Identification of Bioactive Metabolites of Capirona macrophylla by Metabolomic Analysis, Molecular Docking, and In Vitro Antiparasitic Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Analysis by UHPLC/HRMSn
2.4. HPLC Fractionation
2.5. Metabolomic Analysis
2.6. Virtual Screening by Molecular Docking
2.7. Molecular Dynamics
2.8. In Vitro Biological Activity
2.8.1. Antiplasmodial Activity
2.8.2. Antileishmanial Activity
(fluorescence of control with no treatment − fluorescence of Medium RPMI)
2.8.3. Hemolysis Assay
3. Results and Discussion
3.1. Untargeted Metabolomic Analysis
3.2. Virtual Screening by Molecular Docking
3.3. Molecular Dynamics Simulations
3.4. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CID | PubChem Compound Identification |
| CQA | Caffeoylquinic Acid |
| D.E. | Docking Energy |
| DHODH | Dihydroorotate Dehydrogenase enzyme |
| DiCQA | Di Caffeoylquinic Acid |
| HESI | Heated Electrospray Ionization |
| GNPS | Global Natural Products Social Molecular Networking |
| HPLC | High Performance Liquid Chromatography |
| IC50 | Half maximal inhibitory concentration |
| MeOH | Methanol |
| ORO | Orotate substrate |
| SisGen | Brazilian National System of Genetic Heritage Management and Associated Traditional Knowledge |
| UHPLC/HRMSn | Ultra High-Performance Liquid Chromatography/High Resolution Tandem Mass Spectrometry |
References
- Delprete, P.G. A New Revision of Capirona (Rubiaceae, Ixoroideae, Condamineeae), with a New Combination and Additional Notes on Typification of the Names Involved. Phytotaxa 2020, 443, 101–106. [Google Scholar] [CrossRef]
- Taylor, C.M.; Campos, M.T.V.A.; Zappi, D. Flora Da Reserva DUCKE, Amazonas, Brasil: Rutaceae. Rodriguésia 2005, 56, 189–204. [Google Scholar] [CrossRef][Green Version]
- Cardoso, P.H. Capirona Spruce Capirona Macrophylla (Poepp). Delprete 2020, 58, 1–3. [Google Scholar]
- Pianowski, L.F.; Dudycz, L.W.; Pianowski Filho, L.F. WO2017019713A1—Mulateiro-Derived Compositions and Use Thereof for Preventing Hair Loss and Promoting Hair Growth—Google Patents. U.S. Patent Application 15/747,968, 13 September 2018. Available online: https://patents.google.com/patent/WO2017019713A1/en?oq=WO2017019713A1 (accessed on 13 January 2025).
- Da Silva, C.P. Hope Shampoo—The Herbal of the Rainforest. BRPI0504746A, 2005. Available online: https://patents.google.com/patent/BRPI0504746A/pt?oq=BRPI0504746A (accessed on 13 January 2025).
- Azba Cosmetics GmbH. Composition Comprising Extracts. DE202021100508U1, 2001. Available online: https://patents.google.com/patent/DE202021100508U1/de?oq=DE202021100508U1 (accessed on 13 January 2025).
- Zielinski-Habershaw, B.; Spartichino, J. Compositions and Methods for Treating Wounds. US20200390841A1, 2020. Available online: https://patents.google.com/patent/WO1993004691A1/en (accessed on 13 January 2025).
- Pereira, O.E.; Bento, A.D. Lotion Aux Herbes Pour Stimuler Les Racines Des Cheveux. WO2008154714A1, 2008. Available online: https://patents.google.com/patent/WO2008154714A1/en?oq=WO2008154714A1 (accessed on 13 January 2025).
- Moreira, N.J. Composição Anti-Alopécia, Produto Anti-Alopécia e Uso de Composição Anti-Alopécia. BRPI0802665A2, 2008. pp. 1–9. Available online: https://www.escavador.com/patentes/329707/composicao-anti-alopecia-produto-anti-alopecia-e-uso-de-composicao (accessed on 13 January 2025).
- Estacio, P.O.; Bento, A.D. Herb Lotion to Activate Hair Roots. WO2008154714A1, 2008. p. 13. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2008154714 (accessed on 13 January 2025).
- Hisayuki, K.; Yuki, S.; Eiichiro, Y.; Masako, N.; Minoru, F. Skin External Preparation. JPH0812565A, 1996. p. 1. Available online: https://patents.google.com/patent/JPH10147539A/en (accessed on 13 January 2025).
- Rikako, S.; Kenichi, U.; Osamu, M. Cyclic AMP (CAMP) Production Inhibitor. JP2004359571A, 2004. p. 1. Available online: https://patents.google.com/patent/JP2004359571A/en (accessed on 13 January 2025).
- Vásquez-Ocmín, P.; Cojean, S.; Rengifo, E.; Suyyagh-Albouz, S.; Amasifuen Guerra, C.A.; Pomel, S.; Cabanillas, B.; Mejía, K.; Loiseau, P.M.; Figadère, B.; et al. Antiprotozoal Activity of Medicinal Plants Used by Iquitos-Nauta Road Communities in Loreto (Peru). J. Ethnopharmacol. 2018, 210, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Estevez, Y.; Castillo, D.; Pisango, M.T.; Arevalo, J.; Rojas, R.; Alban, J.; Deharo, E.; Bourdy, G.; Sauvain, M. Evaluation of the Leishmanicidal Activity of Plants Used by Peruvian Chayahuita Ethnic Group. J. Ethnopharmacol. 2007, 114, 254–259. [Google Scholar] [CrossRef]
- Caetano, R.S.; De Souza, A.C.R.; Feitozao, L.F. O Uso De Plantas Medicinais Utilizadas Por Frequentadores Dos Ambulatórios Santa Marcelina, Porto Velho—Ro. Rev. Saúde E Pesqui. 2014, 7, 55–63. [Google Scholar]
- Peixoto, H.; Roxo, M.; Koolen, H.; Da Silva, F.; Silva, E.; Braun, M.S.; Wang, X.; Wink, M. Calycophyllum Spruceanum (Benth.), the Amazonian “Tree of Youth” Prolongs Longevity and Enhances Stress Resistance in Caenorhabditis Elegans. Molecules 2018, 23, 534. [Google Scholar] [CrossRef]
- Santos, A.B.; Ribeiro-Oliveira, J.P.; Carvalho, C.M. Sobre a Botânica, a Etnofarmacologia e a Química de Calycophyllum Spruceanum (Benth.) Hook. f. Ex K. Schum. Rev. Bras. Plantas Med. 2016, 18, 383–389. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Sugui, M.M.; Sinhorin, V.D.G.; Bicudo, R.d.C.; de Moura, F.R.; Sinhorin, A.P. First Phytochemical and Biological Study of the Ethanolic Extract from Leaves of Capirona decorticans (Rubiaceae). Acta Amazon. 2018, 48, 338–346. [Google Scholar] [CrossRef]
- Martins, D.; Nunez, C. Secondary Metabolites from Rubiaceae Species. Molecules 2015, 20, 13422–13495. [Google Scholar] [CrossRef] [PubMed]
- Chibli, L.A.; Schmidt, T.J.; Nonato, M.C.; Calil, F.A.; Da Costa, F.B. Natural Products as Inhibitors of Leishmania Major Dihydroorotate Dehydrogenase. Eur. J. Med. Chem. 2018, 157, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Chibli, L.A.; Rosa, A.L.; Nonato, M.C.; Da Costa, F.B. Untargeted LC–MS Metabolomic Studies of Asteraceae Species to Discover Inhibitors of Leishmania Major Dihydroorotate Dehydrogenase. Metabolomics 2019, 15, 59. [Google Scholar] [CrossRef]
- Kato, K.C. Universidade Federal de Ouro Preto, Escola de Farmácia, Avaliação Da Toxicidade Do Antimoniato de Meglumina; Universidade Federal de Ouro Preto: Ouro Preto, Minas Gerais, Brazil, 2008. [Google Scholar]
- Boulos, M.; Dutra, A.P.; DiSanti, S.M.; Shiroma, M.; Amato Neto, V. Avaliação Clínica Do Quinino Para o Tratamento de Malária Por Plasmodium Falciparum. Rev. Soc. Bras. Med. Trop. 1997, 30, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis Treatment—A Challenge That Remains: A Review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.S.; Dias, P.A.R.; Peixoto, F.H.d.S.; Dos Santos, I.X.P.; Gardone, D.S.; Corrêa, M.I. (Eds.) Doenças Tropicais E Negligenciadas; Editora Pasteur: Irati, Paraná, Brazil, 2021. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2021; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240040496. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 18 December 2023).
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Phillips, M.A.; Lotharius, J.; Marsh, K.; White, J.; Dayan, A.; White, K.L.; Njoroge, J.W.; El Mazouni, F.; Lao, Y.; Kokkonda, S.; et al. A Long-Duration Dihydroorotate Dehydrogenase Inhibitor (DSM265) for Prevention and Treatment of Malaria. Sci. Transl. Med. 2015, 7, 296ra111. [Google Scholar] [CrossRef]
- Schöning-Stierand, K.; Diedrich, K.; Ehrt, C.; Flachsenberg, F.; Graef, J.; Sieg, J.; Penner, P.; Poppinga, M.; Ungethüm, A.; Rarey, M. ProteinsPlus: A Comprehensive Collection of Web-Based Molecular Modeling Tools. Nucleic Acids Res. 2022, 50, W611–W615. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Vieira, I.H.P.; Botelho, E.B.; de Souza Gomes, T.J.; Kist, R.; Caceres, R.A.; Zanchi, F.B. Visual Dynamics: A WEB Application for Molecular Dynamics Simulation Using GROMACS. BMC Bioinform. 2023, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Kagami, L.; Wilter, A.; Diaz, A.; Vranken, W. The ACPYPE Web Server for Small-Molecule MD Topology Generation. Bioinformatics 2023, 39, btad350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic Atom Type and Bond Type Perception in Molecular Mechanical Calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium Falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Bagavan, A.; Rahuman, A.A.; Kaushik, N.K.; Sahal, D. In Vitro Antimalarial Activity of Medicinal Plant Extracts against Plasmodium Falciparum. Parasitol. Res. 2011, 108, 15–22. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Loset, J.R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug Screening for Kinetoplastid Diseases; DNDi: Geneva, Switzerland, 2009. [Google Scholar]
- Wang, W.; Xiong, W.; Zhu, Y.; Xu, H.; Yang, X. Protective Effect of PEGylation against Poly(Amidoamine) Dendrimer-Induced Hemolysis of Human Red Blood Cells. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93B, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Suzumura, Y.; Tomoda, H.; Masuma, R.; Haneda, K.; Kisni, M.; Iwai, Y.; Omura, S. Xanthoquinodins, New Anticoccidial Agents Produced by Humicola Sp. Production, Isolation and Physico-Chemical and Biological Properties. J. Antibiot. 1993, 46, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Boonruangprapa, T.; Rachtawee, P.; Pittayakhajonwut, P. Antimicrobial Activity and Cytotoxicity of Xanthoquinodin Analogs from the Fungus Cytospora Eugeniae BCC42696. Phytochemistry 2018, 151, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Marin-Felix, Y.; Surup, F.; Stchigel, A.M.; Stadler, M. Seven New Cytotoxic and Antimicrobial Xanthoquinodins from Jugulospora Vestita. J. Fungi 2020, 6, 188. [Google Scholar] [CrossRef]
- Rahman, M.M.; Basta, T.; Teng, J.; Lee, M.; Worrell, B.T.; Stowell, M.H.B.; Hibbs, R.E. Structural Mechanism of Muscle Nicotinic Receptor Desensitization and Block by Curare. Nat. Struct. Mol. Biol. 2022, 29, 386–394. [Google Scholar] [CrossRef]
- Patel, S.; Shukla, J.; Jain, S.; Paliwal, V.; Tripathi, N.; Paliwal, S.; Sharma, S. Repositioning of Tubocurarine as Analgesic and Anti-Inflammatory Agent: Exploring beyond Myorelaxant Activity. Biochem. Pharmacol. 2022, 205, 115248. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, W.; Lin, Y.C. Gossypol Inhibits the Growth of MAT-LyLu Prostate Cancer Cells by Modulation of TGFβ/Akt Signaling. Int. J. Mol. Med. 2009, 24, 69–75. [Google Scholar] [CrossRef]
- Coutinho, E.M. Gossypol: A Contraceptive for Men. Contraception 2002, 65, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Von Hagen, V.; Moustafa, Y.; Montmasson, M.P.; Monet, J.D. Effects of Gossypol on the Cell Cycle Phases in T-47D Human Breast Cancer Cells. Anticancer Res. 1991, 11, 1469–1475. [Google Scholar] [PubMed]
- Rucinska, A.; Kirko, S.; Gabryelak, T. Effect of the Phytoestrogen, Genistein-8-C-glucoside, on Chinese Hamster Ovary Cells in Vitro. Cell Biol. Int. 2007, 31, 1371–1378. [Google Scholar] [CrossRef]
- Antosiak, A.; Milowska, K.; Maczynska, K.; Rozalska, S.; Gabryelak, T. Cytotoxic Activity of Genistein-8-C-Glucoside Form Lupinus Luteus L. and Genistein against Human SK-OV-3 Ovarian Carcinoma Cell Line. Med. Chem. Res. 2017, 26, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Banyal, A.; Tiwari, S.; Sharma, A.; Chanana, I.; Patel, S.K.S.; Kulshrestha, S.; Kumar, P. Vinca Alkaloids as a Potential Cancer Therapeutics: Recent Update and Future Challenges. 3 Biotech 2023, 13, 211. [Google Scholar] [CrossRef]
- Kumar, S.; Swamy, N.; Tuli, H.S.; Rani, S.; Garg, A.; Mishra, D.; Abdulabbas, H.S.; Sandhu, S.S. Myricetin: A Potential Plant-Derived Anticancer Bioactive Compound—An Updated Overview. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2179–2196. [Google Scholar] [CrossRef] [PubMed]
- Rong, N.; Yang, R.; Ibrahim, I.A.A.; Zhang, W. Cardioprotective Role of Scopoletin on Isoproterenol-Induced Myocardial Infarction in Rats. Appl. Biochem. Biotechnol. 2023, 195, 919–932. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A Review of Its Source, Biosynthesis, Methods of Extraction, and Pharmacological Activities. Z. Naturforschung C 2022, 77, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Mojarrab, M.; Kazemi-Afrakoti, S.; Farzaei, M.H. Isofraxidin: Synthesis, Biosynthesis, Isolation, Pharmacokinetic and Pharmacological Properties. Molecules 2020, 25, 2040. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Q.-L.; Wu, L.-H.; Jawaid, P.; Jiao, Y.-F.; Kadowaki, M.; Kondo, T. Isofraxidin, a Potent Reactive Oxygen Species (ROS) Scavenger, Protects Human Leukemia Cells from Radiation-Induced Apoptosis via ROS/Mitochondria Pathway in P53-Independent Manner. Apoptosis 2014, 19, 1043–1053. [Google Scholar] [CrossRef]
- Cardona Zuleta, L.M.; Cavalheiro, A.J.; Siqueira Silva, D.H.; Furlan, M.; Marx Young, M.C.; Albuquerque, S.; Castro-Gamboa, I.; da Silva Bolzani, V. Seco-Iridoids from Calycophyllum Spruceanum (Rubiaceae). Phytochemistry 2003, 64, 549–553. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.A.B.; Amorim, R.M.F.; de Freitas Lopes, R.; Mota, M.R.L.; da Silva, F.M.A.; Koolen, H.H.F.; Lima, E.S.; Assreuy, A.M.S.; da Cunha, R.M. Calycophyllum Spruceanum BENTH Ameliorates Acute Inflammation in Mice. J. Ethnopharmacol. 2018, 219, 103–109. [Google Scholar] [CrossRef]
- Safdar, M.A.; Aslam, R.M.N.; Shakeel, A.; Shiza; Waqar, M.; Jmail, A.; Mehmood, M.H.; Gul, H. Cyanidin as Potential Anticancer Agent Targeting Various Proliferative Pathways. Chem. Biol. Drug Des. 2023, 101, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MS(n). J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Boschi, D.; Pippione, A.C.; Sainas, S.; Lolli, M.L. Dihydroorotate Dehydrogenase Inhibitors in Anti-Infective Drug Research. Eur. J. Med. Chem. 2019, 183, 111681. [Google Scholar] [CrossRef] [PubMed]
- Brandão, G.C.; Rocha Missias, F.C.; Arantes, L.M.; Soares, L.F.; Roy, K.K.; Doerksen, R.J.; Braga de Oliveira, A.; Pereira, G.R. Antimalarial Naphthoquinones. Synthesis via Click Chemistry, in Vitro Activity, Docking to Pf DHODH and SAR of Lapachol-Based Compounds. Eur. J. Med. Chem. 2018, 145, 191–205. [Google Scholar] [CrossRef] [PubMed]
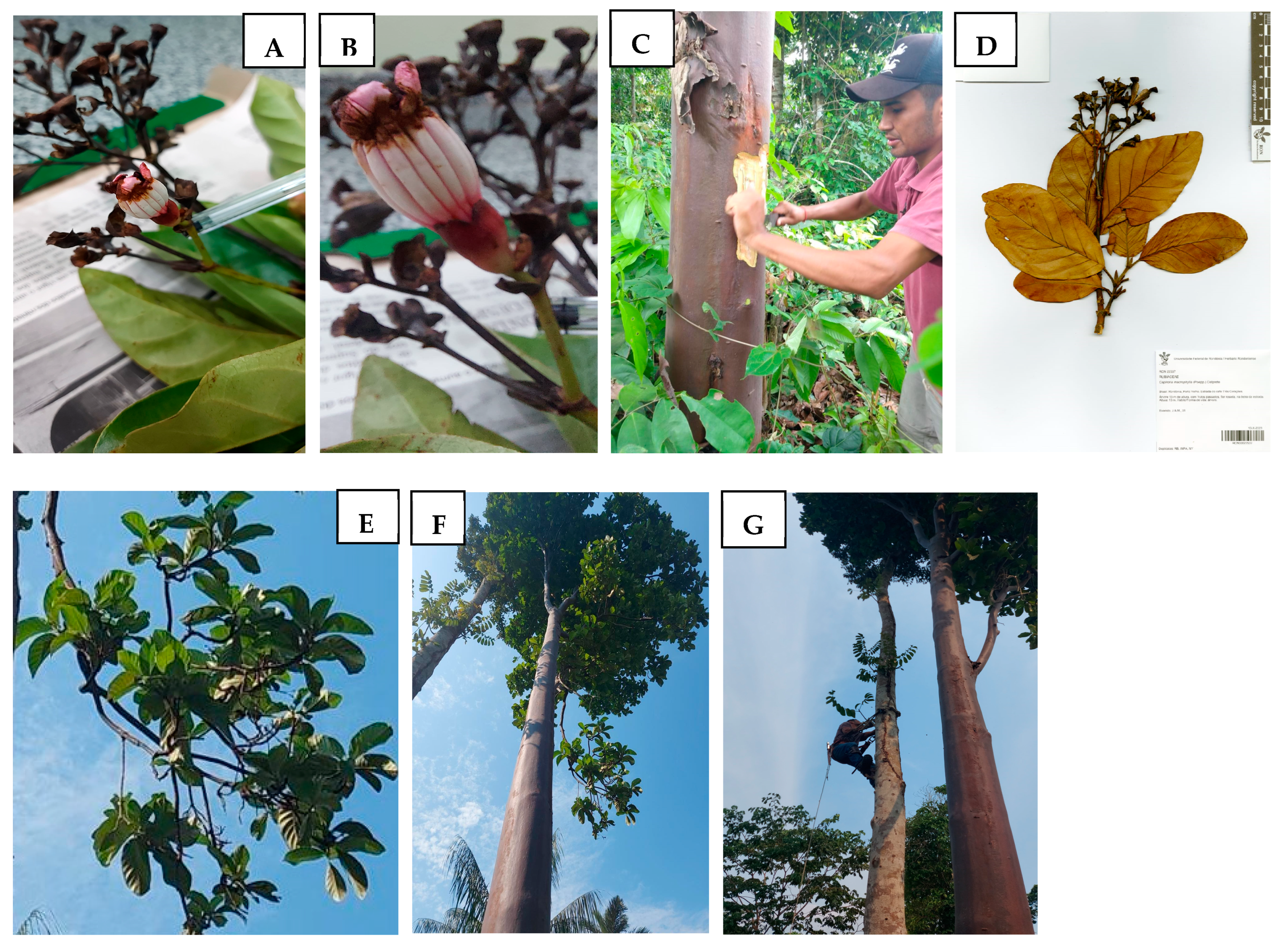



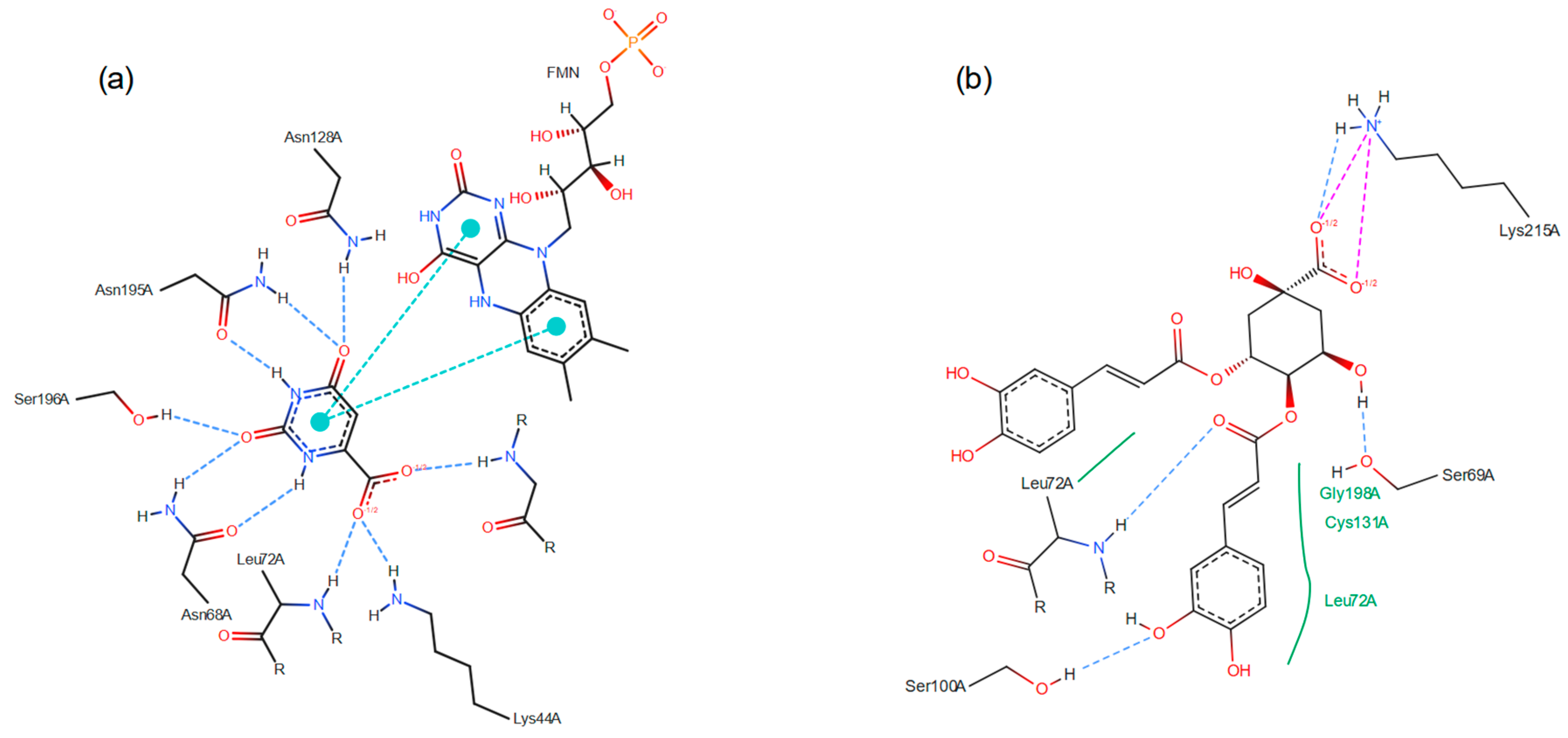

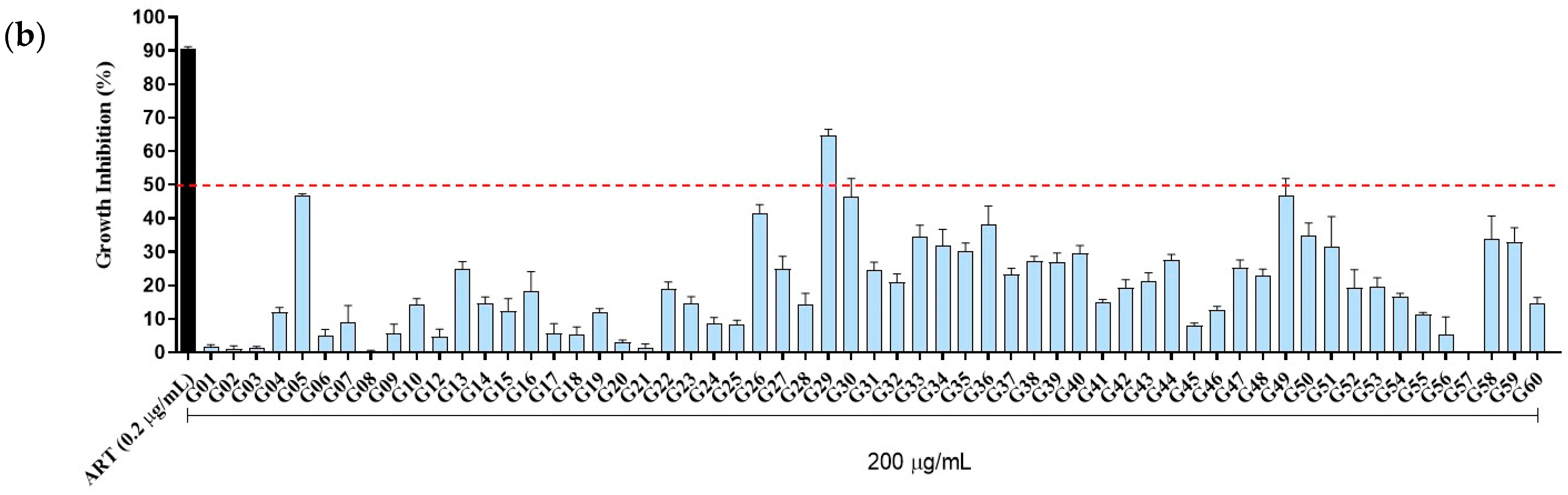
| ID | tR (min) Average | Formula [M + H]+ | Experimental Mass (m/z) | Error (ppm) | Peak Area Leaf/Stem/Wood Bark | Name | CID PubChem | MS2 Ions and Percentages (m/z, %) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | - | 254.1606 | - | 9.2 × 106/1.5 × 108/8.8 × 108 | Unknown | - | 254.1609 (100%), 237.1342 (3.5%), 196.1163 (3.3%), and 195.1127 (32.0%) |
| 2 | 1.09 | C11H13N2O2 | 205.0971 | −2.75 | 1.7 × 108/1.7 × 108/3.8 × 108 | Tryptophan | 6305 | 188.0705 (100%), 146.06 (32.7%), 189.0740 (13.0%), 144.0807 (5.4%) and 159.0916 (5.3%) |
| 3 | 2.62 | C16H19O9 | 355.1026 | −0.96 | 1.6 × 108/2 × 108/4.6 × 108 | Chlorogenic acid | 1794427 | 163.0389 (100%), and 355.1026 (44.9%) |
| 4 | 2.87 | - | 289.1182 | - | 2.3 × 106/1.1 × 107/3 × 107 | Unknown | - | 216.1017 (100%), 272.0914 (53.7%), 289.1182 (44.9%), 188.0706 (20.5%) and 180.0803 (7.7%) |
| 5 | 3.29 | C30H27O12 | 579.1484 | −3.24 | 3 × 107/9.8 × 103/0 | Procyanidin | 130556 | 127.0389 (100%), 579.1385 (88.9%), 291.0858 (78.8%), 427.1019 (78.7%), and 409.0906 (55.3%) |
| 6 | 3.72 | - | 496.2035 | - | 8.2 × 105/8.7 × 106/8.6 × 107 | Unknown | - | 185.0808 (100%), 308.8935 (44.1%), 371.3235 (42.4%), and 330.3095 (37.6%) |
| 7 | 3.83 | C15H15O6 | 291.0855 | −4.55 | 5.5 × 107/7 × 105/5.5 × 104 | Epicatechin | 72276 | 139.0388 (100%), 123.0440 (52.5%), 291.0857 (28.8%), 165.0544 (26.2%), and 147.0438 (55.3%) |
| 8 | 4.46 | C24H15N2O5 | 291.0974 | −2.36 | 2.4 × 106/1.5 × 108/1.5 × 108 | Malonyltryptophan | 5199636 | 188.0867 (100%), 245.0919 (91.2%), 130.0652 (40.7%), 227.0814 (27.9%), 273.0867 (18.6%), and 291.0979 (17.4%) |
| 9 | 4.78 | C10H9O4 | 193.0497 | −2.1 | 1.9 × 107/4.5 × 108/3.3 × 108 | Scopoletin | 5280460 | 193.0495 (100%), 194.0528 (10.6%), 133.0284 (4.4%), and 178.0260 (1.9%) |
| 10 | 5.07/5.42 | C11H11O5 | 223.0597 | −4.48 | 4.4 × 106/5.3 × 108/6 × 107/1.2 × 106/1.3 × 108/1.2 × 107 | Isofraxidin | 5318565 | 223.0599 (100%), 224.0631 (11.2%), 208.0363 (4.6%), and 163.0387 (2.3%) |
| 11 | 5.13 | C11H17O3 | 197.1170 | −4.11 | 1.4 × 108/3.3 × 107/1.1 × 107 | Loliolide | 14334 | 197.1169 (100%), 179.1064 (83.7%), 135.1167 (44.4%), 104.0857 (19.9%), and 161.0958 (18.7%) |
| 12 | 5.89 | C28H35N2O11 | 575.2247 | 1.01 | 7.7 × 106/1 × 108/1.9 × 108 | 5-Carboxystrictosidine | 10483216 | 575.2227 (100%), 413.1701 (32.6%), 188.0704 (14%), 395.1589 (8.4%), and 165. 0811 (7.3%) |
| 13 | 7.32 | - | 181.1223 | - | 2.8 × 107/5.8 × 106/5.2 × 106 | Unknown | - | 181.1221 (100%), 135.1167 (15.4%), 163.1115 (13.5%), 139.0752 (4.1), 121.1012 (4.1%), and 95.0858 (3.76) |
| 14 | 8.30 | - | 367.1180 | - | 1.2 × 104/2.9 × 107/4.9 × 106 | Unknown | - | 367.1172 (100%), 336.0986 (54.3%), 337.1018 (7.9%), 321.0753 (1.4%), and 352.0924 (1.1%) |
| 15 | 10.08 | - | 335.2184 | - | 1.6 × 106/4 × 107/7.6 × 107 | Unknown | - | 335.2193 (100%), 195.0990 (0.3%), 71.17 (0.2%), and 203.3772 (0.1%) |
| 16 | 10.79 | C18H31O2 | 279.2315 | −3.31 | 1.7 × 106/2.7 × 107/7.3 × 107 | Linolenic acid | 5280934 | 279.2315 (100%), 95.0859 (44.1%), 81.0704 (43.9%), 67.0548 (26.7%), 109.1014 (23.5%), and 123.1169 (20.8%) |
| ID | tR (min) Average | Formula [M-H]− | Experimental Mass m/z | Error (ppm) | Peak Area Leaf/Stem/Wood Bark | Name | CID PubChem | MS2 Ions and Percentages (m/z, %) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.28 2.63 | C7H11O6 | 191.0551 | −2.54 | 5.9 × 107/7.3 × 107/1.5 × 108/5.4 × 108/1.3 × 109/1.2 × 109 | Quinic acid | 6508 | 191.0546 (100%), 85.0278 (2.5%), 173.0440 (0.9%), 127.0385 (0.9%) and 93.0329 (0.7%) |
| 2 | 1.08 | C16H17O9 | 353.0870 | −0.62 | 6 × 107/7.3 × 107/2.4 × 108 | 5-O-CQA * | 5280633 | 353.0867 (100%), 191.0546 (72.3%), 179.0334 (49.2%) and 135.0434 (8.03%) |
| 3 | 2.63 | C16H17O9 | 353.0873 | 0.06 | 1.4 × 108/1.8 × 108/3.7 × 108 | 3-O-CQA * | 1794427 | 191.0545 (100%), 353.0864 (19.9%), 179.0337 (4.5%), 192.0579 (3.5%) and 161.7553 (1%) |
| 4 | 2.90 | C16H17O9 | 353.0867 | −1.5 | 7.1 × 107/7.9 × 107/2.2 × 108 | 4-O-CQA * | 9798666 | 173.0438 (100%), 353.0864 (89.8%), 179.0333 (68.3%), 191.0545 (39%) and 135.0434 (13.5%) |
| 5 | 3.30 | C30H25O12 | 577.1356 | 1.76 | 3.1 × 107/3 × 103/2.1 × 103 | Procyanidin B2 | 122738 | 577.1346 (100%), 425.0876 (50.1%), 289.0715 (40.8%), 407.0769 (37.4%) and 125.0230 (33%) |
| 6 | 3.85 | C15H13O6 | 289.0716 | −0.81 | 3.6 × 107/4.1 × 104/5.5 × 103 | Epicatechin | 1203 | 289.0715 (100%), 245.0813 (20.3%), 125.0229 (5.6%), 205.0496 (5.5%) and 179.0339 (5%) |
| 7 | 4.48 | C13H13N2O3 | 245.0923 | −1.42 | 1.7 × 107/6.2 × 107/6.8 × 107 | N-Acetyltryptophan | 2002 | 245.0921 (100%), 203.0811 (64%), 74.0231 (27.3%) and 201.1022 (8.1%) |
| 8 | 4.56 | C17H19O9 | 367.1026 | −0.84 | 1.8 × 107/2.2 × 107/6.1 × 107 | 3-O-Feruloylquinic acid | 9799386 | 173.0443 (100%), 191.0546 (76.7%), 367.1020 (50%), 193.0491 (18.7%) and 93.0329 (7%) |
| 9 | 5.22 | - | 581.2208 | - | 5.6 × 104/7.8 × 105/8.3 × 106 | Unknown | - | 581.2225 (100%), 419.1700 (12.9%), 101.0227 (4.3%), 153.0542 (3.3%) |
| 10 | 5.41 | C26H27O16 | 595.1309 | 1.69 | 8.4 × 106/4.8 × 105/1 × 104 | Quercetin-3-O-vicianoside | 13887800 | 595.1301 (100%), 300.0271 (52.2%), 301.0329 (10.9%), 178.9975 (0.9%) and 302.0391 (0.6%) |
| 11 | 5.74 | C25H23O12 | 515.1192 | 0.48 | 2.5 × 107/1.6 × 107/9.1 × 106 | 1,5-DiCQA ** | 122685 | 515.1188 (100%), 353.0874 (60%), 179.0338 (32.3%), 173.0443 (25.3%) and 191.0550 (24.9%) |
| 12 | 5.89 | C27H29O16 | 609.1456 | 0.11 | 2.4 × 107/8.6 × 105/2.2 × 105 | Quercetin-3-O-rutinose | 5293655 | 609.1452 (100%), 300.0271 (32.8%), 301.0343 (32.8%), 67.4327 (2.4%) and 302.0387 (1.1%) |
| 13 | 6.19 | C25H23O12 | 515.1193 | 0.71 | 2.2 × 107/8.7 × 106/3.5 × 106 | 3,4-DiCQA ** | 3802778 | 515.1189 (100%), 353.0874 (72.1%), 173.0443 (41.8%), 179.0338 (29.2%) and 191.0549 (9.4%) |
| 14 | 6.27 | - | 187.0964 | - | 3.6 × 106/2.4 × 107/6.8 × 107 | Unknown | - | 125.0957 (100%), 126.0991 (5.4%), 169.0857 (5.0%) and 143.1065 (1.8%) |
| 15 | 6.30 | C33H39O19 | 739.2066 | −2.65 | 4.8 × 105/2.2 × 106/3.7 × 106 | Kaempferol-3-O-robinoside-7-O-rhamnoside | 5351997 | 739.2043 (100%), 577.1774 (75.3%), 173.0442 (30.2%), 578.1737 (21.1%) 191.0555 (20.3) |
| 16 | 6.91 | - | 241.1077 | - | 4.2 × 105/7.2 × 105/3.2 × 107 | Unknown | - | 241.1065 (100%), 197.1165 (74.5%), 67.5990 (6.8%) and 179.1060 (6.6%) |
| 17 | 7.25 | C10H17O4 | 201.1123 | −1.96 | 1.4 × 106/1.1 × 107/2.3 × 107 | Decanedioic acid | 5192 | 201.1118 (100%), 67.3854 (24.8%), 139.1109 (8.7%), 183.1008 (7.7%%) and 111.0799 (5.4%) |
| 18 | 8.80 | C18H33O5 | 329.2322 | −1.79 | 5.4 × 106/5.5 × 107/6.3 × 107 | FA 18:1 + 3O | 153001 | 329.2323 (100%), 171.1010 (6.6%), 211.1326 (4.6%), 229.1432 (3.86%) and 311.2209 (0.5%) |
| 19 | 10.08 | C18H31O4 | 311.2223 | 0.18 | 1.3 × 106/2.9 × 107/7.2 × 107 | FA 18:2 + 2O | 1928 | 311.2218 (100%), 171.1011 (24.9%), 293.2113 (10.9%), 185.1168 (5.9%) and 201.1118 (4.7%) |
| 20 | 10.79 | - | 295.2260 | - | 4.8 × 105/1.5 × 107/4.2 × 107 | Unknown | - | 183.1373 (100%), 277.2157 (71.4%), 68.0670 (20.5%) and 171.1007 (19.6%) |
| DHODH Leishmania major | DHODH Plasmodium falciparum | ||||
|---|---|---|---|---|---|
| CID | Name | kcal/mol | CID | Name | kcal/mol |
| 5281780 | 3,4-Dicaffeoylquinic acid | −8.78 | 51347395 | DSM265 * | −9.82 |
| 1492348 | Orotate (ORO*) | −8.56 | 5280863 | Kaempferol | −8.82 |
| 9799386 | 3-Feruloylquinic acid | −8.35 | 5280343 | Quercetin | −8.62 |
| 12358846 | 1,4-Dicaffeoylquinic acid | −8.08 | 161313 | Anabasamine | −8.57 |
| 10466307 | Loganetin | −7.41 | 6476139 | Methyl chlorogenate | −7.78 |
| 6476139 | Methyl chlorogenate | −7.34 | 5282944 | 9-HODE | −7.59 |
| 14334 | Loliolide | −7.08 | 1794427 | 3-Caffeoylquinic acid | −7.5 |
| 1794427 | 3-O-Caffeoylquinic acid | −7.02 | 348159 | (-)-5-Caffeoylquinic acid | −7.43 |
| 348159 | (-)-5-Caffeoylquinic acid | −6.77 | 9799386 | 3-Feruloylquinic acid | −7.38 |
| 58427569 | 4-O-Caffeoylquinic acid | −6.67 | 14334 | Loliolide | −7.01 |
| 5280460 | Scopoletin | −6.58 | 10466307 | Loganetin | −6.99 |
| 5199636 | N-Malonyltryptophan | −6.54 | 5199636 | N-Malonyltryptophan | −6.95 |
| 23760099 | Diderroside | −6.51 | 101756906 | Diderroside methyl ester | −6.77 |
| 5280633 | 5-Caffeoylquinic acid | −6.28 | 58427569 | 4-Caffeoylquinic acid | −6.72 |
| 14158101 | 3-p-Coumaroylquinic acid | −6.18 | 5281780 | 3,4-Dicaffeoylquinic acid | −6.67 |
| 5280863 | Kaempferol | −6.16 | 1148 | DL-Tryptophan | −6.45 |
| 5280343 | Quercetin | −6.12 | 5460026 | beta-Gentiobiose | −5.66 |
| 161313 | Anabasamine | −5.98 | 12358846 | 1,4-Dicaffeoylquinic acid | −5.65 |
| 6508 | Quinic acid | −5.75 | 5429 | Theobromine | −5.59 |
| 1148 | DL-Tryptophan | −5.52 | 798 | Indole | −5.37 |
| 10483216 | 5(S)-5-Carboxystrictosidine | −5.51 | 23760099 | Diderroside | −5.07 |
| 101756906 | Diderroside methyl ester | −5.14 | 22563 | Desethylatrazine | −5.07 |
| 5282944 | 9-HODE | −5.06 | 998 | Phenylacetaldehyde | −4.9 |
| 5429 | Theobromine | −4.96 | 135398660 | Pterin | −4.69 |
| 5281769 | 1,5-Dicaffeoylqunic acid | −4.8 | 6508 | Quinic acid | −4.65 |
| 5460026 | beta-Gentiobiose | −4.69 | 75368781 | 4,5-Dycaffeoylquinic acid | −4.63 |
| 798 | Indole | −4.65 | 5281769 | 1,5-Dicaffeoylquinic acid | −3.10 |
| 998 | Phenylacetaldehyde | −4.51 | 10483216 | 5(S)-5-Carboxystrictosidine | −2.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evaristo, J.; Laia, E.d.; Tavares, B.; Mendonça, E.; Grisostenes, L.; Rodrigues, C.; do Nascimento, W.; Garcia, C.; Guterres, S.; Nogueira, F.; et al. Identification of Bioactive Metabolites of Capirona macrophylla by Metabolomic Analysis, Molecular Docking, and In Vitro Antiparasitic Assays. Metabolites 2025, 15, 157. https://doi.org/10.3390/metabo15030157
Evaristo J, Laia Ed, Tavares B, Mendonça E, Grisostenes L, Rodrigues C, do Nascimento W, Garcia C, Guterres S, Nogueira F, et al. Identification of Bioactive Metabolites of Capirona macrophylla by Metabolomic Analysis, Molecular Docking, and In Vitro Antiparasitic Assays. Metabolites. 2025; 15(3):157. https://doi.org/10.3390/metabo15030157
Chicago/Turabian StyleEvaristo, Joseph, Elise de Laia, Bruna Tavares, Esdras Mendonça, Larissa Grisostenes, Caroline Rodrigues, Welington do Nascimento, Carolina Garcia, Sheila Guterres, Fábio Nogueira, and et al. 2025. "Identification of Bioactive Metabolites of Capirona macrophylla by Metabolomic Analysis, Molecular Docking, and In Vitro Antiparasitic Assays" Metabolites 15, no. 3: 157. https://doi.org/10.3390/metabo15030157
APA StyleEvaristo, J., Laia, E. d., Tavares, B., Mendonça, E., Grisostenes, L., Rodrigues, C., do Nascimento, W., Garcia, C., Guterres, S., Nogueira, F., Zanchi, F., & Evaristo, G. (2025). Identification of Bioactive Metabolites of Capirona macrophylla by Metabolomic Analysis, Molecular Docking, and In Vitro Antiparasitic Assays. Metabolites, 15(3), 157. https://doi.org/10.3390/metabo15030157






