Amelioration of Metabolic Syndrome by Co-Administration of Lactobacillus johnsonii CRL1231 and Wheat Bran in Mice via Gut Microbiota and Metabolites Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. The Bacterial Strain Preparation
2.2. Experimental Design: Animals and Diet
2.3. Oral Glucose and Sucrose Tolerance Test
2.4. Microbiota Characterization
2.4.1. Bacterial DNA Extraction
2.4.2. Metagenomic Sequencing
2.4.3. Microbial Analysis by Quantitative PCR (qPCR)
2.5. Animal Sacrifice and Sample Collection
2.6. Biochemical Evaluations and Cardiovascular Risk Indices Calculations
2.7. Cytokines Measurement
2.8. Plasma Lipoperoxidation
2.9. Histopathological Analysis and Adiposity Index
2.10. Assessment of Hepatic Oxidative Stress
2.11. Determination of Short-Chain Fatty Acids (SCFAs), FA Metabolites, and FE Esterase Activity in Large Intestine Contents
2.12. Statistical Analysis
3. Results
3.1. Effect of Lj CRL1231 on Body Weight Gain (BWG), Food Efficiency Ratio (FER), Adiposity Index and Leptin Levels
3.2. Effect of Lj CRL1231 on Adipocyte Size
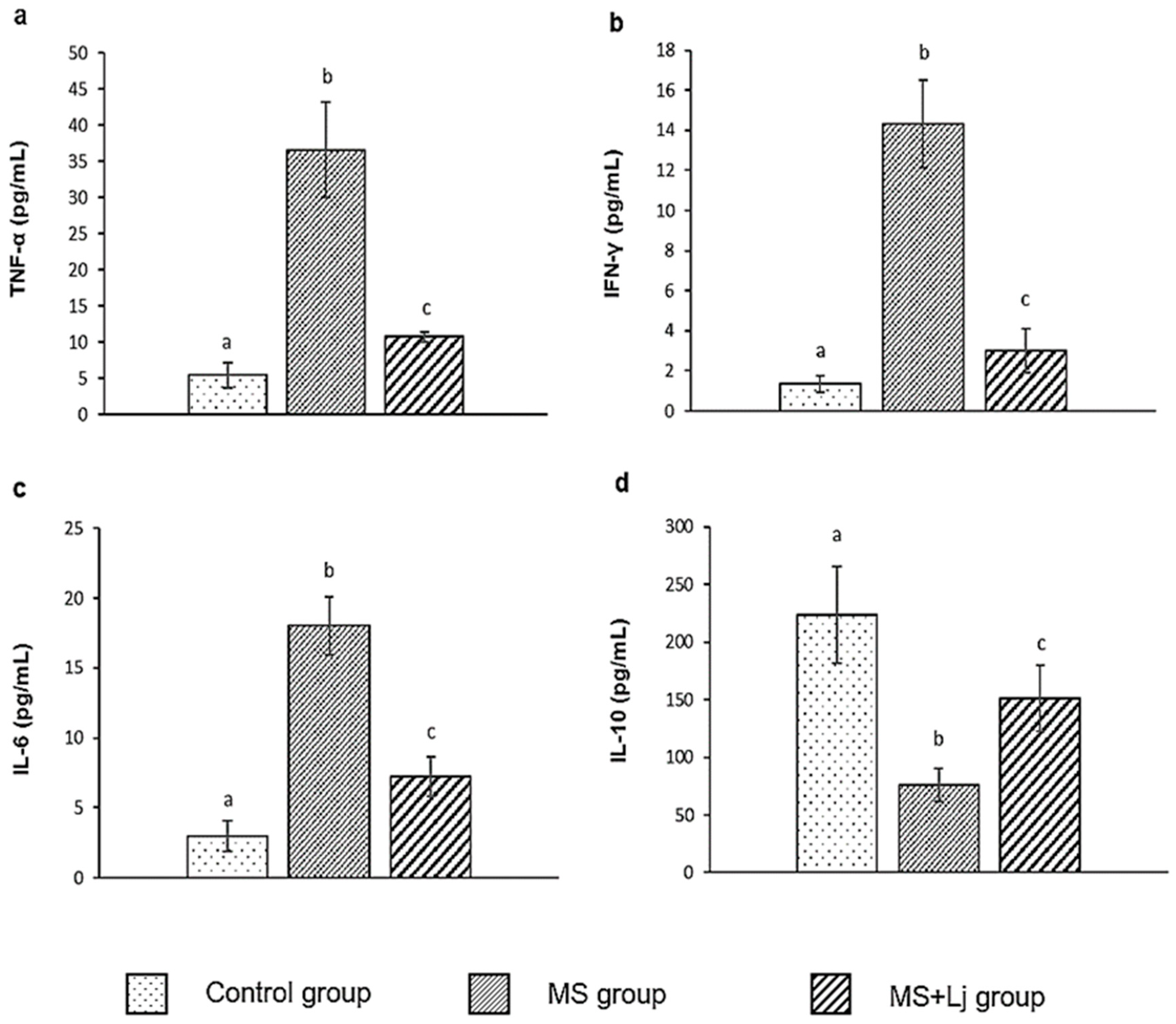
3.3. Effect of Lj CRL1231 on Inflammatory Profile
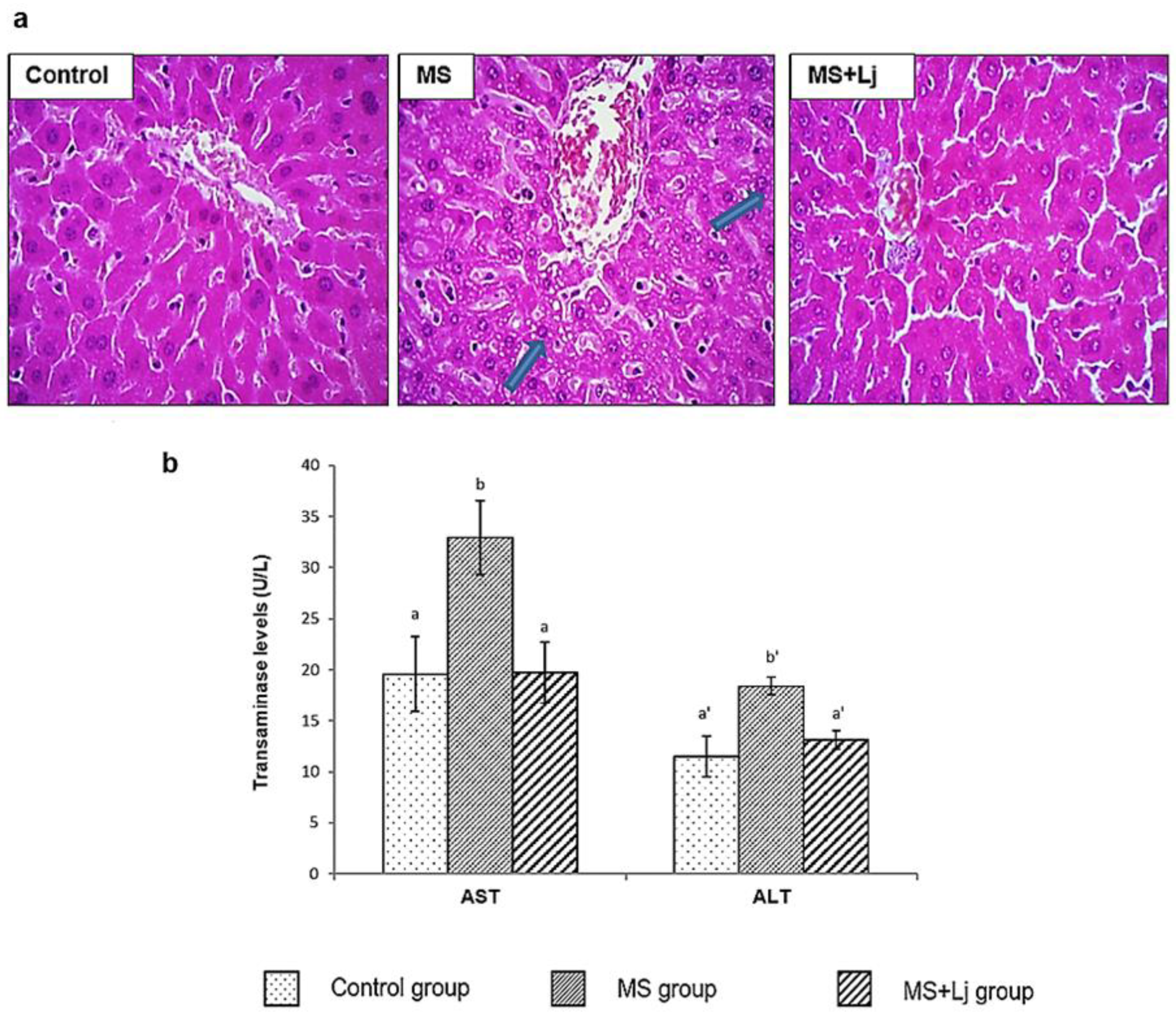
3.4. Effect of Lj CRL1231 on Liver Injury
3.5. Effect of Lj CRL1231 on Intestinal FE Activity and Oxidative Status
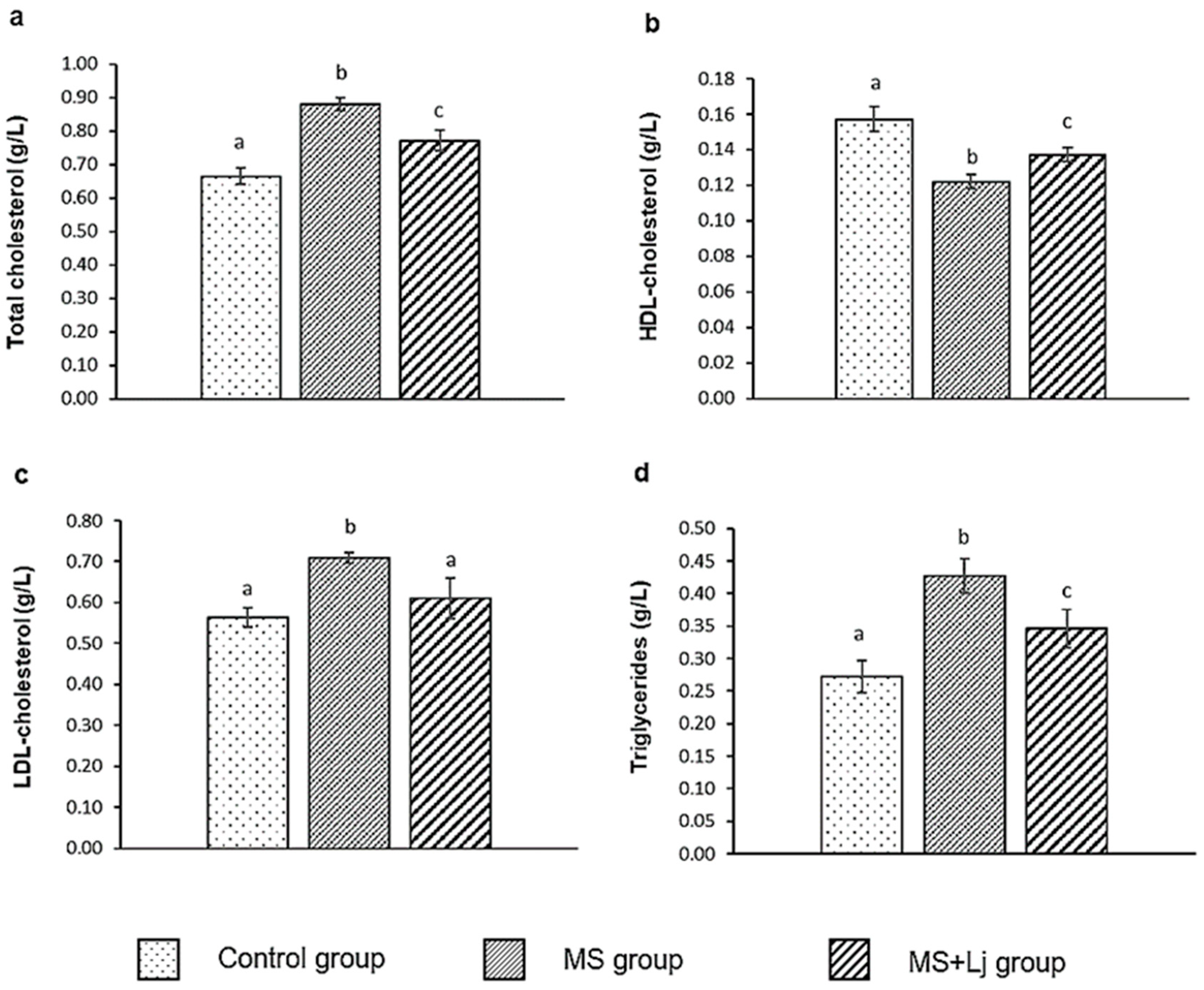
3.6. Effect of Lj CRL1231 on Plasma Lipid and Parameters in Assessing Cardiovascular Risk
3.7. Effect of Lj CRL1231 on Glucose Metabolic Disorders
3.8. Influence of Lj CRL1231 on Metabolites from FA and SCFA Production in Colon Contents
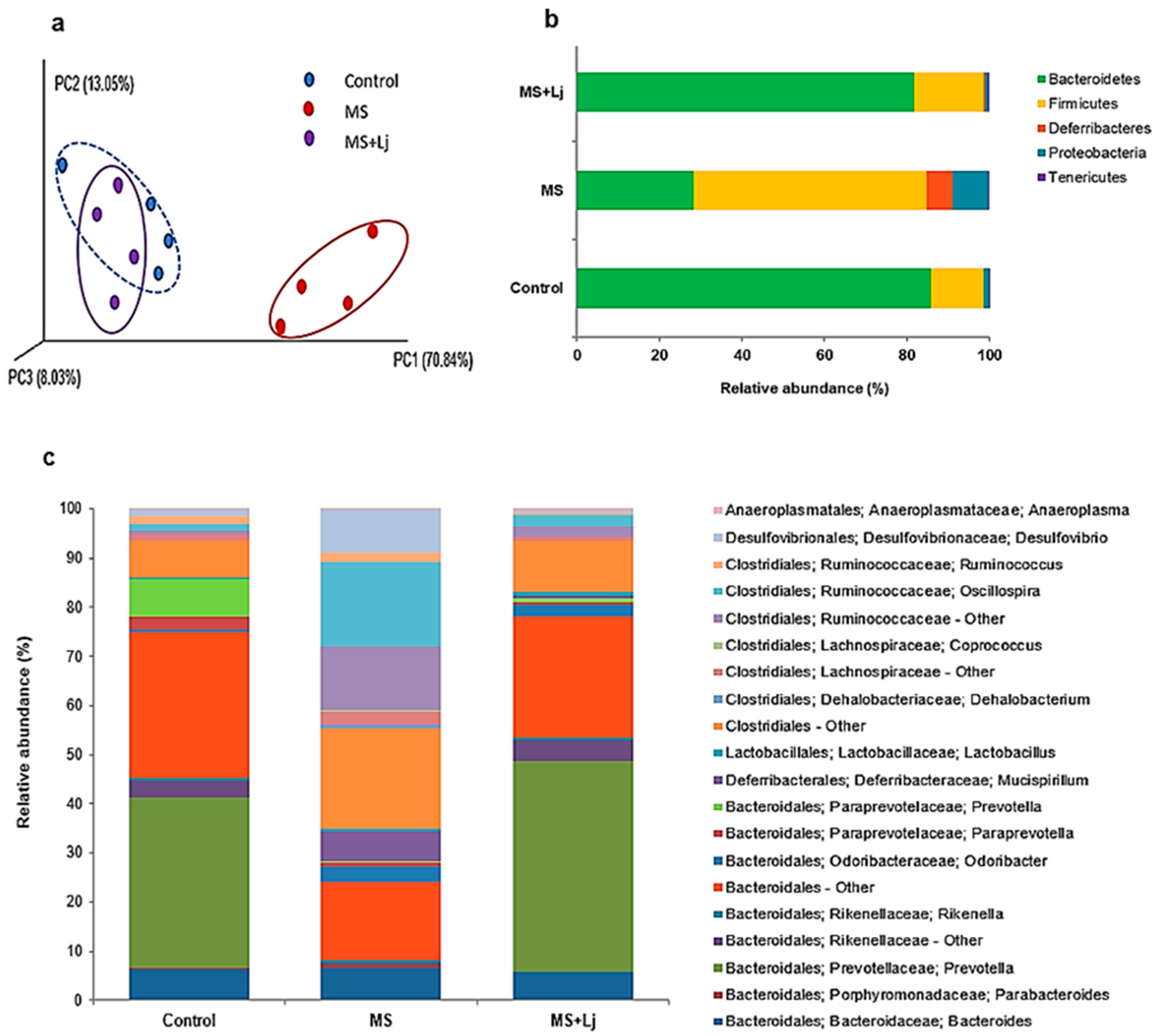
3.9. Modulation of IM by Lj CRL1231 and Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Atherogenic Coefficient |
| AIP | Atherogenic Index of Plasma |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| AUC | Areas Under the Curve |
| BA | Benzoic Acid |
| BWG | Body Weight Gain |
| CRI | Castelli Risk Index |
| CVD | Cardiovascular Disease |
| DHF | Dihydroferulic Acid |
| DHPPA | 3,4-Dihydroxyphenylpropionic acid |
| FA | Ferulic Acid |
| FE | Feruloyl Esterase |
| FER | Food Efficiency Ratio |
| GPx | Glutathione Peroxidase |
| GR | Glutathione Reductase |
| HFD | High Fat Diet |
| HFD+WB | High-Fat Diet Supplemented with Wheat Bran |
| HOMA-IR | Homeostasis Model Assessment of Basal Insulin Resistance |
| HPPA | 3-Hydroxyphenylpropionic Acid |
| IFE | Intestinal Feruloyl Esterase |
| IM | Intestinal Microbiota |
| Lj CRL1231 | Lactobacillus Johnsonii CRL1231 |
| MS | Metabolic Syndrome |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| OGTT | Oral Glucose Tolerance Test |
| OSTT | Oral Sucrose Tolerance Test |
| SCFAs | Short Chain Fatty Acids |
| T2D | Type 2 Diabetes |
| TBARS | Thiobarbituric Acid Reactive Substances |
| WB | Wheat Bran |
References
- Tilg, H.; Moschen, A.R. Gut Microbiome, Obesity, and Metabolic Syndrome. In Metabolic Syndrome: A Comprehensive Textbook; Ahima, R.S., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 373–384. ISBN 978-3-031-40116-9. [Google Scholar]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and Much More. J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Gil, Á.; Gómez-Llorente, C. Effects of Probiotics on Metabolic Syndrome: A Systematic Review of Randomized Clinical Trials. Nutrients 2020, 12, 124. [Google Scholar] [CrossRef]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and Tea Consumption in Relation with Non-Alcoholic Fatty Liver and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of Oils and Solid Fats on Blood Lipids: A Systematic Review and Network Meta-Analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, F.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri FYNLJ109L1 Attenuating Metabolic Syndrome in Mice via Gut Microbiota Modulation and Alleviating Inflammation. Foods 2021, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, Z.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Lactobacillus rhamnosus FJSYC4-1 and Lactobacillus reuteri FGSZY33L6 Alleviate Metabolic Syndrome via Gut Microbiota Regulation. Food Funct. 2021, 12, 3919–3930. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.d.M.; Tur, J.A. Dietary Fat Intake and Metabolic Syndrome in Adults: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 887–905. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, L.; Xu, X.; Liu, Z.; Zhan, H.; Tao, X.; Shah, N.P.; Wei, H. Beneficial Effects of Probiotic Cholesterol-Lowering Strain of Enterococcus faecium WEFA23 from Infants on Diet-Induced Metabolic Syndrome in Rats. J. Dairy Sci. 2017, 100, 1618–1628. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Yang, G.; Hong, E.; Oh, S.; Kim, E. Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods 2020, 9, 1494. [Google Scholar] [CrossRef]
- Russo, M.; Marquez, A.; Abeijón-Mukdsi, M.C.; Santacruz, A.; López-Malo, A.; Gauffin-Cano, P.; Medina, R. Microencapsulated Feruloyl Esterase-Producing Lactobacilli Ameliorate Lipid Profile and Glycaemia in High Fat Diet-Induced Obese Mice. Benef. Microbes 2019, 10, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, X.; Zhang, M.; Zu, X.; Shen, F.; Hou, X.; Hao, E.; Bai, G. Ferulic Acid Targets ACSL1 to Ameliorate Lipid Metabolic Disorders in Db/Db Mice. J. Funct. Foods 2022, 91, 105009. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Li, H. Ferulic Acid Confers Protection on Islet Î2 Cells and Placental Tissues of Rats with Gestational Diabetes Mellitus. Cell Mol. Biol. 2020, 66, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the health grain Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.L.; Michaelson, L.V.; Shewry, P.R.; Lovegrove, A.; Spencer, J.P.E. Increased Bioavailability of Phenolic Acids and Enhanced Vascular Function Following Intake of Feruloyl Esterase-Processed High Fibre Bread: A Randomized, Controlled, Single Blind, Crossover Human Intervention Trial. Clin. Nutr. 2021, 40, 788–795. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus plantarum X1 with α-Glucosidase Inhibitory Activity Ameliorates Type 2 Diabetes in Mice. RSC Adv. 2016, 6, 63536–63547. [Google Scholar] [CrossRef]
- Russo, M.; Marquez, A.; Herrera, H.; Abeijon-Mukdsi, C.; Saavedra, L.; Hebert, E.; Gauffin-Cano, P.; Medina, R. Oral Administration of Lactobacillus fermentum CRL1446 Improves Biomarkers of Metabolic Syndrome in Mice Fed a High-Fat Diet Supplemented with Wheat Bran. Food Funct. 2020, 11, 3879–3894. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-Filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Gauffin Cano, P.; Santacruz, A.; Trejo, F.M.; Sanz, Y. Bifidobacterium CECT 7765 Improves Metabolic and Immunological Alterations Associated with Obesity in High-Fat Diet Fed Mice. Obesity 2013, 21, 2310–2321. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Q.; Hao, C.; Wang, H.H.; Ma, T.; Chen, X.; Ngai, F.-W.; Xie, Y.J. Combined Lifestyle Factors and Metabolic Syndrome Risk: A Systematic Review and Meta-Analysis. Int. J. Obes. 2025, 49, 226–236. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–17. ISBN 978-3-319-48382-5. [Google Scholar]
- Croci, S.; D’Apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary Strategies for Management of Metabolic Syndrome: Role of Gut Microbiota Metabolites. Nutrients 2021, 13, 1389. [Google Scholar] [CrossRef]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, M.H.; Kim, S.H. Selection and Characterization of Probiotic Bacteria Exhibiting Antiadipogenic Potential in 3T3-L1 Preadipocytes. Probiotics Antimicro. Prot. 2022, 14, 72–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, T.; Yang, Y.; Zhang, J.; Ren, F.; Wu, Z. Lactobacillus johnsonii Attenuates Citrobacter rodentium–Induced Colitis by Regulating Inflammatory Responses and Endoplasmic Reticulum Stress in Mice. J. Nutr. 2021, 151, 3391–3399. [Google Scholar] [CrossRef]
- Han, S.; Zheng, H.; Han, F.; Zhang, X.; Zhang, G.; Ma, S.; Liu, K.; Qin, W.; Wu, G. Lactobacillus johnsonii 6084 Alleviated Sepsis-Induced Organ Injury by Modulating Gut Microbiota. Food Sci. Nutr. 2022, 10, 3931–3941. [Google Scholar] [CrossRef]
- Fändriks, L. Roles of the Gut in the Metabolic Syndrome: An Overview. J. Intern. Med. 2017, 281, 319–336. [Google Scholar] [CrossRef]
- Hu, B.; Ye, C.; Leung, E.L.-H.; Zhu, L.; Hu, H.; Zhang, Z.; Zheng, J.; Liu, H. Bletilla striata Oligosaccharides Improve Metabolic Syndrome through Modulation of Gut Microbiota and Intestinal Metabolites in High Fat Diet-Fed Mice. Pharmacol. Res. 2020, 159, 104942. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zeng, D.; Wang, H.; Ni, X.; Yi, D.; Pan, K.; Jing, B. Preventing Non-Alcoholic Fatty Liver Disease through Lactobacillus johnsonii BS15 by Attenuating Inflammation and Mitochondrial Injury and Improving Gut Environment in Obese Mice. Appl. Microbiol. Biotechnol. 2014, 98, 6817–6829. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat Phenolic Compounds Regulate Metabolic Syndrome in High Fat Diet-Fed Mice via Gut Microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Zhou, H.; Wang, L.; Chen, X.; Song, G.; Liu, J.; Li, A. Ferulic Acid Suppresses Obesity and Obesity-Related Metabolic Syndromes in High Fat Diet-Induced Obese C57BL/6J Mice. Food Agric. Immunol. 2018, 29, 1116–1125. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Abd El-Twab, S.M. Ferulic Acid Prevents Oxidative Stress, Inflammation, and Liver Injury via Upregulation of Nrf2/HO-1 Signaling in Methotrexate-Induced Rats. Environ. Sci. Pollut. Res. 2020, 27, 7910–7921. [Google Scholar] [CrossRef]
- Teixeira, L.D.; Torrez Lamberti, M.F.; DeBose-Scarlett, E.; Bahadiroglu, E.; Garrett, T.J.; Gardner, C.L.; Meyer, J.L.; Lorca, G.L.; Gonzalez, C.F. Lactobacillus johnsonii N6.2 and Blueberry Phytophenols Affect Lipidome and Gut Microbiota Composition of Rats Under High-Fat Diet. Front. Nutr. 2021, 8, 757256. [Google Scholar] [CrossRef]
- Qing, X.; Zeng, D.; Wang, H.; Ni, X.; Liu, L.; Lai, J.; Khalique, A.; Pan, K.; Jing, B. Preventing Subclinical Necrotic Enteritis through Lactobacillus johnsonii BS15 by Ameliorating Lipid Metabolism and Intestinal Microflora in Broiler Chickens. AMB Expr. 2017, 7, 139. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhattacharjee, J.; Bhatnagar, M.K.; Tyagi, S.; Delhi, N. Atherogenic index of plasma, castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int. J. Pharm. Biol. Sci. 2013, 3, 359–364. [Google Scholar]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic Acid Exerts Its Antidiabetic Effect by Modulating Insulin-Signalling Molecules in the Liver of High-Fat Diet and Fructose-Induced Type-2 Diabetic Adult Male Rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Koh, W.Y.; Utra, U.; Ahmad, R.; Rather, I.A.; Park, Y.-H. Evaluation of Probiotic Potential and Anti-Hyperglycemic Properties of a Novel Lactobacillus Strain Isolated from Water Kefir Grains. Food Sci. Biotechnol. 2018, 27, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Serra-Barcellona, C.; Habib, N.C.; Honoré, S.M.; Sánchez, S.S.; Genta, S.B. Enhydrin Regulates Postprandial Hyperglycemia in Diabetic Rats by Inhibition of α-Glucosidase Activity. Plant Foods Hum. Nutr. 2017, 72, 156–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, P.; Zhao, J. Ferulic Acid Mediates Prebiotic Responses of Cereal-Derived Arabinoxylans on Host Health. Anim. Nutr. 2022, 9, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors Affecting Intake, Metabolism and Health Benefits of Phenolic Acids: Do We Understand Individual Variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- Braune, A.; Bunzel, M.; Yonekura, R.; Blaut, M. Conversion of Dehydrodiferulic Acids by Human Intestinal Microbiota. J. Agric. Food Chem. 2009, 57, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat Bran Promotes Enrichment within the Human Colonic Microbiota of Butyrate-Producing Bacteria That Release Ferulic Acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, G.; Chen, C.; Zheng, Y.; Ma, F.; Zhao, J.; Lee, Y.-K.; Zhang, H.; Chen, W. Lactobacillus Strains Derived from Human Gut Ameliorate Metabolic Disorders via Modulation of Gut Microbiota Composition and Short-Chain Fatty Acids Metabolism. Benef. Microbes 2021, 12, 267–282. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal Relationships among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Tian, B.; Geng, Y.; Wang, P.; Cai, M.; Neng, J.; Hu, J.; Xia, D.; Cao, W.; Yang, K.; Sun, P. Ferulic Acid Improves Intestinal Barrier Function through Altering Gut Microbiota Composition in High-Fat Diet-Induced Mice. Eur. J. Nutr. 2022, 61, 3767–3783. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut Microbiota Dysbiosis Associated with Glucose Metabolism Disorders and the Metabolic Syndrome in Older Adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araújo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics Modulate Gut Microbiota and Improve Insulin Sensitivity in DIO Mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, M.; Tao, G.; Lu, M.; Lin, J.; Huang, J. Feruloylated Oligosaccharides and Ferulic Acid Alter Gut Microbiome to Alleviate Diabetic Syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef]
- Yan, S.; Shi, R.; Li, L.; Ma, S.; Zhang, H.; Ye, J.; Wang, J.; Pan, J.; Wang, Q.; Jin, X.; et al. Mannan Oligosaccharide Suppresses Lipid Accumulation and Appetite in Western-Diet-Induced Obese Mice Via Reshaping Gut Microbiome and Enhancing Short-Chain Fatty Acids Production. Mol. Nutr. Food Res. 2019, 63, 1900521. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Qing, X.; Zeng, D.; Luo, M.; Liu, L.; Li, G.; Pan, K.; Jing, B. Live Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance and Lowers Fat Deposition by Improving Lipid Metabolism, Intestinal Development, and Gut Microflora in Broilers. Front. Microbiol. 2017, 8, 1073. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Lu, J.; Shi, J.; Guan, J.; Yan, F.; Li, B.; Huo, G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wang, L.; Wen, C.; Yang, J.; Song, S.; Liu, X. Sulfated Polysaccharide from Sea Cucumber and Its Depolymerized Derivative Prevent Obesity in Association with Modification of Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1800446. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Adachi, K.; Sugiyama, T.; Shimozato, A.; Ebi, M.; Ogasawara, N.; Funaki, Y.; Goto, C.; Sasaki, M.; Kasugai, K. Association of Intestinal Microbiota with Metabolic Markers and Dietary Habits in Patients with Type 2 Diabetes. Digestion 2016, 94, 66–72. [Google Scholar] [CrossRef]
- Gong, L.; Wang, H.; Wang, T.; Liu, Y.; Wang, J.; Sun, B. Feruloylated Oligosaccharides Modulate the Gut Microbiota in Vitro via the Combined Actions of Oligosaccharides and Ferulic Acid. J. Funct. Foods 2019, 60, 103453. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]



| Experimental Groups | |||
|---|---|---|---|
| Control | MS | MS+Lj | |
| Gavage administration | 100 µL of water | 100 µL of water | 100 µL of L. johnsonii CRL1231 suspension (final dose: 108 cells/day) |
| Diet | * ND: Normal diet. Calorie content: 3.1 Kcal/g (6.5% oil vegetable-derived Kcal). | * HFD+WB: High-fat diet supplemented with wheat bran to 7% (w/w). Calorie content: 5.1 Kcal/g (with 60% lard-derived Kcal). | * HFD+WB: High-fat diet supplemented with wheat bran to 7% (w/w). Calorie content: 5.1 Kcal/g (with 60% lard-derived Kcal). |
| Drinking water | ad libitum | ad libitum | ad libitum |
| Control | MS | MS+Lj | |
|---|---|---|---|
| Cardiovascular risk indices | |||
| *AC | 3.19 ± 0.43 a | 6.20 ± 0.53 b | 4.62 ± 0.54 c |
| **CRI-I | 4.23 ± 0.62 a | 7.21 ± 0.25 b | 5.62 ±0.41 c |
| **CRI-II | 3.59 ±0.33 a | 5.82 ± 0.24 b | 4.45 ± 0.40 a |
| ***AIP | 0.23 ± 0.05 a | 0.53 ± 0.04 b | 0.40 ± 0.05 c |
| Glucose (mmol/L) | 3.97 ± 0.47 a | 7.80 ± 0.92 b | 5.34 ± 0.55 c |
| Insulin (µU/mL) | 8.70 ± 0.70 a | 12.60 ± 0.86 b | 8.96 ± 0.97 a |
| HOMA-IR | 1.54 ± 0.28 a | 4.37 ± 0.42 b | 2.13 ± 0.77 a |
| Control | MS | MS+Lj | |||
|---|---|---|---|---|---|
| Compound | Rt (min) | Molecular Ion [M − H]− (m/z) | |||
| FA | 12.55 | 193.0 | − | − | − |
| DHF | 8.26 | 195.1 | + | + | ++ |
| DHPPA | 7.36 | 181.1 | ++ | + | ++ |
| HPPA | 8.56 | 165.1 | +++ | + | ++++ |
| BA | 13.2 | 121.0 | − | − | − |
| Control | MS | MS+Lj | |
|---|---|---|---|
| SCFA (nmol/g fecal content) | |||
| Acetic acid | 12.24 ± 0.15 a | 7.59 ± 0.45 b | 9.05 ± 0.58 c |
| Propionic acid | 4.45 ± 0.24 a | 2.71 ± 0.09 c | 5.64 ± 0.40 b |
| Butyric acid | 1.40 ± 0.15 a | 0.52 ± 0.04 b | 1.50 ± 0.18 a |
| TOTAL SCFAs | 18.09 ± 0.18 a | 10.82 ± 0.19 c | 16.19 ± 0.39 b |
| Control | MS | MS+Lj | |
|---|---|---|---|
| Chao 1 | 274.26 ± 38.46 a | 255.71 ± 1.02 a | 255.30 ± 8.50 a |
| Shannon | 5.39 ± 0.45 a | 5.31 ± 0.13 a | 5.05 ± 0.03 a |
| Observed OTUs (Species) | 298.49 ± 23.60 a | 266.44 ± 2.76 b | 279.02 ± 15.89 ab |
| PD whole tree | 20.87 ± 1.90 a | 19.28 ± 0.28 a | 19.64 ± 1.08 a |
| Bacterial Counts (log cells/g Fecal Sample) | |||||
|---|---|---|---|---|---|
| Bacterial Groups | Control | MS | p-Values a | MS+Lj | p-Values b |
| Total bacteria | 10.73 | 11.39 | 0.095 | 10.57 | 0.093 |
| Bifidobacterium | 6.71 | 5.40 | 0.028 * | 6.58 | 0.033 * |
| Bacteroides | 7.40 | 7.70 | 0.698 | 7.60 | 0.137 |
| Enterobacteriaceae | 5.44 | 7.08 | 0.039 * | 7.15 | 0.148 |
| Lactobacillus | 7.65 | 8.29 | 0.030 * | 9.21 | 0.723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.; Marquez, A.; Andrada, E.; Torres, S.; Santacruz, A.; Medina, R.; Gauffin-Cano, P. Amelioration of Metabolic Syndrome by Co-Administration of Lactobacillus johnsonii CRL1231 and Wheat Bran in Mice via Gut Microbiota and Metabolites Modulation. Metabolites 2025, 15, 466. https://doi.org/10.3390/metabo15070466
Russo M, Marquez A, Andrada E, Torres S, Santacruz A, Medina R, Gauffin-Cano P. Amelioration of Metabolic Syndrome by Co-Administration of Lactobacillus johnsonii CRL1231 and Wheat Bran in Mice via Gut Microbiota and Metabolites Modulation. Metabolites. 2025; 15(7):466. https://doi.org/10.3390/metabo15070466
Chicago/Turabian StyleRusso, Matias, Antonela Marquez, Estefanía Andrada, Sebastián Torres, Arlette Santacruz, Roxana Medina, and Paola Gauffin-Cano. 2025. "Amelioration of Metabolic Syndrome by Co-Administration of Lactobacillus johnsonii CRL1231 and Wheat Bran in Mice via Gut Microbiota and Metabolites Modulation" Metabolites 15, no. 7: 466. https://doi.org/10.3390/metabo15070466
APA StyleRusso, M., Marquez, A., Andrada, E., Torres, S., Santacruz, A., Medina, R., & Gauffin-Cano, P. (2025). Amelioration of Metabolic Syndrome by Co-Administration of Lactobacillus johnsonii CRL1231 and Wheat Bran in Mice via Gut Microbiota and Metabolites Modulation. Metabolites, 15(7), 466. https://doi.org/10.3390/metabo15070466










