Plasma and Urine Metabolites Associated with Microperimetric Retinal Sensitivity in Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Study Protocol
2.4. AMD Grading
2.5. MAIA Microperimetry Testing
2.6. Metabolomic Profiling
2.7. Statistical Analysis
3. Results
3.1. Demographics
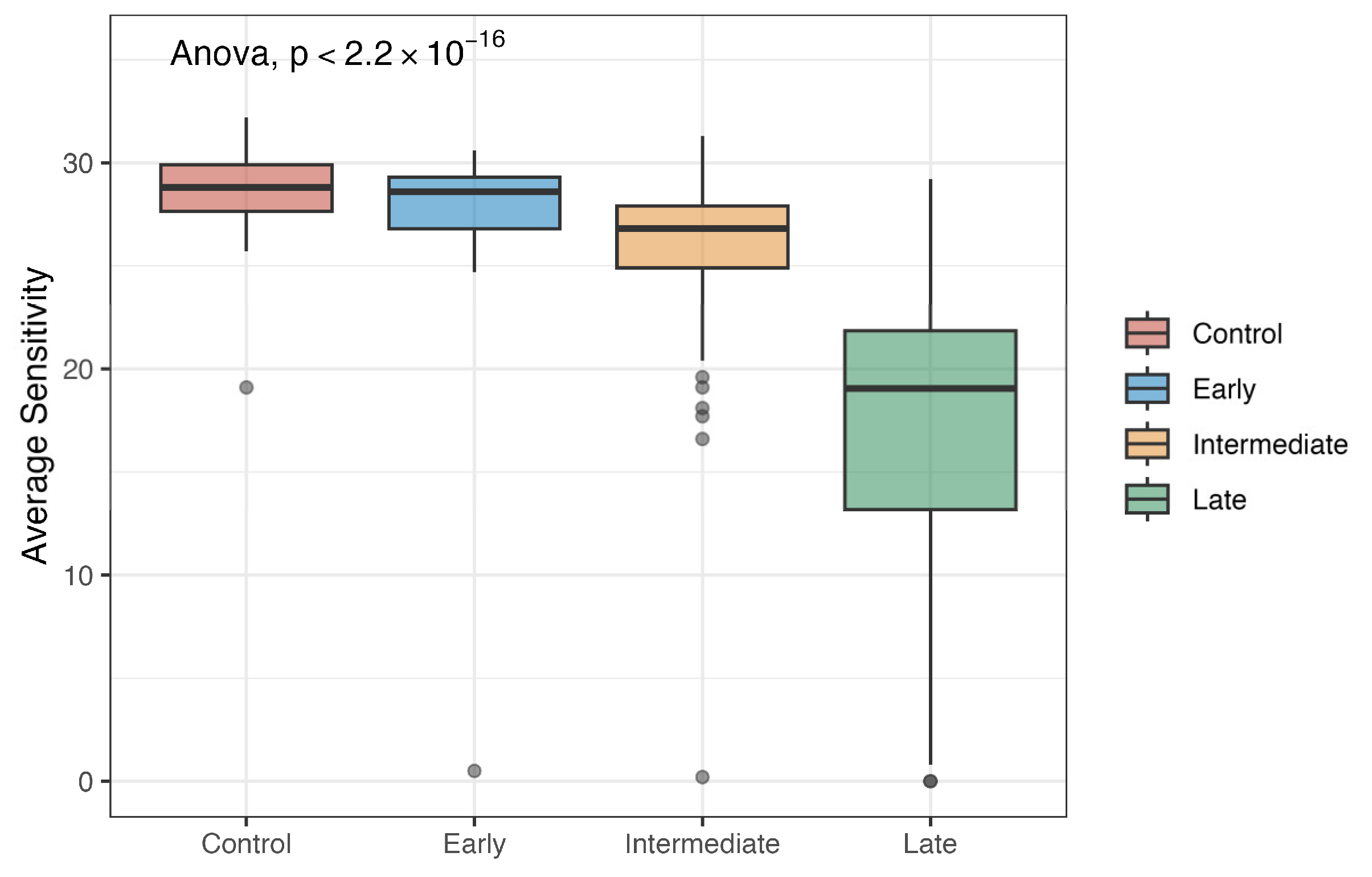
3.2. Average Sensitivity by Stage
3.3. Microperimetry and Plasma Metabolites
3.4. Microperimetry and Urine Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vyawahare, H.; Shinde, P. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 2022, 14, e29583. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, A.; Stinnett, S.S.; Petrowski, J.T.; Schuman, S.G.; Toth, C.A.; Cousins, S.W.; Lad, E.M. Visual Function Measures in Early and Intermediate Age-Related Macular Degeneration. Retina 2016, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Pilotto, E. Microperimetry in Age: Related Macular Degeneration. Eye 2017, 31, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Mendez, K.M.; Kim, J.; Laíns, I.; Nigalye, A.; Katz, R.; Pundik, S.; Kim, I.K.; Liang, L.; Vavvas, D.G.; Miller, J.B.; et al. Association of Human Plasma Metabolomics with Delayed Dark Adaptation in Age-Related Macular Degeneration. Metabolites 2021, 11, 183. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An Emerging but Powerful Tool for Precision Medicine. Mol. Case Stud. 2015, 1, 588. [Google Scholar] [CrossRef]
- Laíns, I.; Gantner, M.; Murinello, S.; Lasky-Su, J.A.; Miller, J.W.; Friedlander, M.; Husain, D. Metabolomics in the Study of Retinal Health and Disease. Prog. Retin. Eye Res. 2019, 69, 57–79. [Google Scholar] [CrossRef]
- Lains, I.; Han, X.; Gil, J.; Providencia, J.; Nigalye, A.; Alvarez, R.; Douglas, V.P.; Mendez, K.; Katz, R.; Tsougranis, G.; et al. Plasma Metabolites Associated with OCT Features of Age-Related Macular Degeneration. Ophthalmol. Sci. 2024, 4, 100357. [Google Scholar] [CrossRef]
- Lains, I.; Mendez, K.M.; Gil, J.Q.; Miller, J.B.; Kelly, R.S.; Barreto, P.; Kim, I.K.; Vavvas, D.G.; Murta, J.N.; Liang, L.; et al. Urinary Mass Spectrometry Profiles in Age-Related Macular Degeneration. J. Clin. Med. 2022, 11, 940. [Google Scholar] [CrossRef]
- Roh, M.; Laíns, I.; Shin, H.J.; Park, D.H.; Mach, S.; Vavvas, D.G.; Kim, I.K.; Miller, J.W.; Husain, D.; Miller, J.B. Microperimetry in Age-Related Macular Degeneration: Association with Macular Morphology Assessed by Optical Coherence Tomography. Br. J. Ophthalmol. 2019, 103, 1769–1776. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Laíns, I.; Chung, W.; Kelly, R.S.; Gil, J.; Marques, M.; Barreto, P.; Murta, J.N.; Kim, I.K.; Vavvas, D.G.; Miller, J.B.; et al. Human Plasma Metabolomics in Age-Related Macular Degeneration: Meta-Analysis of Two Cohorts. Metabolites 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Li, M.-X.; Yeung, J.M.Y.; Cherny, S.S.; Sham, P.C. Evaluating the Effective Numbers of Independent Tests and Significant P-Value Thresholds in Commercial Genotyping Arrays and Public Imputation Reference Datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, D.R. A Simple Correction for Multiple Testing for Single-Nucleotide Polymorphisms in Linkage Disequilibrium with Each Other. Am. J. Hum. Genet. 2004, 74, 765–769. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- Theodoridis, K.; Gika, H.; Kotali, A. Acylcarnitines in Ophthalmology: Promising Emerging Biomarkers. Int. J. Mol. Sci. 2022, 23, 16183. [Google Scholar] [CrossRef]
- Liew, G.; Tse, B.; Ho, I.-V.; Joachim, N.; White, A.; Pickford, R.; Maltby, D.; Gopinath, B.; Mitchell, P.; Crossett, B. Acylcarnitine Abnormalities Implicate Mitochondrial Dysfunction in Patients With Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines-Old Actors Auditioning for New Roles in Metabolic Physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- Fu, Z.; Kern, T.S.; Hellström, A.; Smith, L.E.H. Fatty Acid Oxidation and Photoreceptor Metabolic Needs. J. Lipid Res. 2021, 62, 100035. [Google Scholar] [CrossRef]
- Lains, I.; Mendez, K.; Nigalye, A.; Katz, R.; Douglas, V.P.; Kelly, R.S.; Kim, I.K.; Miller, J.B.; Vavvas, D.G.; Liang, L.; et al. Plasma Metabolomic Profiles Associated with Three-Year Progression of Age-Related Macular Degeneration. Metabolites 2022, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, O.A.; Mohiuddin, S.S. Biochemistry, Oxidative Phosphorylation; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Storey, K.B. Oxidative Stress Concept Updated: Definitions, Classifications, and Regulatory Pathways Implicated. EXCLI J. 2021, 20, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.M.; Akhtar, S.; Currie, Z. Ageing Changes in the Eye. Postgrad. Med. J. 2006, 82, 581–587. [Google Scholar] [CrossRef]
- Zafrilla, P.; Losada, M.; Perez, A.; Caravaca, G.; Mulero, J. Biomarkers of Oxidative Stress in Patients with Wet Age Related Macular Degeneration. J. Nutr. Health Aging 2013, 17, 219–222. [Google Scholar] [CrossRef]
- Ehrlich, R.; Harris, A.; Kheradiya, N.S.; Winston, D.M.; Ciulla, T.A.; Wirostko, B. Age-Related Macular Degeneration and the Aging Eye. Clin. Interv. Aging 2008, 3, 473–482. [Google Scholar] [CrossRef]
| 0 (N = 95) | Early (N = 25) | Intermediate (N = 199) | Late (N = 44) | Overall (N = 363) | |
|---|---|---|---|---|---|
| Eye | |||||
| OD | 49 (51.6%) | 15 (60.0%) | 96 (48.2%) | 24 (54.5%) | 184 (50.7%) |
| OS | 46 (48.4%) | 10 (40.0%) | 103 (51.8%) | 20 (45.5%) | 179 (49.3%) |
| Average Sensitivity | |||||
| Mean (SD) | 28.7 (1.73) | 27.1 (5.79) | 26.1 (3.18) | 17.2 (8.05) | 25.7 (5.26) |
| Median [Min, Max] | 28.8 [19.1,32.2] | 28.6 [0.500, 30.6] | 26.8 [0.200, 31.3] | 19.1 [0, 29.2] | 27.3 [0, 32.2] |
| Age | |||||
| Mean (SD) | 64.7 (6.89) | 66.7 (7.45) | 69.9 (6.32) | 72.6 (10.4) | 68.6 (7.58) |
| Median [Min, Max] | 65.0 [50.0, 83.0] | 67.0 [54.0, 81.0] | 70.0 [54.0, 86.0] | 74.0 [45.0, 91.0] | 69.0 [45.0, 91.0] |
| BMI | |||||
| Mean (SD) | 26.8 (4.10) | 27.2 (4.28) | 27.7 (5.22) | 27.2 (5.86) | 27.4 (4.97) |
| Median [Min, Max] | 25.8 [18.7, 42.9] | 25.7 [22.0, 36.1] | 26.5 [19.2, 53.0] | 26.4 [19.2, 52.9] | 26.3 [18.7, 53.0] |
| Smoking | |||||
| Ex-smoker | 38 (40.0%) | 7 (28.0%) | 103 (51.8%) | 25 (56.8%) | 173 (47.7%) |
| Nonsmoker | 55 (57.9%) | 18 (72.0%) | 90 (45.2%) | 19 (43.2%) | 182 (50.1%) |
| Smoker | 2 (2.1%) | 0 (0%) | 6 (3.0%) | 0 (0%) | 8 (2.2%) |
| AREDS | |||||
| No | 95 (100%) | 22 (88.0%) | 48 (24.1%) | 10 (22.7%) | 175 (48.2%) |
| Yes | 0 (0%) | 3 (12.0%) | 151 (75.9%) | 34 (77.3%) | 188 (51.8%) |
| Analysis | Super Pathway | Sub-Pathway | Metabolite | Coefficient | p-Value |
|---|---|---|---|---|---|
| All | Energy | Oxidative Phosphorylation | Phosphate | −10.0 | 3.72 × 10−5 |
| Amino Acid | Tryptophan Metabolism | Oxindolylalanine | 3.4 | 0.0009 | |
| Controls | Energy | Oxidative Phosphorylation | Phosphate | −6.5 | 0.0004 |
| AMD | Energy | Oxidative Phosphorylation | Phosphate | −11.2 | 0.0005 |
| Amino Acid | Lysine Metabolism | Glutarylcarnitine (C5-DC) | 3.0 | 0.001 |
| Analysis | Super Pathway | Sub-Pathway | Metabolite | Coefficient | p-Value |
|---|---|---|---|---|---|
| All | Amino Acid | Lysine Metabolism | 2-oxoadipate | −0.7 | 3.87 × 10−5 |
| Nucleotide | Purine Metabolism, (Hypo)Xanthine/Inosine containing | urate | −6.1 | 8.24 × 10−5 | |
| Amino Acid | Leucine, Isoleucine, and Valine Metabolism | ethylmalonate | −2.9 | 8.65 × 10−5 | |
| Amino Acid | Lysine Metabolism | N6-acetyllysine | −5.2 | 0.0001 | |
| Amino Acid | Lysine Metabolism | 5-hydroxylysine | −1.8 | 0.0001 | |
| Amino Acid | Tryptophan Metabolism | 3-hydroxyanthranilate | −2.0 | 0.0008 | |
| Lipid | Progestin Steroids | 5alpha-pregnan-3beta, 20alpha-diol disulfate | −1.4 | 0.001 | |
| Carbohydrate | Aminosugar Metabolism | Erythronate * | −4.5 | 0.001 | |
| AMD | Amino Acid | Lysine Metabolism | 2-oxoadipate | −0.7 | 0.0002 |
| Amino Acid | Leucine, Isoleucine, and Valine Metabolism | ethylmalonate | −3.4 | 0.0003 | |
| Nucleotide | Purine Metabolism, (Hypo)Xanthine/Inosine containing | urate | −7.4 | 0.0004 | |
| Amino Acid | Lysine Metabolism | 5-hydroxylysine | −2.3 | 0.0006 | |
| Lipid | Pregnenolone Steroids | pregnenetriol disulfate * | −2.3 | 0.0009 | |
| Amino Acid | Lysine Metabolism | N6-acetyllysine | −5.7 | 0.001 | |
| Carbohydrate | Aminosugar Metabolism | erythronate * | −5.8 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sourirajan, K.; Mendez, K.; Lains, I.; Tsougranis, G.; Kang, H.; Kozak, G.; Bannerman, A.; Bhat, R.; Choi, H.; Nigalye, A.; et al. Plasma and Urine Metabolites Associated with Microperimetric Retinal Sensitivity in Age-Related Macular Degeneration. Metabolites 2025, 15, 232. https://doi.org/10.3390/metabo15040232
Sourirajan K, Mendez K, Lains I, Tsougranis G, Kang H, Kozak G, Bannerman A, Bhat R, Choi H, Nigalye A, et al. Plasma and Urine Metabolites Associated with Microperimetric Retinal Sensitivity in Age-Related Macular Degeneration. Metabolites. 2025; 15(4):232. https://doi.org/10.3390/metabo15040232
Chicago/Turabian StyleSourirajan, Krupa, Kevin Mendez, Ines Lains, Gregory Tsougranis, Haemin Kang, Georgiy Kozak, Augustine Bannerman, Roshni Bhat, Hanna Choi, Archana Nigalye, and et al. 2025. "Plasma and Urine Metabolites Associated with Microperimetric Retinal Sensitivity in Age-Related Macular Degeneration" Metabolites 15, no. 4: 232. https://doi.org/10.3390/metabo15040232
APA StyleSourirajan, K., Mendez, K., Lains, I., Tsougranis, G., Kang, H., Kozak, G., Bannerman, A., Bhat, R., Choi, H., Nigalye, A., Kim, I. K., Vavvas, D. G., Wu, D. M., Liang, L., Miller, J. B., Miller, J. W., Lasky-Su, J., & Husain, D. (2025). Plasma and Urine Metabolites Associated with Microperimetric Retinal Sensitivity in Age-Related Macular Degeneration. Metabolites, 15(4), 232. https://doi.org/10.3390/metabo15040232








