Effect of Four Different Initial Drying Temperatures on Biochemical Profile and Volatilome of Black Tea
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Processing Method
2.2. Sensory Evaluation, Water Extract, Catechins, Amino Acid, and Total Phenol Content Determination
2.3. Volatilome Detection and Analysis by GC-MS
2.4. Data Analysis
3. Results
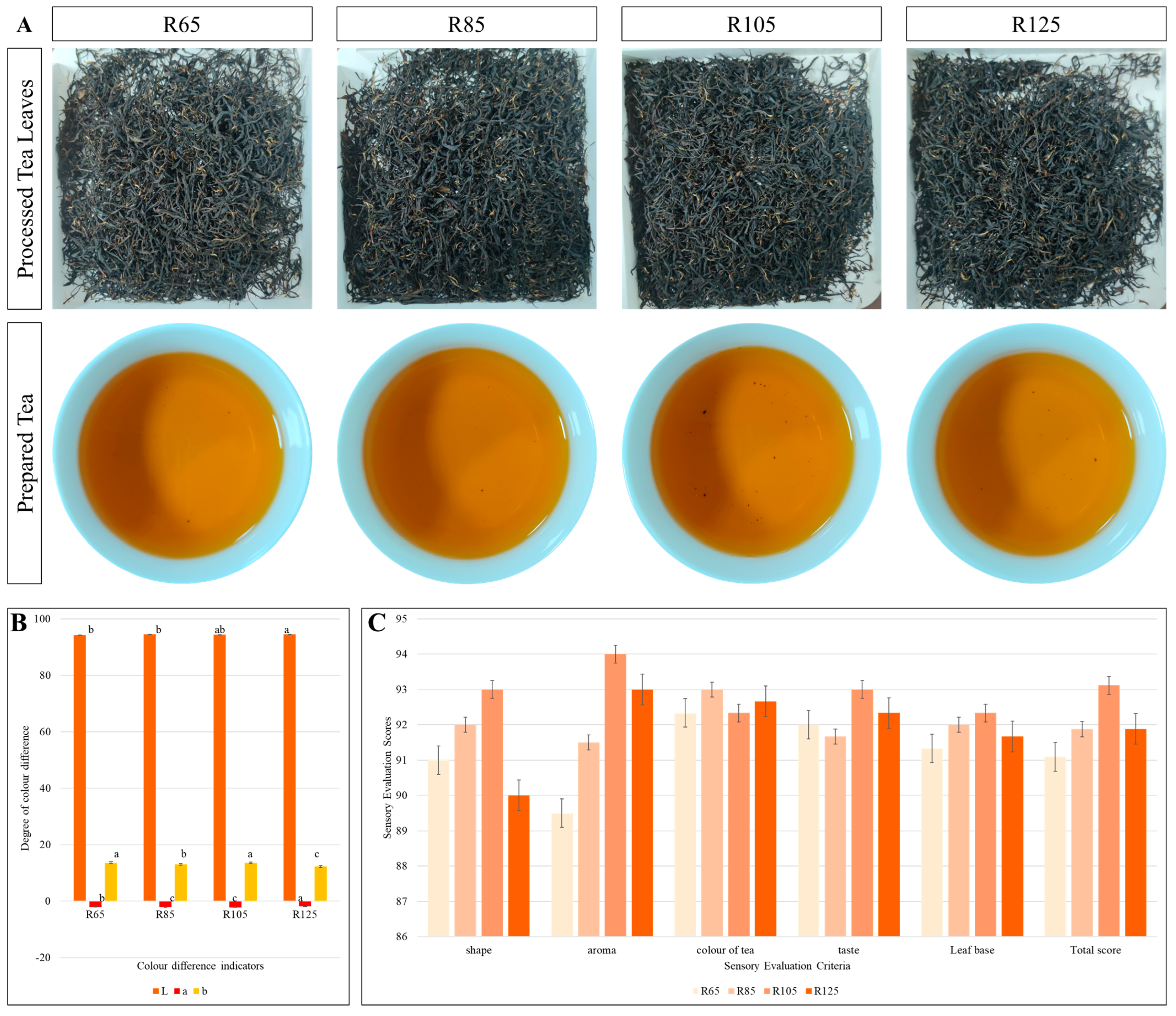
3.1. Sensory Evaluation
3.2. Effect of Processing Temperature on Amino Acids, Catechins, Polyphenols, and Caffeine Contents
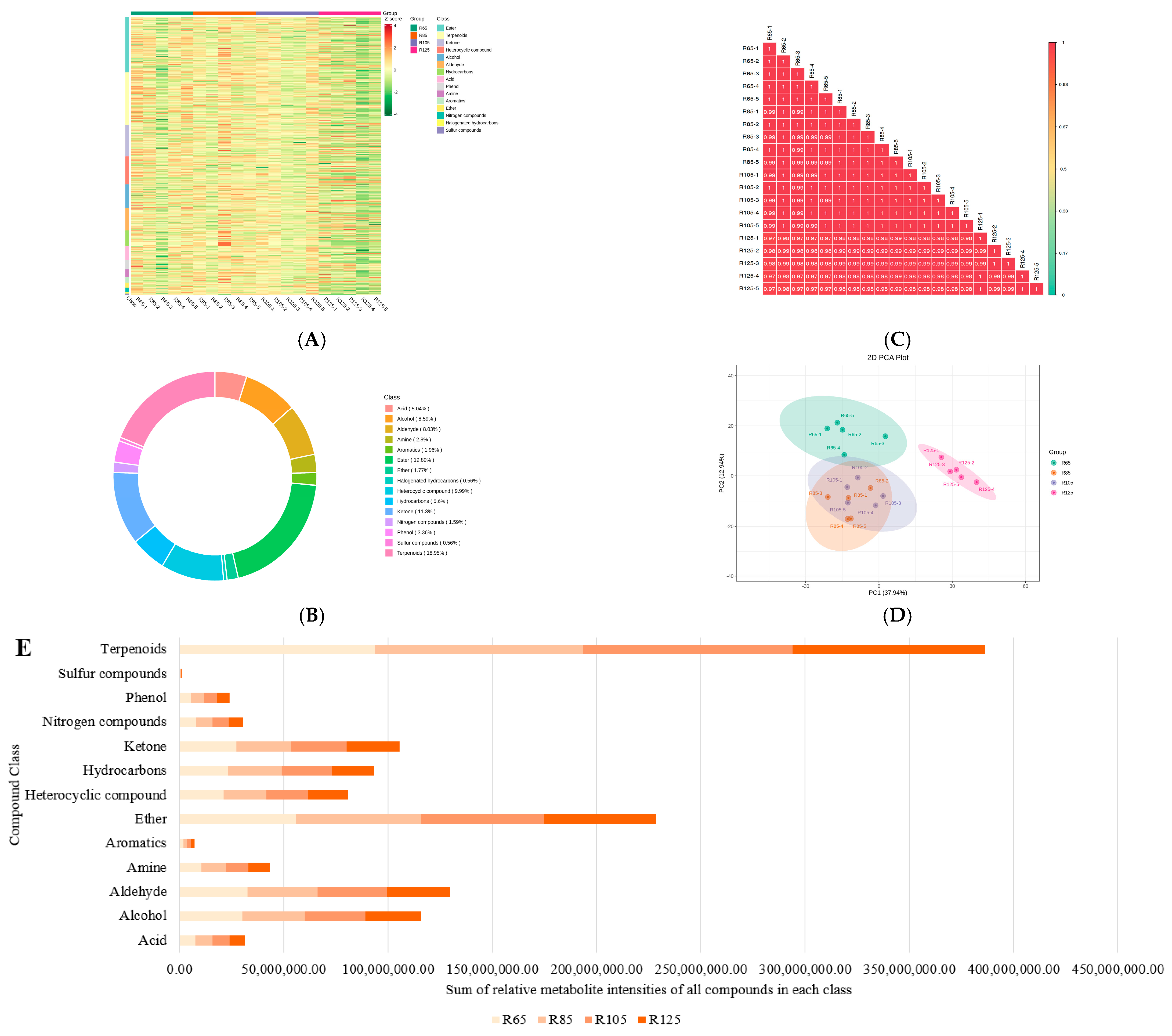
3.3. Global Volatilome Profiles of LWV Processed at Different Temperatures
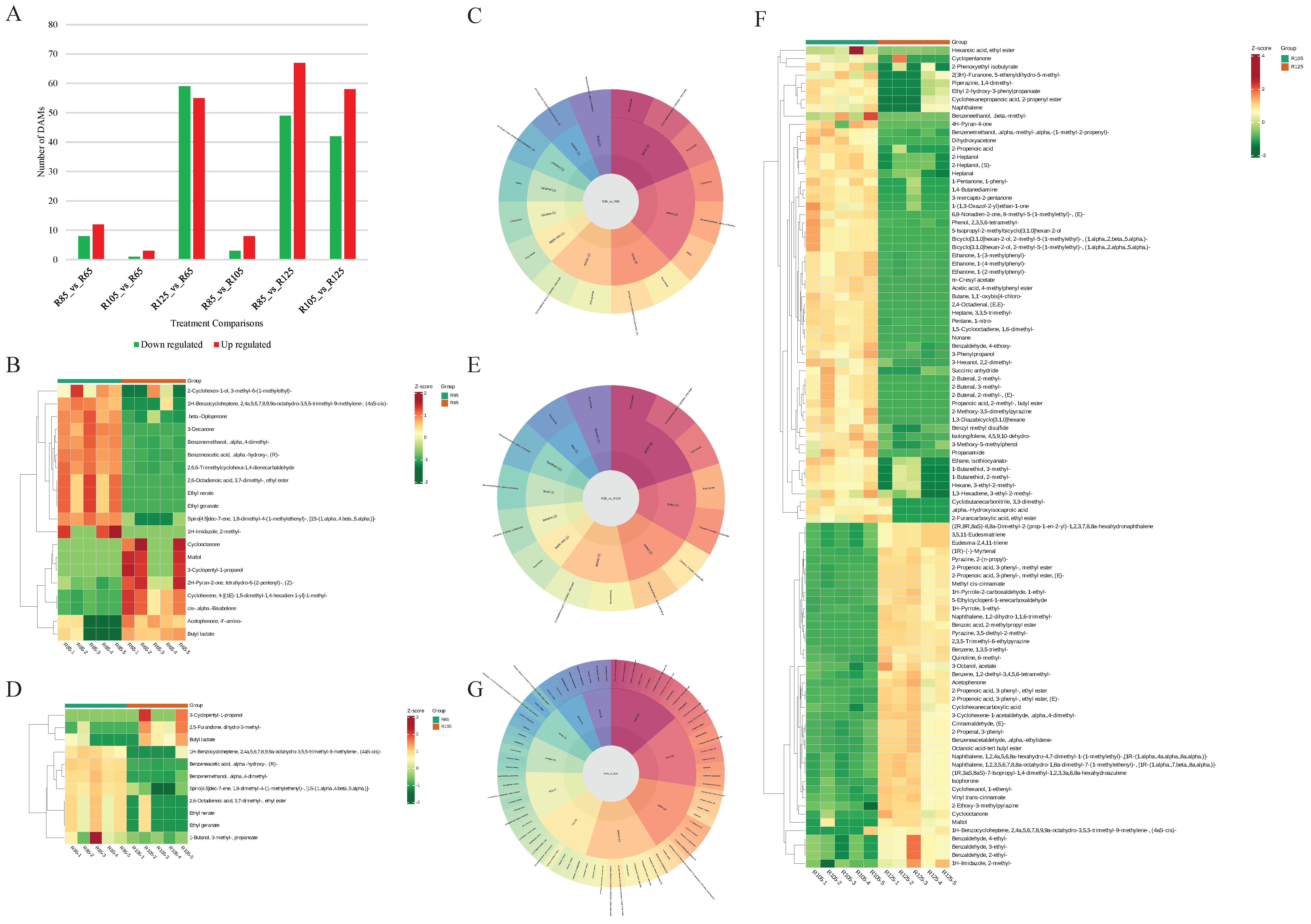
3.4. Differential Volatilome Profiles of LWV Processed at Different Temperatures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, S.-Y.; Nie, Q.; Tai, H.-C.; Song, X.-L.; Tong, Y.-F.; Zhang, L.-J.-F.; Wu, X.-W.; Lin, Z.-H.; Zhang, Y.-Y.; Ye, D.-Y. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef]
- Jason, W. China’s Dominance in Tea Shows No Signs of Waning; Gravesend, Kent DA11 0DF, United Kingdom, 27.06.2024 2024. Available online: https://www.teaandcoffee.net/feature/34515/chinas-dominance-in-tea-shows-no-signs-of-waning/ (accessed on 22 December 2024).
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Aaqil, M.; Peng, C.; Kamal, A.; Nawaz, T.; Zhang, F.; Gong, J. Tea Harvesting and Processing Techniques and Its Effect on Phytochemical Profile and Final Quality of Black Tea: A Review. Foods 2023, 12, 4467. [Google Scholar] [CrossRef] [PubMed]
- Jolvis Pou, K. Fermentation: The key step in the processing of black tea. J. Biosyst. Eng. 2016, 41, 85–92. [Google Scholar] [CrossRef]
- Temple, S.J.; Temple, C.M.; Boxtel, A.J.B.v.; Clifford, M.N. The effect of drying on black tea quality. J. Sci. Food Agric. 2001, 81, 764–772. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. Lwt 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Teshome, K. Effect of tea processing methods on biochemical composition and sensory quality of black tea (Camellia sinensis (L.) O. Kuntze): A review. J. Hortic. For. 2019, 11, 84–95. [Google Scholar]
- Kavish, S.; Botheju, W.S.; De Silva, C.S. Impact of inlet drying temperature in endless chain pressure dryers on the quality characteristics of leafy type of tea produced using different leaf standards. OUSL J. 2016, 10, 73–92. [Google Scholar] [CrossRef]
- Su, S.; Long, P.; Zhang, Q.; Wen, M.; Han, Z.; Zhou, F.; Ke, J.; Wan, X.; Ho, C.-T.; Zhang, L. Chemical, sensory and biological variations of black tea under different drying temperatures. Food Chem. 2024, 446, 138827. [Google Scholar] [CrossRef] [PubMed]
- Polat, A.; Şat, İ.G.; Ilgaz, Ş. Comparison of black tea volatiles depending on the grades and different drying temperatures. J. Food Process. Preserv. 2018, 42, e13653. [Google Scholar] [CrossRef]
- Shinde, A.; Das, S.; Datta, A. Quality improvement of orthodox and CTC tea and performance enhancement by hybrid hot air–radio frequency (RF) dryer. J. Food Eng. 2013, 116, 444–449. [Google Scholar] [CrossRef]
- Duan, D.; Ma, F.; Zhao, L.; Yin, Y.; Zheng, Y.; Xu, X.; Sun, Y.; Xue, Y. Variation law and prediction model to determine the moisture content in tea during hot air drying. J. Food Process Eng. 2022, 45, e13966. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. National Standard of The People’s Republic of China: Beijing, China, 2018. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT23776-2018 (accessed on 24 January 2025).
- GB/T 8305-2013; Tea. Determination of Water Extracts Content. National Standard of The People’s Republic of China: Beijing, China, 2013. Available online: https://www.chinesestandard.net/PDF/GDOC.aspx/GBT8305-2013 (accessed on 24 January 2025).
- GB/T 8314-2013; Tea. Determination of Free Amino Acids Content. National Standard of The People’s Republic of China: Beijing, China, 2013. Available online: https://www.chinesestandard.net/PDF/GDOC.aspx/GBT8314-2013 (accessed on 24 January 2025).
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. National Standard of The People’s Republic of China: Beijing, China, 2018. Available online: https://www.chinesestandard.net/PDF.aspx/GBT8313-2018 (accessed on 24 January 2025).
- GB/T 23193-2017; Determination of Theanine in Tea-Suing High Performance Liquid Chromatograpy. National Standard of The People’s Republic of China: Beijing, China, 2017. Available online: https://gbstandards.org/GB_standard_english.asp?code=GB/T%2023193-2017 (accessed on 24 January 2025).
- Kanehisa, M. KEGG bioinformatics resource for plant genomics and metabolomics. Plant Bioinform. Methods Protoc. 2016, 1374, 55–70. [Google Scholar]
- Arakawa, K.; Kono, N.; Yamada, Y.; Mori, H.; Tomita, M. KEGG-based pathway visualization tool for complex omics data. Silico Biol. 2005, 5, 419–423. [Google Scholar] [CrossRef]
- Liang, S.; Gao, Y.; Fu, Y.-Q.; Chen, J.-X.; Yin, J.-F.; Xu, Y.-Q. Innovative technologies in tea-beverage processing for quality improvement. Curr. Opin. Food Sci. 2022, 47, 100870. [Google Scholar] [CrossRef]
- Pou, K.J.; Paul, S.K.; Malakar, S. Industrial processing of CTC black tea. In Caffeinated and Cocoa Based Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–162. [Google Scholar]
- Zhong, Y.-F.; Chang, R.; Chen, S.-M.; Luo, H.-Y.; Wang, J.; Zhang, Y.; Zhang, L. Quality and characteristic components of different types of Tuo tea. J. Food Saf. Qual. 2022, 13, 3867–3874. [Google Scholar]
- Gramza-Michałowska, A. Caffeine in tea camellia sinensis—Content, absorption, benefits and risks of consumption. J. Nutr. Health Aging 2014, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, Y. Effect of pH on cream particle formation and solids extraction yield of black tea. Food Chem. 2001, 74, 155–160. [Google Scholar] [CrossRef]
- Wang, X.; Wan, X.; Hu, S.; Pan, C. Study on the increase mechanism of the caffeine content during the fermentation of tea with microorganisms. Food Chem. 2008, 107, 1086–1091. [Google Scholar] [CrossRef]
- Kong, X.; Xu, W.; Zhang, K.; Chen, G.; Zeng, X. Effects of reaction temperature, pH and duration on conversion of tea catechins and formation of theaflavins and theasinensins. Food Biosci. 2023, 54, 102911. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. The influence of processing conditions on catechin, caffeine and chlorophyll contents of green tea (Camelia sinensis) leaves and infusions. LWT 2019, 116, 108567. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Li, L.; Sheng, X.; Zan, J.; Yuan, H.; Zong, X.; Jiang, Y. Monitoring the dynamic change of catechins in black tea drying by using near-infrared spectroscopy and chemometrics. J. Food Compos. Anal. 2023, 119, 105266. [Google Scholar] [CrossRef]
- Yin, X.; Huang, J.a.; Huang, J.; Wu, W.; Tong, T.; Liu, S.; Zhou, L.; Liu, Z.; Zhang, S. Identification of volatile and odor-active compounds in Hunan black tea by SPME/GC-MS and multivariate analysis. LWT 2022, 164, 113656. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, J.Y.; Hou, Y.J.; Li, J.H.; Liu, W.H.; Sun, X.; Huang, Y.P. Hot-air full drying driven metabolome changes in white tea (Camellia sinensis L.). Int. J. Food Prop. 2023, 26, 2742–2756. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Jia, W.; Wen, B.; Liao, S.; Zhao, Y.; Tang, Q.; Li, K.; Hua, Y.; Yang, Y. Dynamic changes in the major chemical and volatile components during the “Ziyan” tea wine processing. LWT 2023, 186, 115273. [Google Scholar] [CrossRef]
- Yao, H.; Su, H.; Ma, J.; Zheng, J.; He, W.; Wu, C.; Hou, Z.; Zhao, R.; Zhou, Q. Widely targeted volatileomics analysis reveals the typical aroma formation of Xinyang black tea during fermentation. Food Res. Int. 2023, 164, 112387. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Sheng, C.; Ke, H.; Li, T.; Liu, Q.; Zhang, J.; Li, L.; Wang, Y.; Ning, J. Revealing the differences in aroma of black tea under different drying methods based on GC–MS, GC-O. Food Chem. X 2024, 23, 101782. [Google Scholar] [CrossRef]
- Wang, H.-j.; Hua, J.-j.; Jiang, Y.-w.; Wang, J.-j.; Yuan, H.-b. Effect of different heat transfer modes during secondary drying on quality components, color and taste of Congou black tea. Food Sci. 2020, 41, 148–157. [Google Scholar]
- Yang, Z.; Tang, J.; Xue, L.; Peng, Y. Study on high efficiency black tea fermentation control system based on multiple regression. In Proceedings of the 2021 2nd International Conference on Artificial Intelligence and Information Systems, Chongqing, China, 28–30 May 2021; pp. 1–5. [Google Scholar]
- Kumazawa, K.; Masuda, H. Change in the flavor of black tea drink during heat processing. J. Agric. Food Chem. 2001, 49, 3304–3309. [Google Scholar] [CrossRef]
- Kidist Teshome, K.T.; Adugna Debela, A.D.; Weyessa Garedew, W.G. Effect of drying temperature and duration on biochemical composition and quality of black tea (Camellia sinensis L.) O. Kuntze at Wush Wush, south western Ethiopia. Asian J. Plant Sci. 2013, 12, 235–240. [Google Scholar] [CrossRef]

| Time (min) | Flow Rate (mL/min) | Acetonitrile (%) | Water (%) |
|---|---|---|---|
| 0.000 | 0.8 | 15 | 85 |
| 12.000 | 0.8 | 10 | 90 |
| 12.100 | 0.8 | 15 | 85 |
| 30.000 | 0.8 | 15 | 85 |
| Sample Name | GA | GC | EGC | C | CAF | EC | EGCG | GCG | ECG | CG |
|---|---|---|---|---|---|---|---|---|---|---|
| Mixed Standard | 4.17 | 5.24 | 8.20 | 10.67 | 12.56 | 18.68 | 19.221 | 22.552 | 31.28 | 38.6 |
| R65 | 230.57 ± 7.59 a | 93.775 ± 3.08 a | 18.945 ± 0.83 a | 34.1 ± 1.02 a | 3650.65 ± 107.70 a | 8.7 ± 0.26 a | 274.32 ± 18.88 a | 219.62 ± 5.21 a | 28.25 ± 1.42 a | 94.32 ± 8.70 a |
| R85 | 241.65 ± 18.68 a | 97.15 ± 5.84 a | 21.505 ± 1.17 ab | 36.8 ± 7.03 a | 3583.175 ± 259.10 a | 14.6 ± 0.46 c | 275.05 ± 47.29 a | 228.22 ± 21.73 a | 26.925 ± 2.05 a | 118.1 ± 12.35 b |
| R105 | 257.05 ± 34.68 a | 93.2 ± 10.74 a | 23.56 ± 1.79 b | 33.65 ± 3.56 a | 3631 ± 238.44 a | 15.805 ± 0.46 c | 279.82 ± 30.37 a | 213.45 ± 28.21 a | 31.325 ± 5.19 a | 110.15 ± 15.62 ab |
| R125 | 336.25 ± 12.31 b | 85.125 ± 3.95 a | 19.91 ± 0.85 a | 32.8 ± 2.64 a | 3376 ± 140.80 a | 12.995 ± 1.29 b | 219.72 ± 13.46 b | 208.8 ± 1.87 a | 27.075 ± 1.53 a | 94.27 ± 4.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Z.; Zhou, H.; Chen, L.; Wang, Y.; Ji, Q.; He, W. Effect of Four Different Initial Drying Temperatures on Biochemical Profile and Volatilome of Black Tea. Metabolites 2025, 15, 74. https://doi.org/10.3390/metabo15020074
Shu Z, Zhou H, Chen L, Wang Y, Ji Q, He W. Effect of Four Different Initial Drying Temperatures on Biochemical Profile and Volatilome of Black Tea. Metabolites. 2025; 15(2):74. https://doi.org/10.3390/metabo15020074
Chicago/Turabian StyleShu, Zaifa, Huijuan Zhou, Limin Chen, Yuhua Wang, Qingyong Ji, and Weizhong He. 2025. "Effect of Four Different Initial Drying Temperatures on Biochemical Profile and Volatilome of Black Tea" Metabolites 15, no. 2: 74. https://doi.org/10.3390/metabo15020074
APA StyleShu, Z., Zhou, H., Chen, L., Wang, Y., Ji, Q., & He, W. (2025). Effect of Four Different Initial Drying Temperatures on Biochemical Profile and Volatilome of Black Tea. Metabolites, 15(2), 74. https://doi.org/10.3390/metabo15020074





