Avian Influenza Virus Strain Specificity in the Volatile Metabolome
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Infections
2.3. Sample Collection
2.4. Headspace Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, M.; Wang, L.-F.; Sun, B.-W.; Wan, W.-B.; Ji, X.; Baele, G.; Bi, Y.H.; Suchard, M.A.; Lai, A.; Zhang, M.; et al. Zoonotic infections by avian influenza virus: Changing global epidemiology, investigation, and control. Lancet Infect. Dis. 2024, 24, e522–e531. [Google Scholar] [PubMed]
- CDC Home Page. Available online: https://archive.cdc.gov/www_cdc_gov/bird-flu/virus-transmission/avian-in-birds.html (accessed on 28 May 2025).
- Graziosi, G.; Lupini, C.; Catelli, E.; Carnaccini, S. Highly Pathogenic Avian Influenza (HPAI) H5 Clade 2.3.4.4b Virus Infection in Birds and Mammals. Animals 2024, 24, 1372. [Google Scholar]
- Lin, T.-H.; Zhu, X.; Wang, S.; Zhang, D.; McBride, R.; Yu, W.; Babarinde, S.; Paulson, J.C.; Wilson, I.A. A single mutation in bovine influenza H5N1 hemagglutinin switches specificity to human receptors. Science 2024, 386, 1128–1134. [Google Scholar] [PubMed]
- Kandeil, A.; Patton, C.; Jones, J.C.; Jeevan, T.; Harrington, W.N.; Trifkovic, S.; Seiler, J.P.; Fabrizio, T.; Woodard, K.; Turner, J.C.; et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat. Commun. 2023, 14, 3082. [Google Scholar] [PubMed]
- Musa, E.; Nia, Z.M.; Bragazzi, N.L.; Leung, D.; Lee, N.; Kong, J.D. Avian Influenza: Lessons from Past Outbreaks and an Inventory of Data Sources, Mathematical and AI Models, and Early Warning Systems for Forecasting and Hotspot Detection to Tackle Ongoing Outbreaks. Healthcare 2024, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kimball, B.A.; Yamazaki, K.; Kohler, D.; Bowen, R.A.; Muth, J.P.; Opiekun, M.; Beauchamp, G.K. Avian Influenza Infection Alters Fecal Odor in Mallards. PLoS ONE 2013, 8, e75411. [Google Scholar]
- Golden, G.L.; Grady, M.; McLean, H.E.; Shriner, S.; Hartwig, A.; Bowen, R.A.; Kimball, B.A. Biodetection of a specific odor signature in mallard feces associated with infection by low pathogenic avian influenza A virus. PLoS ONE 2011, 16, e0251841. [Google Scholar]
- Lommen, A. MetAlign: Interface-Driven, Versatile Metabolomics Tool for Hyphenated Full-Scan Mass Spectrometry Data Preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [PubMed]
- Tikunov, Y.M.; Laptenok, S.; Hall, R.D.; Bovy, A.; De Vos, R.C.H. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 2012, 8, 714–718. [Google Scholar] [PubMed]
- Mardia, K.V.; Kent, J.T.; Bibby, J.M. Multivariate Analysis, 1st ed.; Academic Press: Amsterdam, The Netherlands, 1980. [Google Scholar]
- SAS/STAT, 9.4.; SAS Institute Inc.: Cary, NC, USA, 2008.
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate-A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B-Method. 1995, 57, 289–300. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [PubMed]
- Kemmler, E.; Lemfack, M.C.; Goede, A.; Gallo, K.; Toguem, S.M.T.; Ahmed, W.; Millberg, I.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 4.0: A database of microbial volatiles. Nucleic Acids Res. 2024, 53, D1692–D1696. [Google Scholar]
- Hopken, M.W.; Gilfillan, D.; Gilbert, A.T.; Piaggio, A.J.; Hilton, M.S.; Pierce, J.; Kimball, B.; Abdo, Z. Biodiversity indices and Random Forests reveal the potential for striped skunk (Mephitis mephitis) fecal microbial communities to function as a biomarker for oral rabies vaccination. PLoS ONE 2023, 18, e0285852. [Google Scholar]
- Belizário, J.E.; Faintuch, J.; Malpartida, M.G. Breath Biopsy and Discovery of Exclusive Volatile Organic Compounds for Diagnosis of Infectious Diseases. Front. Cell. Infec. Microbiol. 2021, 10, 564194. [Google Scholar]
- Midura, R.J.; Yanagishita, M. Chaotropic solvents increase the critical micellar concentrations of detergents. Anal. Biochem. 1995, 228, 318–322. [Google Scholar] [PubMed]
- Ferreira, P.C.; Ness, F.; Edwards, S.R.; Cox, B.S.; Tuite, M.F. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol. Microbiol. 2001, 40, 1357–1369. [Google Scholar] [PubMed]
- Acosta-Fernández, R.; Heredia-Barbero, A.; Negron, A. Stability of Pyruvic Acid Adsorbed Onto Clays and Exposed to Ionizing Radiation: Relevance in Chemical Evolution. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 2020, 7, 97–101. [Google Scholar][Green Version]
- de Oliveira Paula, M.M.; Buchili, A.F.M.; Rodrigues, L.M.; Guimarães, A.S.; Ramos, A.D.L.S.; Ramos, E.M. Effects of freezing, irradiation, and aging processes on the volatile profile of Nellore beef. Food Mater. Res. 2024, 4, e028. [Google Scholar][Green Version]
- Dawson, P.; Han, I.; Cox, M.; Black, C.; Simmons, L. Residence time and food contact time effects on transfer of Salmonella Typhimurium from tile, wood and carpet: Testing the five-second rule. J. Appl. Microbiol. 2007, 102, 945–953. [Google Scholar] [PubMed][Green Version]

| Virus | Pretreatment Period | Early Infection Period | Mid Infection Period | Late Infection Period | ||||
|---|---|---|---|---|---|---|---|---|
| H2N2 | −2 | 0 | 1 | 3 | 5 | 7 | 14 | 21 |
| H5N2 | −2 | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| Volatile Name | Effect Type | p-Value | FDR-Adjusted Alpha |
|---|---|---|---|
| Ethylbenzyl ether | Period*Day | p < 0.00006 | 0.00041 |
| 2-Phenoxyethanol | Period*Day | p = 0.00017 | 0.00082 |
| 2-Hydroxybenzaldehyde | Period*Day | p = 0.00022 | 0.0012 |
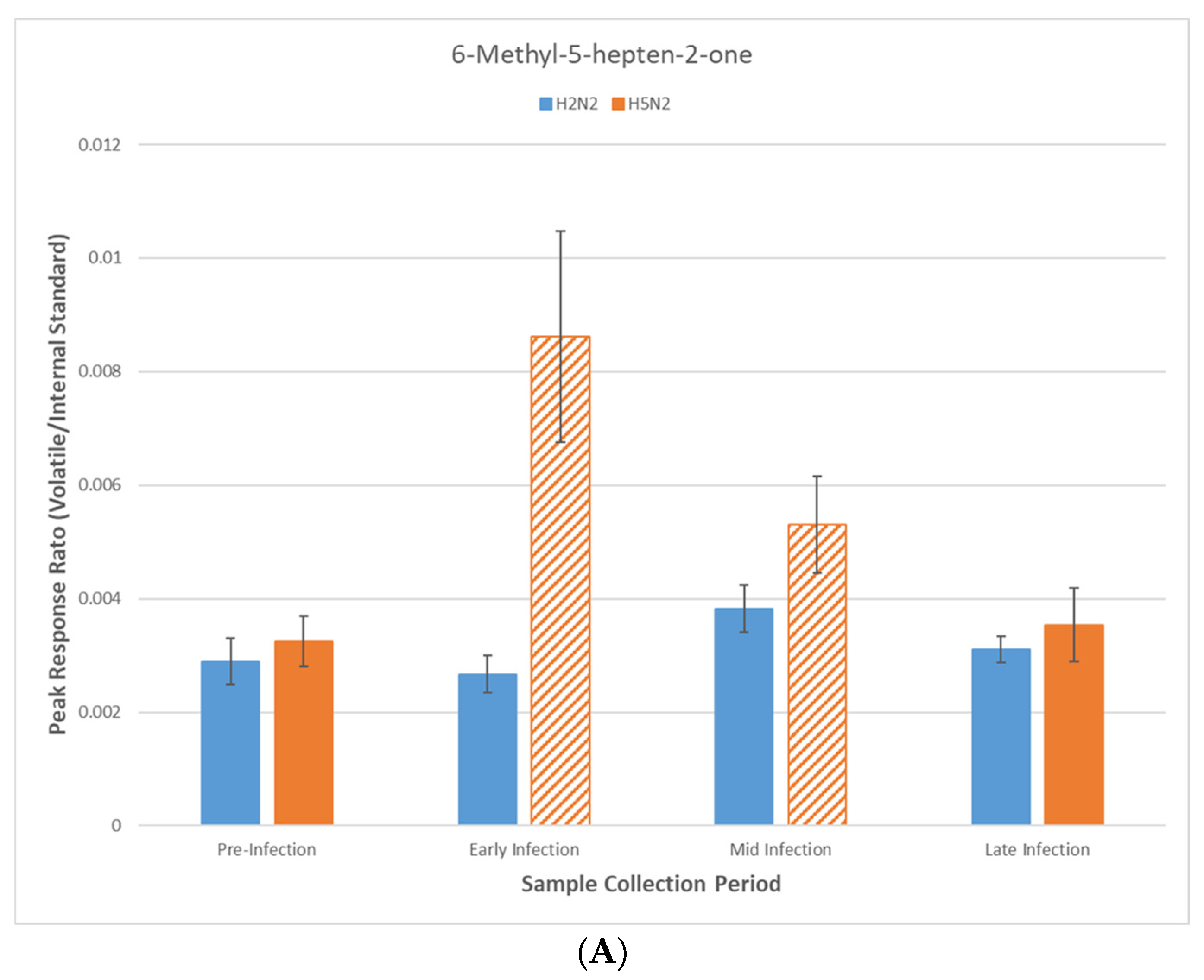
| 6-Methyl-5-hepten-2-one | Period*Day | p = 0.00034 | 0.0016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.E.; Bowen, R.A.; Kimball, B.A. Avian Influenza Virus Strain Specificity in the Volatile Metabolome. Metabolites 2025, 15, 468. https://doi.org/10.3390/metabo15070468
Lee YE, Bowen RA, Kimball BA. Avian Influenza Virus Strain Specificity in the Volatile Metabolome. Metabolites. 2025; 15(7):468. https://doi.org/10.3390/metabo15070468
Chicago/Turabian StyleLee, Young Eun, Richard A. Bowen, and Bruce A. Kimball. 2025. "Avian Influenza Virus Strain Specificity in the Volatile Metabolome" Metabolites 15, no. 7: 468. https://doi.org/10.3390/metabo15070468
APA StyleLee, Y. E., Bowen, R. A., & Kimball, B. A. (2025). Avian Influenza Virus Strain Specificity in the Volatile Metabolome. Metabolites, 15(7), 468. https://doi.org/10.3390/metabo15070468






