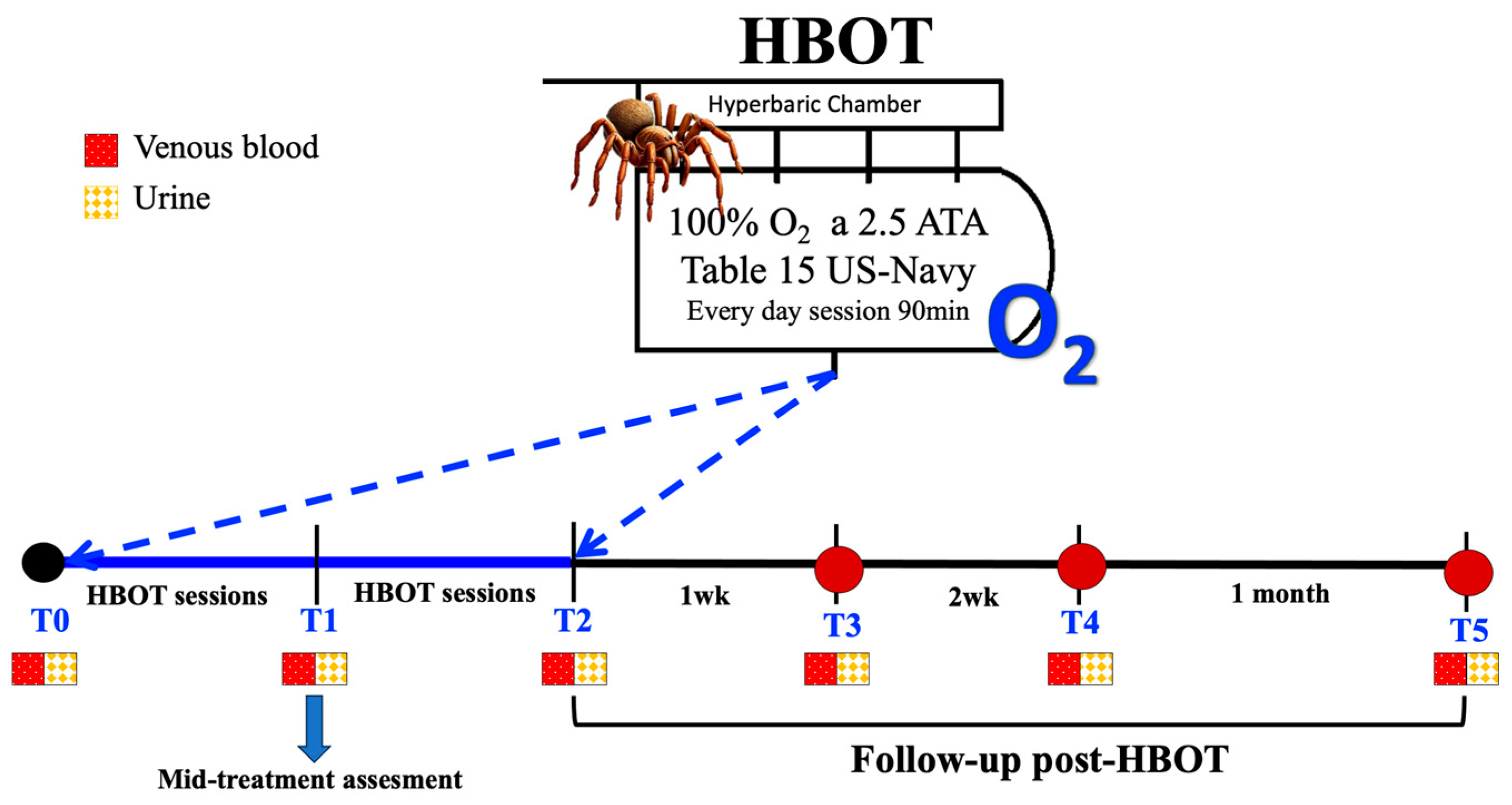
2.2. Blood and Urine Samples
2.3. HBOT Protocol
| ID | Data Bite | Gender | Bite Location | Spider Bite Site | Bite Measure | Symptoms | HBOT |
|---|---|---|---|---|---|---|---|
| 1 | March 2022 | M | Probably in the North Park in Milan | Left leg | 15 L × 15 W × 3 D mm (Blister 30 × 20 mm) | Pain. Erythema, swelling, warmth. Fever 39 °C | n = 16 sessions 2.5 ATA 90 min Table 15 |
| 2 | April 2022 | F | Near Milan (north-west area) | Neck | 70 L × 30 W mm primary lesion and 30 L × 10 W mm secondary lesion | Macular rash, diarrhea, headache, and general malaise. Fever 38 °C | n = 8 sessions 2.5 ATA 90 min Table 15 |
| 3 | April 2022 | M | Milan (north area) | Buttock | 30 L × 20 W × 2 D mm | Vomit, swelling, warmth. Fever 38 °C | n = 9 sessions 2.5 ATA 90 min Table 15 |
| 4 | May 2022 | M | Milan (north area) | Left hip | 20 L × 10 W mm lesion necrotic on the hip and 100 L × 10 W mm on the leg | Burning, swelling. Fever 38 °C | n = 8 sessions 2.5 ATA 90 min Table 15 |
| 5 | July 2022 | M | Milan (north-west area) | Left buttock and arm | 150 L × 100 W mm primary lesion and 10 L × 7 W mm secondary lesion | Pain, swelling. Fever 38 °C. | n = 9 sessions 2.5 ATA 90 min Table 15 |
| 6 | August 2022 | F | Varese | Left shoulder | Ø80 mm × 50 W mm and 5 D mm (Blister) | Swelling, warmth, pus. Fever 38 °C. | n = 14 sessions 2.5 ATA 90 min Table 15 |
| 7 | October 2022 | F | Near Milan (north-east area) | Left ankle | Ø20 mm × 3 D mm (Blister) | With pain. Erythema, pus, swelling. | n = 16 sessions 2.5 ATA 90 min Table 15 |
2.4. Biomarker Measurements
2.4.1. Biochemical and Hematologic Parameters
2.4.2. Oxidative Stress biomarkers
2.4.3. Inflammatory Biomarkers
2.4.4. Creatinine, Neopterin, and Uric Acid Concentration
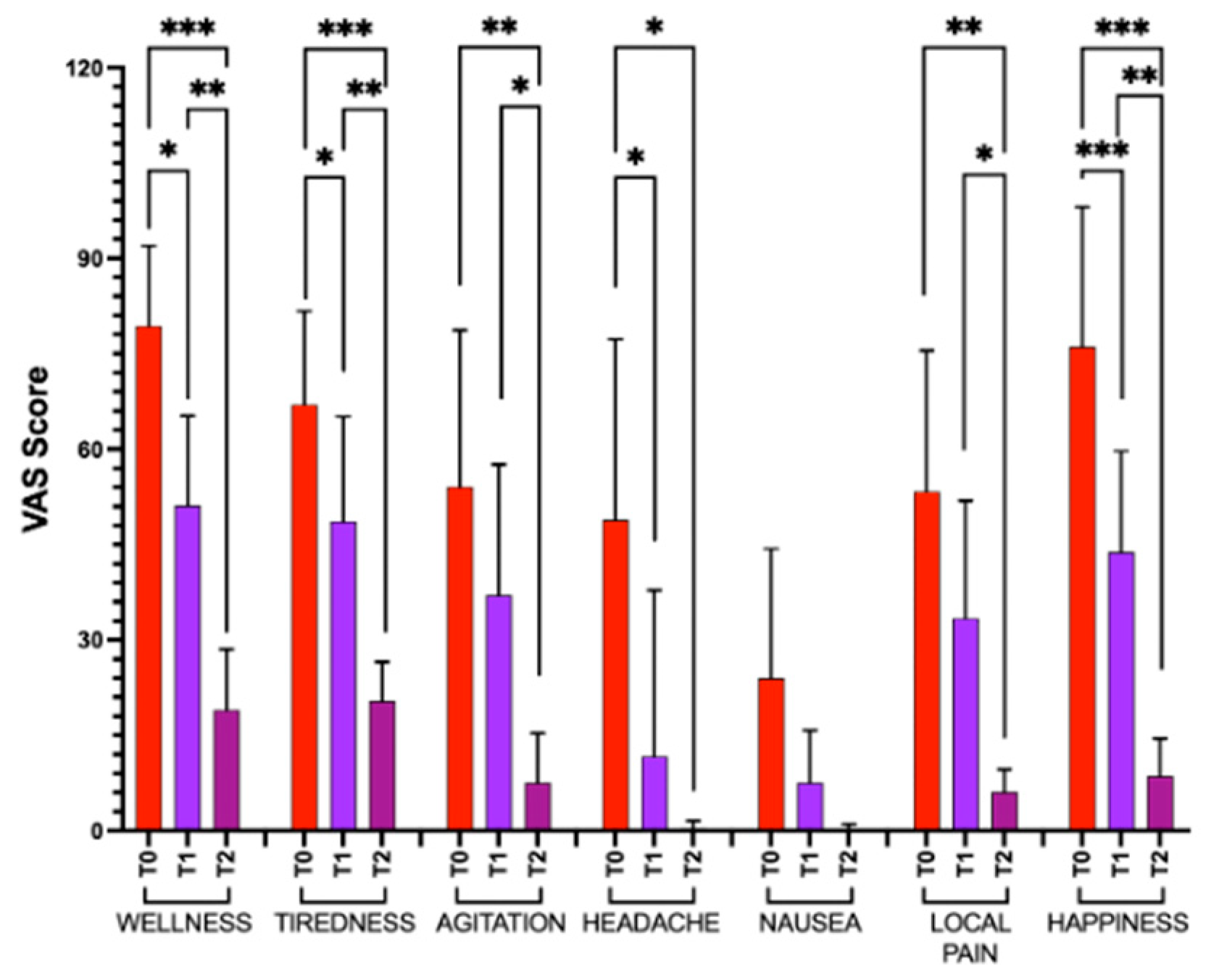
2.5. Visual Analog Scales
2.6. Statistical Analysis
3. Results
3.1. Biochemical–Hematological Parameters
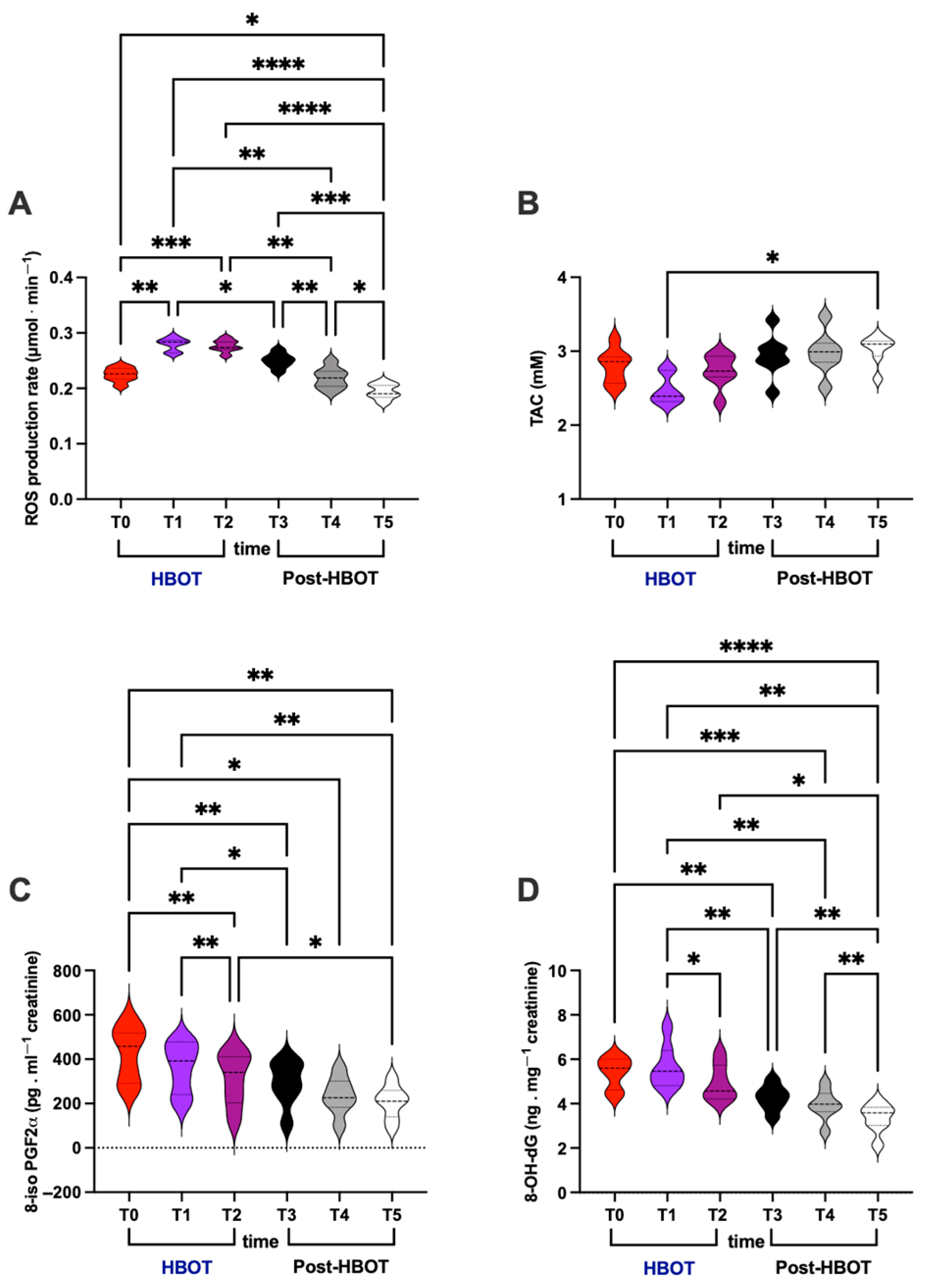
3.2. Oxidative Stress
- (i)
- ROS production rate (μmol⋅min−1; Figure 2A)
- (ii)
- TAC (mM; Figure 2B)
- (iii)
- 8-iso-PGF2α (pg⋅mg−1 creatinine; Figure 2C)
- (iv)
- 8-OH-dG (ng⋅mg−1creatinine; Figure 2D)
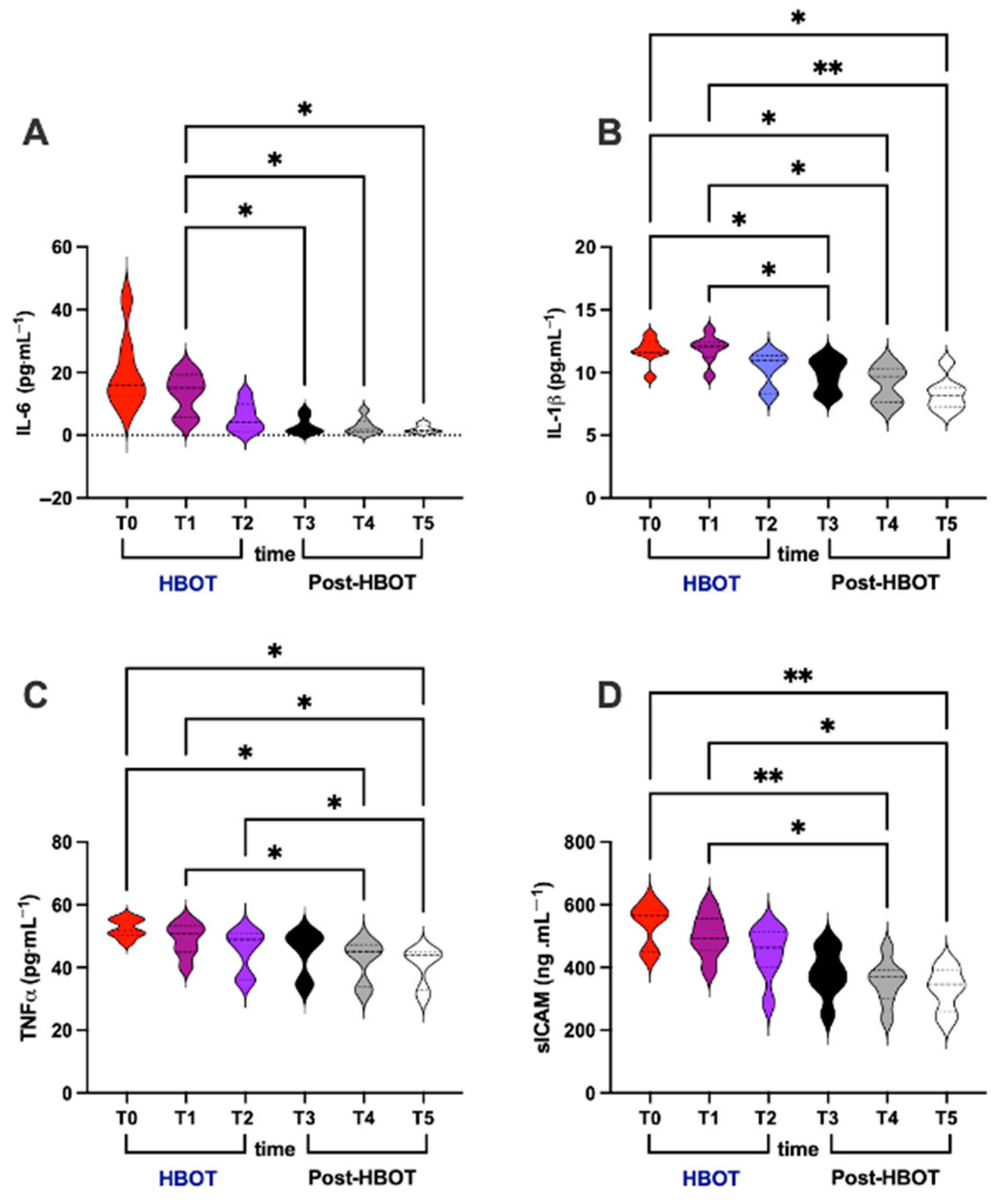
3.3. Inflammation
- (i)
- IL-6 (pg⋅mL−1; Figure 3A)
- (ii)
- IL-1β (pg⋅mL−1; Figure 3B)
- (iii)
- TNF-α (pg⋅mL−1; Figure 3C)
- (iv)
- sICAM (ng⋅mL−1; Figure 3D)
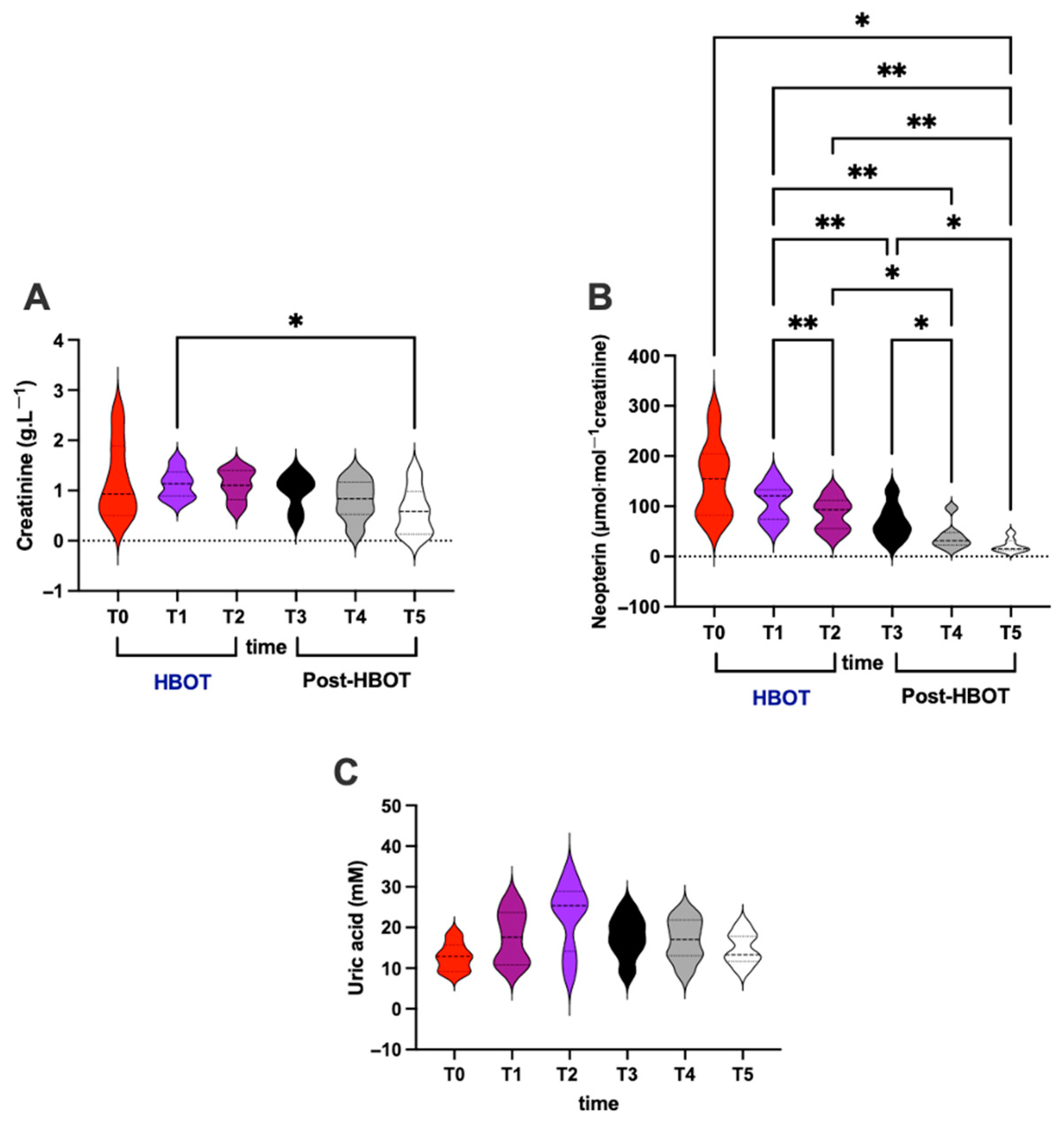
3.4. Renal and Immunological Status
- (i)
- Creatinine (g⋅L−1; Figure 4A)
- (ii)
- Neopterin (μmol⋅mol−1 creatinine; Figure 4B)
3.5. Visual Analog Scale
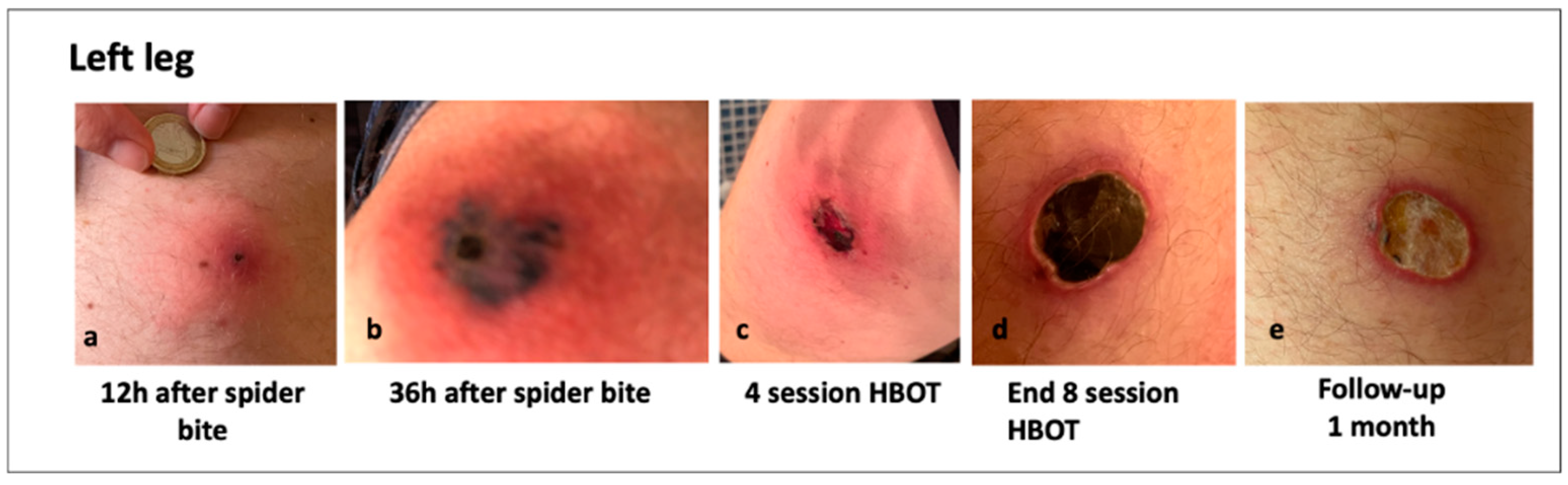
3.6. Clinical Images of Patients with a Loxosceles Rufescens Bite
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPR | Electron Paramagnetic Resonance |
| ER | Emergency Room |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| HBOT | Hyperbaric Oxygen Therapy |
| HPLC | High-pressure Liquid Chromatography |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| LR | Loxosceles rufescens |
| MCH | Mean Corpuscular Hemoglobin |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| OxS | Oxidative Stress |
| PCR | S-Reactive Protein C |
| PTT | Partial Thromboplastin P-Time |
| RBC | Red Blood Cell |
| RDW | Red Cell Distribution Width |
| ROS | Reactive Oxygen Species |
| sICAM1 | Soluble Intercellular Adhesion Molecule-1 |
| TAC | Total Antioxidant Capacity |
| TNF-α | Tumor Necrosis Factor-alpha |
| VAS | Visual Analog Scale |
| WBC | White Blood Cell |
| 8-iso-PGF2α | Lipid Peroxidation |
| 8-OH-dG | DNA Damage |
References
- Nentwig, W.; Pantini, P.; Vetter, R.S. Distribution and medical aspects of Loxosceles rufescens, one of the most invasive spiders of the world Araneae: Sicariidae. Toxicon 2017, 132, 19–28. [Google Scholar] [CrossRef] [PubMed]
- World Spider Catalog. World Spider Catalog, Version 26; Natural History Museum Bern: Bern, Switzerland, 2025. Available online: http://wsc.nmbe.ch (accessed on 1 May 2025).
- Rees, R.; Campbell, D.; Rieger, E.; King, L.E. The diagnosis and treatment of brown recluse spider bites. Ann. Emerg. Med. 1987, 16, 945–949. [Google Scholar] [CrossRef]
- Anoka, I.A.; Robb, E.L.; Baker, M.B. Brown Recluse Spider Toxicity. In StatPearls [Internet]; StatPearls Publishing: Island, FL, USA, 2022. [Google Scholar]
- Chaves-Moreira, D.; Senff-Ribeiro, A.; Wille, A.C.M.; Gremski, L.H.; Chaim, O.M.; Veiga, S.S. Highlights in the knowledge of brown spider toxins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Futrell, J.M. Loxoscelism. Am. J. Med. Sci. 1992, 304, 261–267. [Google Scholar] [CrossRef]
- Veraldi, S.; Schianchi, R.; Nazzaro, G. Necrotic ulcers caused by Loxosceles rufescens bites: A report of seven patients and scanning electron microscopy of the spider. Eur. J. Dermatol. 2024, 34, 267–270. [Google Scholar] [CrossRef]
- Swanson, D.L.; Vetter, R.S. Loxoscelism. Clin Dermatol. 2006, 24, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Garvey, E.M.; DeLong, W.G. Dog bite wounds: Bacterial complications and tissue necrosis. J. Trauma 2002, 52, 390–392. [Google Scholar]
- Boia-Ferreira, M.; Moreno, K.G.; Basílio, A.B.C.; da Silva, L.P.; Vuitika, L.; Soley, B.; Wille, A.C.M.; Donatti, L.; Barbaro, K.C.; Chaim, O.M.; et al. Correction: Boia-Ferreira et al. TCTP fromLoxosceles Intermedia(Brown Spider) Venom Contributes to the Allergic and Inflammatory Response of Cutaneous Loxoscelism. Cells 2019, 8, 1489. [Google Scholar] [CrossRef]
- King, L.E.; Rees, R.S. Dapsone treatment of a brown recluse bite. J. Am. Med. Assoc. 1983, 250, 648. [Google Scholar] [CrossRef]
- Fusto, G.; Bennardo, L.; Del Luca, E.; Mazzucca, D.; Tamburi, F.; Patruno, C.; Nisticò, S.P. Spider bites of medical significance in the Mediterranean area: Misdiagnosis, clinical features and management. J. Venom. Anim. Toxins incl. Trop. Dis. 2020, 26, e20190100. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.P.; de Castro, F.J.; Vuitika, L.; Polli, N.L.C.; Antunes, B.C.; Bóia-Ferreira, M.; Minozzo, J.C.; Mariutti, R.B.; Matsubara, F.H.; Arni, R.K.; et al. Brown Spiders’ Phospholipases-D with Potential Therapeutic Applications: Functional Assessment of Mutant Isoforms. Biomedicines 2021, 9, 320. [Google Scholar] [CrossRef]
- Gremskim, L.H.; Trevisan-Silva, D.; Ferrer, V.P.; Matsubara, F.H.; Meissner, G.O.; Wille, A.C.M. Recent Advances in the Understanding of Brown Spider Venoms: From the Biology of Spiders to the Molecular Mechanisms of Toxins. Toxicon 2014, 83, 91–120. [Google Scholar] [CrossRef]
- Gremskim, L.H.; Da Justam, H.C.; Da Silvam, T.P.; Pollim, N.L.C.; Antunesm, B.C.; Minozzom, J.C. Forty Years of the Description of Brown Spider Venom Phospholipases-D. Toxins 2020, 12, 164. [Google Scholar] [CrossRef]
- Dunbar, J.P.; Sulpice, R.; Dugon, M.M. The kiss of (cell) death: Can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin. Toxicol. 2019, 57, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Rekow, M.A.; Civello, D.J.; Geren, C.R. Enzymatic and hemolytic properties of brown recluse spider (Loxosceles reclusa) toxin and extracts of venom apparatus, cephalothorax and abdomen. Toxicon 1983, 21, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, K.; Hemshekhar, M.; Thushara, R.M.; Santhosh, M.S.; Shanmuga, M.; Sundaram, M.; Kemparaju, K.; Girish, K.S. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon 2015, 98, 89–97. [Google Scholar] [CrossRef]
- Petricevichm, V.L. Scorpion venom and the inflammatory response. Mediat. Inflamm. 2010, 2010, 903295. [Google Scholar]
- Hobbs, G.D.; Anderson, A.R.; Greene, T.J.; Yealy, D.M. Comparison of hyperbaric oxygen and dapsone therapy for Loxosceles envenomation. Acad. Emerg. Med. 1996, 3, 758–761. [Google Scholar] [CrossRef]
- Andersen, R.J.; Campoli, J.; Johar, S.K.; Schumaker, K.A.; Allison, E.J. Suspected brown recluse envenomation: A case report and review of different treatment modalities. J. Emerg. Med. 2011, 41, e31–e37. [Google Scholar] [CrossRef]
- Hadanny, A.; Fishlev, G.; Bechor, Y.; Meir, O.; Efrati, S. Nonhealing wounds caused by brown spider bites: Application of hyperbaric oxygen therapy. Adv. Ski. Skin. Wound Care 2016, 29, 560–566. [Google Scholar] [CrossRef]
- Zanon, V.; Morri, A.; Lonati, D.; Paoli, A.; Camporesi, E.M.; Bosco, G. HBO2 in snake envenomation (atrox albinus rattlesnake): A case report in a human. Undersea Hyperb. Med. 2016, 43, 4. [Google Scholar]
- Marmo, M.; Villani, R.; Di Minno, R.M.; Noschese, G.; Paganini, M.; Quartesan, S.; Rizzato, A.; Bosco, G. Cave canem: HBO2 therapy efficacy on Capnocytophaga canimorsus infections: A case series. Undersea Hyperb. Med. 2017, 44, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.M.; Brunetti, E.; Barruscotti, S.; Brazzelli, V. Brown recluse (L. rufescens) can bite in Northern Italy, too: First case report and review of the literature. BMJ Case Rep 2019, 12, e230000. [Google Scholar] [CrossRef]
- Starace, M.; Ferrari, T.; Bruni, F.; Patrizi, A.; Alessandrini, A. Report of cutaneous loxoscelism caused by violin spider bite in Northern Italy. Int. J. Dermatol. 2021, 61, E88–E89. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.H.; Da Silveira, R.B.; Helena Appel, M.; Mangili, O.C.; Gremski, W.; Veiga, S.S. Brown Spiders and Loxoscelism. Toxicon 2004, 44, 693–709. [Google Scholar] [CrossRef]
- Isbister, G.K.; Fan, H.W. Spider Bite. Lancet 2021, 378, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.H.; Squaiella-Baptistão, C.C.; Marques, M.O.T.; Tambourgi, D.V. Clinical Aspects, Diagnosis and Management of Loxosceles Spider Envenomation: Literature and Case Review. Arch. Toxicol. 2020, 94, 1461–1477. [Google Scholar] [CrossRef]
- Manríquez, J.J.; Silva, S. Cutaneous and Visceral Loxoscelism: A Systematic Review. Rev. Chil. Infectol. 2009, 26, 420–432. [Google Scholar] [CrossRef]
- Miranda, A.L.S.; de Guerra-Duarte, C.; Guerra-Duart, S.d.A.; Chávez-Olórtegui, C.; Soto-Blanco, B. History, Challenges and Perspectives on Loxosceles (Brown Spiders) Antivenom Production in Brazil. Toxicon 2021, 192, 40–45. [Google Scholar] [CrossRef]
- Svendsen, F.J. Treatment of clinically diagnosed brown recluse spider bites with hyperbaric oxygen: A clinical observation. J. Ark. Med. Soc. 1986, 83, 199–204. [Google Scholar] [PubMed]
- Strain, G.M.; Snider, T.G.; Tedford, B.L.; Cohn, G.H. Hyperbaric oxygen effects on brown reclus espider (Loxosceles reclusa) envenomation in rabbits. Toxicon 1991, 29, 989–996. [Google Scholar] [CrossRef]
- Hobbs , G.D. Brown recluse spider envenomation: Is hyperbaric oxygen the answer? Hobbs GD. Acad Emerg Med. 1997, 4, 165–166. [Google Scholar] [CrossRef]
- Bangasser, R. Treatment of the brown recluse spider (Loxosceles reclusa) bite with hyperbaric oxygen Therapy. In Hyperbaric Medicine Practice; Kindwall, E.P., Whelan, H.T., Eds.; Best Publishing Co.: Flagstaff, Ariz, 1999; pp. 869–877. [Google Scholar]
- Bosco, G.; Brizzolari, A.; Paganini, M.; Camporesi, E.; Vezzoli, A.; Mrakic-Sposta, S. Oxy-inflammation in hyperbaric oxygen therapy applications. Eur. J. Transl. Myol. 2025, 35, 12783. [Google Scholar] [CrossRef]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L.; et al. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Vezzoli, A.; Garetto, G.; Paganini, M.; Camporesi, E.; Giacon, T.A.; Dellanoce, C.; Agrimi, J.; Bosco, G. Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes. Metabolites 2023, 13, 1032. [Google Scholar] [CrossRef]
- Bosco, G.; Landolfi, A.; Giacon, T.A.; Vezzoli, A.; Paolocci, N.; Mrakic-Sposta, S. Short-term suborbital space flight curtails astronauts’ dopamine levels increasing cortisol/BDNF and prompting pro-oxidative/inflammatory milieu. Mil. Med. Res. 2025, 12, 2. [Google Scholar] [CrossRef]
- Montorsi, M.; Vezzoli, A.; Mrakic Sposta, F.; Gussoni, M.; Brizzolari, A.; Bosco, G.; Dellanoce, C.; Barassi, A.; Picconi, B.; Ranuncoli, C.; et al. Systemic Responses Towards Oxy-Inflammation, Hormones, and Mood in Breast Cancer Survivors: Preliminary Evidences from Dragon Boat Endurance Race. J. Clin. Med. 2025, 14, 2532. [Google Scholar] [CrossRef] [PubMed]
- Mrakic Sposta, S.; Ferrucci, R.; Marceglia, S.; Vezzoli, A.; Parazzini, M.; Fiocchi, S.; Vergari, M.; Floro, S.; Deriz, M.; Priori, A. Spinal tDCS on Oxy-Inflammation in patients with Multiple Sclerosis: A pilot study. Brain Stimul. 2025, 18, 1131–1133. [Google Scholar] [CrossRef]
- Collins, S.L.; Moore, R.A.; McQuay, H.J. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain 1997, 72, 95–97. [Google Scholar] [CrossRef]
- Rees, R.S.; Altenbern, D.P.; Lynch, J.; King, L.E., Jr. Brown recluse spider bites. A comparison of early surgical excision versus dapsone and delayed surgical excision. Ann. Surg. 1985, 202, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Pandey, M. Loxoscelism: Cutaneous and Hematologic Manifestations. Adv. Hematol. 2019, 2019, 4091278. [Google Scholar] [CrossRef]
- Anwar, S.; Torosyan, R.; Ginsberg, C.; Liapis, H.; Morrison, A.R. Clinicopathological course of acute kidney injury following brown recluse (Loxoscles reclusa) envenomation. Clin. Kidney J. 2013, 6, 609–612. [Google Scholar] [CrossRef]
- de-Almeida, D.M.; Squaiella-Baptistão, C.C.; Lopes, P.H.; van den Berg, C.W.; Tambourgi, D.V. Loxosceles venom Sphingomyelinase D activates human blood leukocytes: Role of the complement system. Mol. Immunol. 2018, 94, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.H.; van den Berg, C.W.; Tambourgi, D.V. Sphingomyelinases D From Loxosceles Spider Venoms and Cell Membranes: Action on Lipid Rafts and Activation of Endogenous Metalloproteinases. Front. Pharmacol. 2020, 11, 636. [Google Scholar] [CrossRef]
- de Souza, A.L.; Malaque, C.M.; Sztajnbok, J.; Romano, C.C.; Duarte, A.J.; Seguro, A.C. Loxosceles venom-induced cytokine activation, hemolysis, and acute kidney injury. Toxicon 2008, 51, 151–156. [Google Scholar] [CrossRef]
- Peterson, M.E. Brown spider envenomation. Clin. Tech. Small Anim. Pract. 2006, 21, 191–193. [Google Scholar] [CrossRef]
- Maynor, M.L.; Moon, R.E.; Klitzman, B.; Fracica, P.J.; Canada, A. Brown recluse spider envenomation: A prospective trial of hyperbaric oxygen therapy. Acad. Emerg. Med. 1997, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- da Silvam, M.S.; Lopesm, P.H.; Eliasm, M.C.; Tambourgim, D.V. Cytotoxic and genotoxic effects on human keratinocytes triggered by sphingomyelinase D from Loxosceles venom. Arch. Toxicol. 2020, 94, 3563–3577. [Google Scholar] [CrossRef]

| Biochemical Hematological Parameters | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Leukocytes (WBC) (109/L) (range 4.5 to 11.0 × 109/L) | 11.71 H | 9.53 | 18.29 H | 8.83 | 16.65 H | 12.05 H | 9.95 |
| Erythrocytes (RBC) (1012/µL) (range M: 4.7–6.1 1012/µL; F: 4.2–5.4 1012/µL) | 4.83 | 4.81 | 5.21 | 4.73 | 4.66 | 4.50 | 3.91 L |
| Hemoglobin (Hb) (g/dL) (range M: 13.8–17.2 g/dL; F: 12.1–15.1 g/dL) | 14 | 13.5 | 16.6 | 14.4 | 14.1 | 14.9 | 11.9 L |
| Hematocrit (Hct) (%) (range M: 40–54%; F: 36–48%) | 42.8 | 41.8 | 48.7 | 42.5 | 42 | 43.6 | 36.0 L |
| Corpuscular Volume (fL) (range 80–100 fL) | 88.6 | 86.9 | 93.5 | 89.9 | 90.1 | 96.9 | 92.1 |
| MCH (pg) (range 27–33 pg) | 29 | 28.1 | 31.9 | 30.4 | 30.3 | 33.1 H | 30.4 |
| MCHC (g/dL) (range 32–26 g/dL) | 32.7 | 32.3 | 34.1 | 33.9 | 33.6 | 34.2 | 33.1 |
| RDW (%) (range 11.5–14.5%) | 13 | 14.4 | 13.2 | 12.6 | 13 | 14.4 | 13 |
| Platelets (109/L) (range 150–450 109/L) | 151 | 298 | 220 | 273 | 218 | 212 | 305 |
| Neutrophils (%) (range 40–60%) | 76.1 H | 65.3 H | 78.5 H | 82.2 H | 88.3 H | 70.3 H | 71.2 H |
| Lymphocytes (%) (range 20–40%) | 12.7 L | 24.3 | 11.4 L | 8.9 L | 4.3 L | 19.7 L | 21.3 |
| Monocytes (%) (range 2–10%) | 10.9 H | 6.8 | 9.7 | 7.1 | 6.7 | 9.5 | 6.9 |
| Eosinophilis (%) (range 0–6%) | 0.0 | 2.8 | 0.1 | 1.7 | 0.5 | 0.3 | 0.4 |
| Basophiles (%) (range 0.5–1%) | 0.3 | 0.8 | 0.3 | 0.1 | 0.2 | 0.2 | 0.2 |
| Neutrophils (109/L) (range 2.0–7.0 × 109/L) | 8.15 H | 7.21 H | 14.36 H | 7.25 H | 14.70 H | 8.46 H | 7.08 H |
| Lymphocytes (109/L) (range 1.5–3.5 × 109/L) | 1.36 | 2.32 | 2.8 | 0.79 L | 0.72 L | 2.37 | 2.12 |
| Monocytes (109/L) (range 0.2–0.8 × 109/L) | 1.17 H | 0.65 | 1.77 H | 0.63 | 1.11 H | 1.15 | 0.69 |
| Eosinophilis (109/L) (range 0.04–0.4 × 109/L) | 0.00 | 0.27 | 0.02 | 0.15 | 0.09 | 0.04 | 0.04 |
| Basophiles (109/L) (range 0.01–0.1 × 109/L) | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Prothrombin Time (PT) | |||||||
| INR (range 0.8–1.1) | 1.08 | 0.97 | 1.13 | 1.08 | 1.14 H | 1.16 H | 1.22 H |
| PT sec (range 11–13.5 s) | 13 | 12 | 14 | 13 | 13 | 13 | 14 |
| Partial Thromboplastin P-Time (PTT) s (range 25–35 s) | 27 | 28.7 | 39.7 H | 27.7 | 25.1 | 29 | 33.5 |
| S-Alanine Aminotransferase (U/L) (range M: 30 U/L; F: 19 U/L) | 16 | 17 | 29 | 58 H | 50 H | 12 | 188 H |
| S-Creatine Kinase (U/L) (range 20–170 U/L) | 140 | 51 | 114 | 52 | 67 | 170 H | 85 |
| S-Creatinine (mg/dL) (range M: 0.7–1.3 mg/dL; F: 0.6–1.1 mg/dL) | 1.17 | 0.72 | 1.04 | 1.26 H | 1.11 | 1.1 H | 0.6 |
| S-Glucose (mg/dL) (range 70–100 mg/dL) | 112 H | 86 | 117 H | 100 H | 131 H | 153 H | 94 |
| S-Urea (mg/dL) (range 15–55 mg/dL) | 27 | 29 | 41 | 39 | 45 | 26 | 25 |
| S-Total Ca (mg/dL) (range 8.5–10.5 mg/dL) | 9.5 | 9.5 | 10 | 9.5 | 9.4 | 9.6 | 9.4 |
| S-Na (mmoli/L) (range 136–145 mmoli/L) | 136 | 140 | 137 | 138 | 143 | 133 | 141 |
| S-K (mmoli/L) (range 3.5–5.5 mmoli/L) | 4.03 | 4.64 | 4.17 | 3.75 | 4.21 | 4.89 | 3.61 |
| S-Alpha-Amylase (U/L) (range 30–110 U/L) | 64 | - | 84 | 84 | 42 | - | - |
| S-Total Biliruin (mg/dL) (range 0.2–1.0 mg/dL) | 0.68 | 0.45 | 5.87 H | 0.81 | 0.55 | 1.07 H | 0.35 |
| S-Reactive Protein C (PRC) (mg/dL) (range < 0.3 mg/dL) | 2.8 H | 2.0 H | 8.7 H | 6.5 H | 3.5 H | 11.1 H | 4.6 H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrakic-Sposta, S.; Vezzoli, A.; Graci, C.; Gussoni, M.; Cimmino, A.; Dellanoce, C.; Camporesi, E.M.; Sesana, G.; Bosco, G. The Adjuvant Effect of Hyperbaric Oxygenation for Loxosceles rufescens Bite: A Case Series. Metabolites 2025, 15, 470. https://doi.org/10.3390/metabo15070470
Mrakic-Sposta S, Vezzoli A, Graci C, Gussoni M, Cimmino A, Dellanoce C, Camporesi EM, Sesana G, Bosco G. The Adjuvant Effect of Hyperbaric Oxygenation for Loxosceles rufescens Bite: A Case Series. Metabolites. 2025; 15(7):470. https://doi.org/10.3390/metabo15070470
Chicago/Turabian StyleMrakic-Sposta, Simona, Alessandra Vezzoli, Carmela Graci, Maristella Gussoni, Attilio Cimmino, Cinzia Dellanoce, Enrico Maria Camporesi, Giovanni Sesana, and Gerardo Bosco. 2025. "The Adjuvant Effect of Hyperbaric Oxygenation for Loxosceles rufescens Bite: A Case Series" Metabolites 15, no. 7: 470. https://doi.org/10.3390/metabo15070470
APA StyleMrakic-Sposta, S., Vezzoli, A., Graci, C., Gussoni, M., Cimmino, A., Dellanoce, C., Camporesi, E. M., Sesana, G., & Bosco, G. (2025). The Adjuvant Effect of Hyperbaric Oxygenation for Loxosceles rufescens Bite: A Case Series. Metabolites, 15(7), 470. https://doi.org/10.3390/metabo15070470









