Chemical Informatics Combined with Kendrick Mass Analysis to Enhance Annotation and Identify Pathways in Soybean Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Sample Preparation
2.3. ESI FT-ICR Mass Spectrometry
2.4. Data Processing and Analysis
3. Results and Discussion
3.1. ESI-FTICR of Soybean Leaf Extracts
3.2. Data Processing to Identify Matches in SoyCyc
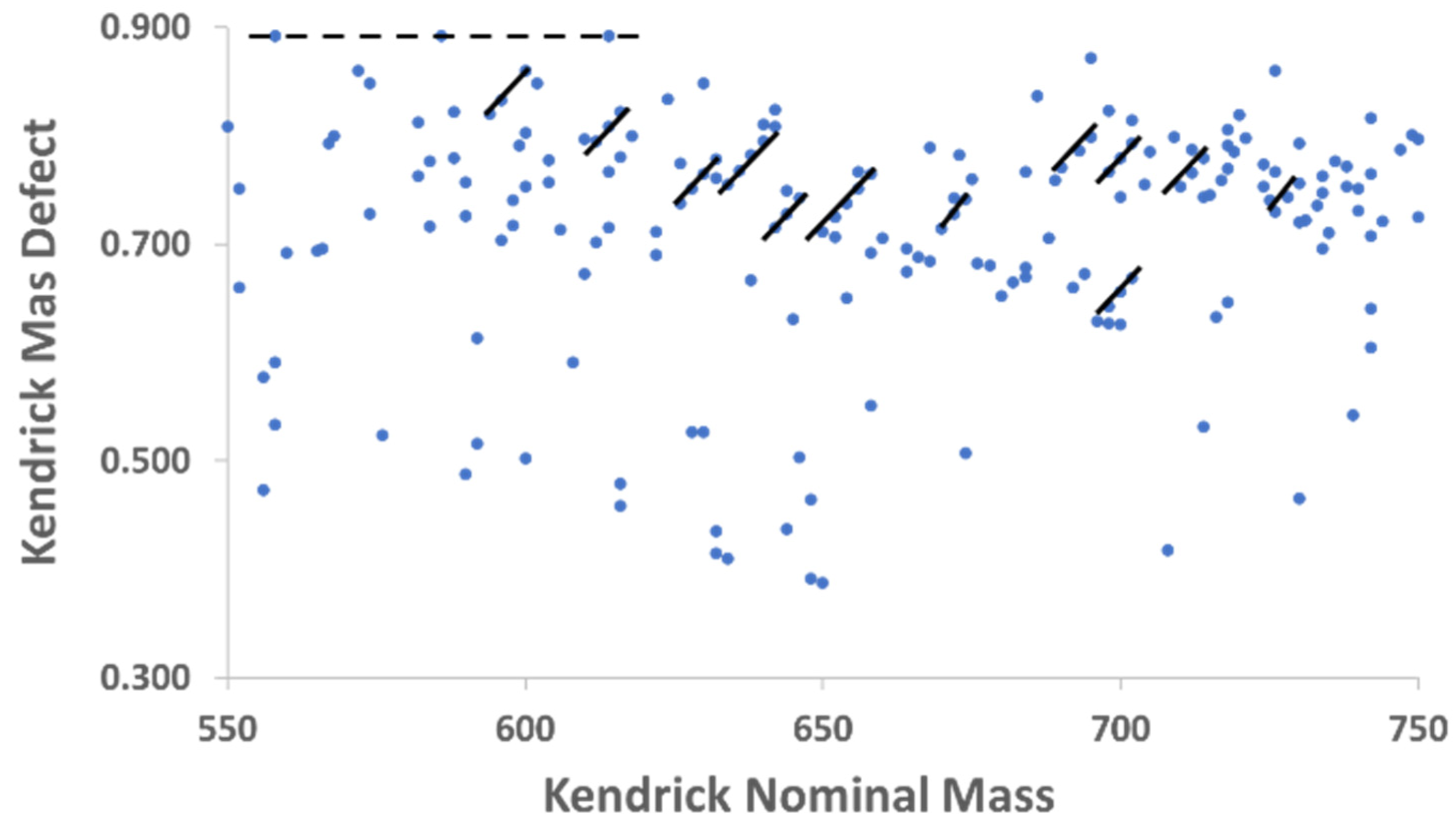
3.3. Kendrick Mass Defect (KMD) Analysis
3.4. Metabolic Pathways Analysis of Cultivars
3.5. Implications on Previous Metabolomics Investigation of Drought Stress on Cultivars [14]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Frederick, J.R.; Camp, C.R.; Bauer, P.J. Drought-Stress Effects on Branch and Mainstem Seed Yield and Yield Components of Determinate Soybean. Crop Sci. 2001, 41, 759–763. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic Profiling of Soybeans (Glycine max L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Song, L.; Schapaugh, W.T.; Ali, L.; Sinclair, T.R.; Riar, M.K.; Raymond, R.N.; Li, Y.; Vuong, T.; Valliyodan, B.; et al. The importance of slow canopy wilting in drought tolerance in soybean. J. Exp. Bot. 2020, 71, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.M.; Lee, J.-D.; Sleper, D.A.; Fritschi, F.B.; Sharp, R.E.; Carter, T.E.; Nelson, R.L.; King, C.A.; Schapaugh, W.T.; Ellersieck, M.R.; et al. Two Soybean Plant Introductions Display Slow Leaf Wilting and Reduced Yield Loss under Drought. J. Agron. Crop Sci. 2014, 200, 231–236. [Google Scholar] [CrossRef]
- Du, Y.L.; Zhao, Q.; Chen, L.R.; Yao, X.D.; Zhang, H.J.; Wu, J.J.; Xie, F.T. Effect of Drought Stress during Soybean R2–R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Ncube, E.; Mohale, K.; Nogemane, N. Metabolomics as a Prospective Tool for Soybean (Glycine max) Crop Improvement. Curr. Issues Mol. Biol. 2022, 44, 4181–4196. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Belinato, J.R.; Porto, C.; Nunes, E.; Guimaraes, F.; Meyer, M.C.; Pilau, E.J. Soybean Metabolomics Based in Mass Spectrometry: Decoding the Plant’s Signaling and Defense Responses under Biotic Stress. J. Agric. Food Chem. 2021, 69, 7257–7267. [Google Scholar] [CrossRef]
- Jervis, J.; Kastl, C.; Hildreth, S.B.; Biyashev, R.; Grabau, E.A.; Saghai-Maroof, M.A.; Helm, R.F. Metabolite Profiling of Soybean Seed Extracts from Near-Isogenic Low and Normal Phytate Lines Using Orthogonal Separation Strategies. J. Agric. Food Chem. 2015, 63, 9879–9887. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Nakamura, T.; Sugimoto, Y.; Sakamoto, K. Proteomic and Metabolomic Analyses of Soybean Root Tips Under Flooding Stress. Protein Pept. Lett. 2014, 21, 865–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, Y.P.; Wang, X.J.; Li, X.M.; Dong, S.K. Physiology and metabonomics reveal differences in drought resistance among soybean varieties. Bot. Stud. 2022, 63, 8. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, D.; Agtuca, B.J.; A Stopka, S.; Vertes, A.; Koppenaal, D.W.; Pasa-Tolic, L.; Stacey, G.; Anderton, C.R. Observed metabolic asymmetry within soybean root nodules reflects unexpected complexity in rhizobacteria-legume metabolite exchange. ISME J. 2018, 12, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Rudolph, H.L.; Hurst, J.J.; Wood, T.D. High-Throughput Metabolic Profiling of Soybean Leaves by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem. 2016, 88, 1188–1194. [Google Scholar] [CrossRef]
- Zemaitis, K.J.; Ye, H.; Nguyen, H.T.; Wood, T.D. Direct Infusion Metabolomics of the Photosystem and Chlorophyll Related Metabolites within a Drought Tolerant Plant Introduction of Glycine max. Metabolites 2021, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Bragagnolo, F.S.; Funari, C.S.; Ibanez, E.; Cifuentes, A. Metabolomics as a Tool to Study Underused Soy Parts: In Search of Bioactive Compounds. Foods 2021, 10, 1308. [Google Scholar] [CrossRef]
- Kendrick, E. A Mass Scale Based on CH2 = 14.0000 for High Resolution Mass Spectrometry of Organic Compounds. Anal. Chem. 1963, 35, 2146–2154. [Google Scholar] [CrossRef]
- Hughey, C.A.; Hendrickson, C.L.; Rodgers, R.P.; Marshall, A.G. Elemental Composition Analysis of Processed and Unprocessed Diesel Fuel by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2001, 15, 1186–1193. [Google Scholar] [CrossRef]
- Hughey, C.A.; Hendrickson, C.L.; Rodgers, R.P.; Marshall, A.G.; Qian, K. Kendrick Mass Defect Spectrum: A Compact Visual Analysis for Ultrahigh-Resolution Broadband Mass Spectra. Anal. Chem. 2001, 73, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Chainet, F.; Ponthus, J.; Lienemann, C.-P.; Courtiade, M.; Donard, O.F.X. Combining Fourier Transform-Ion Cyclotron Resonance/Mass Spectrometry Analysis and Kendrick Plots for Silicon Speciation and Molecular Characterization in Petroleum Products at Trace Levels. Anal. Chem. 2012, 84, 3998–4005. [Google Scholar] [CrossRef]
- Cody, R.B.; Fouquet, T. Resolution-Enhanced Kendrick Mass Defect Analysis of Polycyclic Aromatic Hydrocarbons and Fullerenes in the Diffusion Flame from a Butane Torch. J. Am. Soc. Mass Spectrom. 2018, 29, 2110–2113. [Google Scholar] [CrossRef]
- Spiegel, M.T.; Anthony, I.G.M.; Brantley, M.R.; Hassell, A.; Farmer, P.J.; Solouki, T. Reactivities of Aromatic Protons in Crude Oil Fractions toward Br2 Tagging for Structural Characterization by Nuclear Magnetic Resonance and Electron Paramagnetic Resonance Spectroscopy and Mass Spectrometry. Energy Fuels 2018, 32, 10549–10555. [Google Scholar] [CrossRef]
- Sato, H.; Nakamura, S.; Teramoto, K.; Sato, T. Structural Characterization of Polymers by MALDI Spiral-TOF Mass Spectrometry Combined with Kendrick Mass Defect Analysis. J. Am. Soc. Mass Spectrom. 2014, 25, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, T.; Sato, H. Convenient visualization of high-resolution tandem mass spectra of synthetic polymer ions using Kendrick mass defect analysis—The case of polysiloxanes. Rapid Commun. Mass Spectrom. 2016, 30, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Fouquet, T. Paper spray and Kendrick mass defect analysis of block and random ethylene oxide/propylene oxide copolymers. Anal. Chim. Acta 2017, 989, 38–44. [Google Scholar] [CrossRef]
- Fouquet, T.; Sato, H. Improving the Resolution of Kendrick Mass Defect Analysis for Polymer Ions with Fractional Base Units. Mass Spectrom. 2017, 6, A0055. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, T.; Sato, H. Extension of the Kendrick Mass Defect Analysis of Homopolymers to Low Resolution and High Mass Range Mass Spectra Using Fractional Base Units. Anal. Chem. 2017, 89, 2682–2686. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, T.; Shimada, H.; Maeno, K.; Ito, K.; Ozeki, Y.; Kitagawa, S.; Ohtani, H.; Sato, H. High-resolution Kendrick Mass Defect Analysis of Poly(ethylene oxide)-based Non-ionic Surfactants and Their Degradation Products. J. Oleo Sci. 2017, 66, 1061–1072. [Google Scholar] [CrossRef]
- Cody, R.B.; Fouquet, T. “Reverse Kendrick Mass Defect Analysis”: Rotating Mass Defect Graphs to Determine Oligomer Compositions for Homopolymers. Anal. Chem. 2018, 90, 12854–12860. [Google Scholar] [CrossRef]
- Fouquet, T.N.J.; Cody, R.B.; Ozeki, Y.; Kitagawa, S.; Ohtani, H.; Sato, H. On the Kendrick Mass Defect Plots of Multiply Charged Polymer Ions: Splits, Misalignments, and How to Correct Them. J. Am. Soc. Mass Spectrom. 2018, 29, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Korf, A.; Fouquet, T.; Schmid, R.; Hayen, H.; Hagenhoff, S. Expanding the Kendrick Mass Plot Toolbox in MZmine 2 to Enable Rapid Polymer Characterization in Liquid Chromatography-Mass Spectrometry Data Sets. Anal. Chem. 2020, 92, 628–633. [Google Scholar] [CrossRef]
- He, H.; Conrad, C.A.; Nilsson, C.L.; Ji, Y.J.; Schaub, T.M.; Marshall, A.G.; Emmett, M.R. Method for Lipidomic Analysis: p53 Expression Modulation of Sulfatide, Ganglioside, and Phospholipid Composition of U87 MG Glioblastoma Cells. Anal. Chem. 2007, 79, 8423–8430. [Google Scholar] [CrossRef] [PubMed]
- Lerno, L.A., Jr.; German, J.B.; Lebrilla, C.B. Method for the Identification of Lipid Classes Based on Referenced Kendrick Mass Analysis. Anal. Chem. 2010, 82, 4236–4245. [Google Scholar] [CrossRef]
- Korf, A.; Vosse, C.; Schmid, R.; Helmer, P.O.; Jeck, V.; Hayen, H. Three-dimensional Kendrick mass plots as a tool for graphical lipid identification. Rapid Commun. Mass Spectrom. 2018, 32, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Ricart, E.; Hanozin, E.; Pupin, M.; Jacques, P.; Smargiasso, N.; De Pauw, E.; Lisacek, F.; Leclere, V.; Flahaut, C. Kendrick Mass Defect Approach Combined to NORINE Database for Molecular Formula Assignment of Nonribosomal Peptides. J. Am. Soc. Mass Spectrom. 2019, 30, 2608–2616. [Google Scholar] [CrossRef]
- Blanc, L.; Ferraro, G.B.; Tuck, M.; Prideaux, B.; Dartois, V.; Jain, R.K.; Desbenoit, N. Kendrick Mass Defect Variation to Decipher Isotopic Labeling in Brain Metastases Studied by Mass Spectrometry Imaging. Anal. Chem. 2021, 93, 16314–16319. [Google Scholar] [CrossRef]
- Muller, W.H.; Verdin, A.; Kune, C.; Far, J.; De Pauw, E.; Malherbe, C.; Eppe, G. Dual-polarity SALDI FT-ICR MS imaging and Kendrick mass defect data filtering for lipid analysis. Anal. Bioanal. Chem. 2021, 413, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Zarev, Y.; Popova, P.; Foubert, K.; Ionkova, I.; Pieters, L. Comparative LC-MS analysis of tropolone alkaloids from in vitro cultures and native sources of Gloriosa superba by Kendrick mass defect plots. Phytochem. Anal. 2021, 32, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.T.; Neumann, E.K.; Caprioli, R.M.; Spraggins, J.M.; Solouki, T. Referenced Kendrick Mass Defect Annotation and Class-Based Filtering of Imaging MS Lipidomics Experiments. Anal. Chem. 2022, 94, 5504–5513. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Liu, Y.; Bianchi, T.S.; Tolic, N.; Jansson, C.; Pasa-Tolic, L. Moving beyond the van Krevelen Diagram: A New Stoichiometric Approach for Compound Classification in Organisms. Anal. Chem. 2018, 90, 6152–6160. [Google Scholar] [CrossRef]
- Brown, A.V.; I Conners, S.; Huang, W.; Wilkey, A.P.; Grant, D.; Weeks, N.T.; Cannon, S.B.; A Graham, M.; Nelson, R.T. A new decade and new data at SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 2021, 49, D1496–D1501. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Karp, P.D.; E Midford, P.; Billington, R.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Ong, W.K.; Subhraveti, P.; Caspi, R.; Fulcher, C.; et al. Pathway Tools version 23.0 update: Software for pathway/genome informatics and systems biology. Briefings Bioinform. 2021, 22, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef] [PubMed]
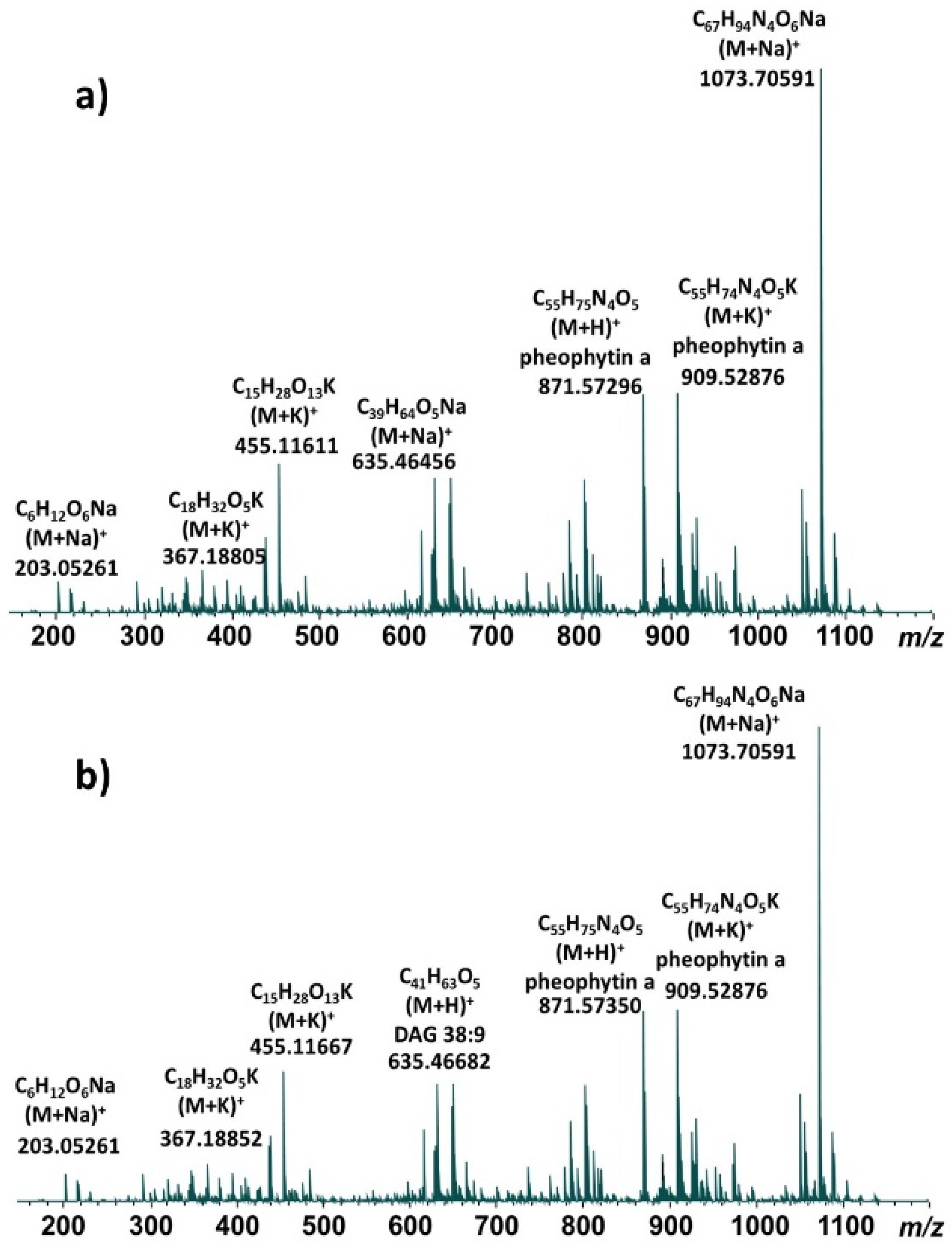
| Measured m/z | Ion Formula | Mass Error (ppm) | Possible Identification |
|---|---|---|---|
| 309.20358 | C16H30NaO4 | −0.16 | Hexdecanedioic acid |
| 325.17755 | C19H26NaO3 | 0.14 | Carlactone |
| 351.17563 | C18H32KO2S | 0.49 | Carboxylic acid class |
| 353.22983 | C18H34NaO5 | −0.04 | Stearic acid isomers |
| 359.01734 | C15H12KO8 | −2.59 | Carboxylic acid class |
| 365.06327 | C15H18KO8 | −0.15 | Carboxylic acid class |
| 367.10843 | C10H23O14 | 0.54 | Carboxylic acid class |
| 395.17342 | C21H28KN2O3 | 0.68 | Galatopinitol |
| 435.25066 | C23H40KO5 | −0.17 | 5-isomers |
| 471.25074 | C26H40KO5 | 0.02 | Glucoside class |
| 497.18664 | C20H33O14 | −0.32 | 3 isomers |
| 585.37007 | C40H50NaO2 | −0.40 | 15-cis-phytoene |
| 609.27067 | C29H46NaO10S | 0.46 | 3 isomers |
| 647.46492 | C46H66NaO2 | −0.40 | Epoxypheophorbide a |
| 649.18953 | C29H38KO14 | 0.33 | Glucoside class |
| 675.49608 | C51H96KO6 | 0.05 | 2 isomers |
| 741.57946 | C17H25NaNO6 | 0.10 | Menaquinol-8 |
| 771.60506 | C56H96KO3 | 0.01 | 34:5-monoglactosyldiacylglycerol |
| 893.55467 | C55H74NaN4O5 | −0.53 | Pheophytin a |
| 907.52140 | C55H71MgN4O6 | −0.50 | Chlorophyll b |
| 923.50859 | C55H72KN4O6 | 0.27 | Pheophytin b |
| 945.47643 | C55H70KMgN4O6 | −1.38 | Chlorophyll b |
| Measured m/z | Ion Formula | Mass Error (ppm) | Possible Identification |
|---|---|---|---|
| 277.08988 | C9H18NaO8 | 1.77 | 3-β-D-galactosyl-sn-glycerol |
| 481.36516 | C30H50NaO3 | −0.12 | Soyasapogenol B |
| 527.15854 | C18H92NaO16 | 0.54 | Trisaccharide class |
| 543.13251 | C18H92KO16 | 0.74 | Trisaccharide class |
| 771.60547 | C50H84KO3 | 0.34 | Plastoquinone |
| Pathway | Covered Compounds |
|---|---|
| Chlorophyll a degradation II | Pheophytin a Chlorophyll a |
| Chlorophyll cycle | Chlorophyll a Chlorophyll b |
| Glycolipid desaturation | 1-18:2-2-18:2-monogalactosyldiacylglycerol 1-18:3-2-18:3-monogalactosyldiacylglycerol 1-18:3-2-18:3-digalactosyldiacylglycerol |
| Medicarpin conjugates interconversion | Medicarpin-3-O-glucoside |
| Phytosterol biosynthesis | Cycloeucalenone |
| Plant sterol biosynthesis II | Squalene |
| Superpathway of carotenoid biosynthesis | Antheraxanthin Plastoquinone 15-cis-phytoene |
| Violdelphin biosynthesis | Delphinidin-3-O-rutioside-7-O-glucoside |
| Pathway | Covered Compounds |
|---|---|
| Chlorophyll a degradation II | Pheophytin a Chlorophyll a |
| Chlorophyll cycle | Chlorophyll a Chlorophyll b |
| Glycolipid desaturation | 1-18:2-2-18:2-monogalactosyldiacylglycerol 1-18:3-2-18:3-monogalactosyldiacylglycerol 1-18:3-2-18:3-digalactosyldiacylglycerol |
| Phytosterol biosynthesis | Cycloeucalenone |
| Plant sterol biosynthesis II | Squalene |
| Superpathway of carotenoid biosynthesis | Antheraxanthin Plastoquinone 15-cis-phytoene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, T.D.; Tiede, E.R.; Izydorczak, A.M.; Zemaitis, K.J.; Ye, H.; Nguyen, H.T. Chemical Informatics Combined with Kendrick Mass Analysis to Enhance Annotation and Identify Pathways in Soybean Metabolomics. Metabolites 2025, 15, 73. https://doi.org/10.3390/metabo15020073
Wood TD, Tiede ER, Izydorczak AM, Zemaitis KJ, Ye H, Nguyen HT. Chemical Informatics Combined with Kendrick Mass Analysis to Enhance Annotation and Identify Pathways in Soybean Metabolomics. Metabolites. 2025; 15(2):73. https://doi.org/10.3390/metabo15020073
Chicago/Turabian StyleWood, Troy D., Erin R. Tiede, Alexandra M. Izydorczak, Kevin J. Zemaitis, Heng Ye, and Henry T. Nguyen. 2025. "Chemical Informatics Combined with Kendrick Mass Analysis to Enhance Annotation and Identify Pathways in Soybean Metabolomics" Metabolites 15, no. 2: 73. https://doi.org/10.3390/metabo15020073
APA StyleWood, T. D., Tiede, E. R., Izydorczak, A. M., Zemaitis, K. J., Ye, H., & Nguyen, H. T. (2025). Chemical Informatics Combined with Kendrick Mass Analysis to Enhance Annotation and Identify Pathways in Soybean Metabolomics. Metabolites, 15(2), 73. https://doi.org/10.3390/metabo15020073








