Associations of S-Adenosylmethionine and S-Adenosylhomocysteine with Hepatocellular Carcinoma
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Population
2.2. Mass Spectrometry Assay for SAM and SAH
2.3. Statistical Analysis
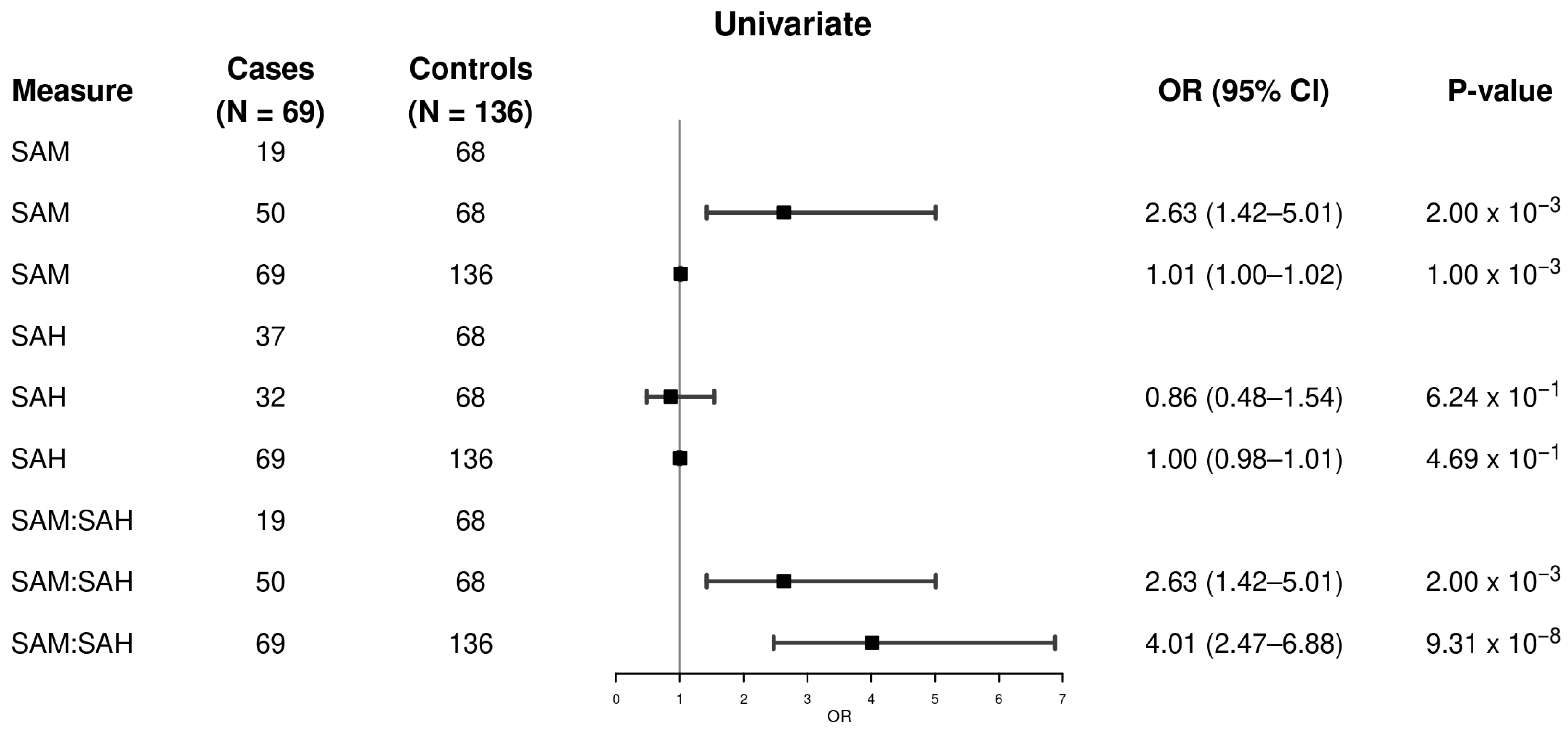
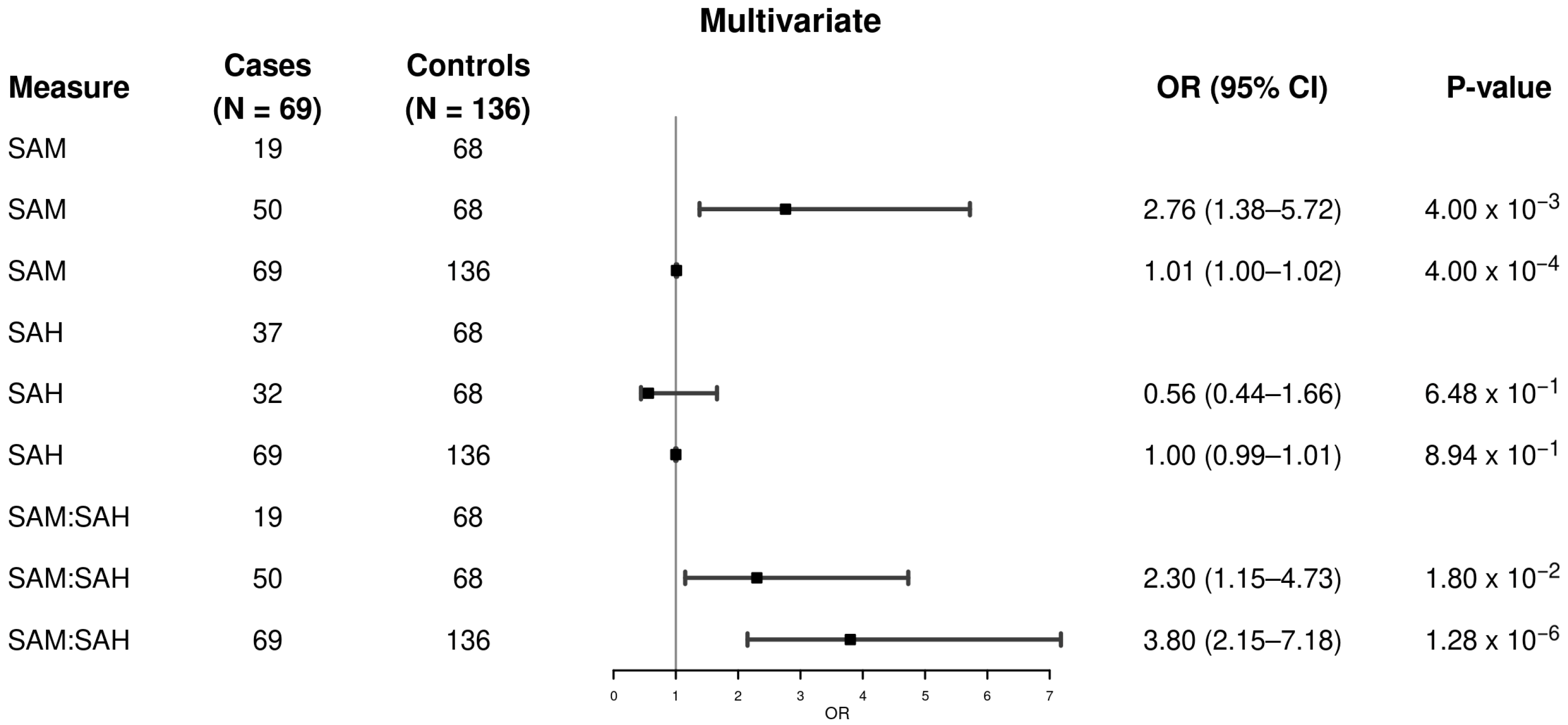
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BCLC | Barcelona Clinic Liver Cancer Group |
| BMI | Body mass index |
| CI | Confidence interval |
| CpG | Cytosine-phosphate-Guanine |
| DNA | Deoxyribonucleic acid |
| ER | Endoplasmic reticulum |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C |
| IBIS | Institute of Biomedicine of Sevilla |
| IISB | Instituto de Investigación Sanitaria Biogipuzkoa |
| IRB | Institutional Review Board |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.F.; Soerjomataram, I. Globalcancer statistics2018: GLOBOCAN estimatesofincidence andmortalityworldwidefor36cancersin185countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [PubMed]
- Antwi, S.O.; Craver, E.C.; Nartey, Y.A.; Sartorius, K.; Patel, T. Metabolic Risk Factors for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: A Prospective Study. Cancers 2022, 14, 6234. [Google Scholar] [CrossRef]
- Antwi, S.O.; Siaw, A.D.J.; Armasu, S.M.; Frank, J.A.; Yan, I.K.; Ahmed, F.Y.; Izquierdo-Sanchez, L.; Boix, L.; Rojas, A.; Banales, J.M.; et al. Genome-wide DNA methylation markers associated with metabolic liver cancer. Gastro Hep Adv. 2025, 4, 100621. [Google Scholar] [CrossRef]
- Hlady, R.A.; Zhao, X.; Pan, X.; Yang, J.D.; Ahmed, F.; Antwi, S.O.; Giama, N.H.; Patel, T.; Roberts, L.R.; Liu, C.; et al. Genome-wide discovery and validation of diagnostic DNA methylation-based biomarkers for hepatocellular cancer detection in circulating cell free DNA. Theranostics 2019, 9, 7239–7250. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nishida, N.; Ueshima, K.; Minami, Y.; Komeda, Y.; Aoki, T.; Takita, M.; Morita, M.; Chishina, H.; Yoshida, A.; et al. Accumulation of Genetic and Epigenetic Alterations in the Background Liver and Emergence of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease. Cells 2021, 10, 3257. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Rusyn, I. Role of epigenetic aberrations in the development and progression of human hepatocellular carcinoma. Cancer Lett. 2014, 342, 223–230. [Google Scholar] [CrossRef]
- Zeisel, S.H. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am. J. Clin. Nutr. 2009, 89, 1488S–1493S. [Google Scholar] [CrossRef]
- Puszyk, W.M.; Trinh, T.L.; Chapple, S.J.; Liu, C. Linking metabolism and epigenetic regulation in development of hepatocellular carcinoma. Lab. Investig. 2013, 93, 983–990. [Google Scholar] [CrossRef]
- Wong, C.C.; Qian, Y.; Yu, J. Interplay between epigenetics and metabolism in oncogenesis: Mechanisms and therapeutic approaches. Oncogene 2017, 36, 3359–3374. [Google Scholar] [CrossRef]
- Mai, J.; Virtue, A.; Maley, E.; Tran, T.; Yin, Y.; Meng, S.; Pansuria, M.; Jiang, X.; Wang, H.; Yang, X.F. MicroRNAs and other mechanisms regulate interleukin-17 cytokines and receptors. Front. Biosci. (Elite Ed.) 2012, 4, 1478–1495. [Google Scholar] [CrossRef]
- McCabe, D.C.; Caudill, M.A. DNA methylation, genomic silencing, and links to nutrition and cancer. Nutr. Rev. 2005, 63, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, M.; Huang, S.Y.; Liu, Z.Y.; He, T.T.; Fang, A.P.; Li, M.C.; Yang, M.T.; Wang, C.; Zhang, Y.J.; Zhu, H.L. Serum S-adenosylhomocysteine, rather than homocysteine, is associated with hepatocellular carcinoma survival: A prospective cohort study. Am. J. Clin. Nutr. 2024, 120, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhang, M.; Shi, K.H. Does dietary L-methionine serve as a foe or friend for global DNA hypomethylation? Food Chem. Toxicol. 2014, 71, 282–283. [Google Scholar] [CrossRef]
- Delgado, M.; Garrido, F.; Pérez-Miguelsanz, J.; Pacheco, M.; Partearroyo, T.; Pérez-Sala, D.; Pajares, M.A. Acute liver injury induces nucleocytoplasmic redistribution of hepatic methionine metabolism enzymes. Antioxid. Redox Signal. 2014, 20, 2541–2554. [Google Scholar] [CrossRef]
- Frau, M.; Feo, F.; Pascale, R.M. Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J. Hepatol. 2013, 59, 830–841. [Google Scholar] [CrossRef]
- Ramani, K.; Mato, J.M.; Lu, S.C. Role of methionine adenosyltransferase genes in hepatocarcinogenesis. Cancers 2011, 3, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Peitta, G.; Simile, M.M.; Feo, F. Alterations of Methionine Metabolism as Potential Targets for the Prevention and Therapy of Hepatocellular Carcinoma. Medicina 2019, 55, 296. [Google Scholar] [CrossRef]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Liang, B.; Su, Y.; Sun, S.; Xia, S.; Shao, J.; Zhang, Z.; Hong, M.; Zhang, F.; et al. Methionine metabolism in chronic liver diseases: An update on molecular mechanism and therapeutic implication. Signal Transduct. Target. Ther. 2020, 5, 280. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Cueto, R.; Shen, W.; Liu, L.; Wang, X.; Wu, S.; Mohsin, S.; Yang, L.; Khan, M.; Hu, W.; Snyder, N.; et al. SAH is a major metabolic sensor mediating worsening metabolic crosstalk in metabolic syndrome. Redox Biol. 2024, 73, 103139. [Google Scholar] [CrossRef]
- Li, T.; Yu, G.; Guo, T.; Qi, H.; Bing, Y.; Xiao, Y.; Li, C.; Liu, W.; Yuan, Y.; He, Y.; et al. The plasma S-adenosylmethionine level is associated with the severity of hepatitis B-related liver disease. Medicine 2015, 94, e489. [Google Scholar] [CrossRef]
- Antwi, S.O.; Heckman, M.; White, L.; Yan, I.; Sarangi, V.; Lauer, K.P.; Reddy, J.; Ahmed, F.; Veliginti, S.; Mejías Febres, E.D.; et al. Metabolic liver cancer: Associations of rare and common germline variants in one-carbon metabolism and DNA methylation genes. Hum. Mol. Genet. 2023, 32, 2646–2655. [Google Scholar] [CrossRef]
- Arning, E.; Wasek, B.; Bottiglieri, T. Quantification of Plasma S-adenosylmethionine and S-adenosylhomocysteine Using Liquid Chromatography-Electrospray-Tandem Mass Spectrometry. Methods Mol. Biol. 2022, 2546, 35–43. [Google Scholar] [PubMed]
- Qiu, S.; Cai, J.; Yang, Z.; He, X.; Xing, Z.; Zu, J.; Xie, E.; Henry, L.; Chong, C.R.; John, E.M.; et al. Trends in Hepatocellular Carcinoma Mortality Rates in the US and Projections Through 2040. JAMA Netw. Open 2024, 7, e2445525. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, X.; Chen, Q.; Xiao, J.; Mi, J.; Liu, Q.; You, Y.; Chen, Y.; Ling, W. Association of serum methionine metabolites with non-alcoholic fatty liver disease: A cross-sectional study. Nutr. Metab. 2022, 19, 21. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wu, Q.; Li, J.; Sun, S.; Sun, S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020, 53, e12891. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hall, M.N. Metabolic reprogramming in hepatocellular carcinoma: Mechanisms and therapeutic implications. Exp. Mol. Med. 2025, 57, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, H.; Lin, Z.; Liu, X.; Dong, L.; Jiang, T.; Tan, Y.; Ning, Z.; Ye, Y.; Tan, G.; et al. Metabolic Reprogramming and Risk Stratification of Hepatocellular Carcinoma Studied by Using Gas Chromatography-Mass Spectrometry-Based Metabolomics. Cancers 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
| MASLD-HCC Cases (N = 69) | Metabolic Controls (N = 136) | p-Value b | |
|---|---|---|---|
| Age, years a | 0.402 | ||
| Mean (SD) | 68 (12) | 67 (10) | |
| Sex | 0.794 | ||
| Female | 21 (30%) | 39 (29%) | |
| Male | 48 (70%) | 97 (71%) | |
| Site | 5.78 × 10−26 | ||
| Mayo Clinic | 30 (44%) | 125 (92%) | |
| Karolinska University Hospital | 21 (30%) | 3 (2%) | |
| BCLC, Barcelona, and IISB, San Sebastian, Spain | 18 (26%) | 8 (6%) | |
| BMI, kg/m2 | |||
| ≤24.9 | 9 (13%) | 17 (13%) | 0.000001 |
| 25–29.9 | 42 (61%) | 35 (26%) | |
| ≥30 | 18 (26%) | 84 (62%) | |
| Mean (SD) | 29 (4) | 32 (7) | 0.0000781 |
| Diabetes Mellitus, Yes | 47 (68%) | 75 (55%) | 0.074 |
| Smoking | 0.036 | ||
| Never | 24 (35%) | 66 (50%) | |
| Former | 38 (55%) | 63 (47%) | |
| Current | 7 (10%) | 4 (3.0%) | |
| Unknown | 0 | 3 | |
| SAM, nmol/L | 0.001 | ||
| Mean (SD) | 121 (60) | 96 (43) | |
| Median (Q1, Q3) | 103 (86, 132) | 87 (67, 118) | |
| SAH, nmol/L | 0.113 | ||
| Mean (SD) | 63 (30) | 65 (22) | |
| Median (Q1, Q3) | 59 (45, 71) | 61 (51, 73) | |
| SAM/SAH, nmol/L | 6.42 × 10−7 | ||
| Mean (SD) | 2.09 (0.88) | 1.48 (0.47) | |
| Median (Q1, Q3) | 1.98 (1.33, 2.68) | 1.44 (1.21, 1.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalley, N.N.; Armasu, S.M.; Fan, W.Z.; Yan, I.K.; Ahmed, F.Y.; Stål, P.; Roberts, L.R.; Patel, T.; Antwi, S.O. Associations of S-Adenosylmethionine and S-Adenosylhomocysteine with Hepatocellular Carcinoma. Metabolites 2025, 15, 740. https://doi.org/10.3390/metabo15110740
Yalley NN, Armasu SM, Fan WZ, Yan IK, Ahmed FY, Stål P, Roberts LR, Patel T, Antwi SO. Associations of S-Adenosylmethionine and S-Adenosylhomocysteine with Hepatocellular Carcinoma. Metabolites. 2025; 15(11):740. https://doi.org/10.3390/metabo15110740
Chicago/Turabian StyleYalley, Naana N., Sebastian M. Armasu, Winnie Z. Fan, Irene K. Yan, Fowsiyo Y. Ahmed, Per Stål, Lewis R. Roberts, Tushar Patel, and Samuel O. Antwi. 2025. "Associations of S-Adenosylmethionine and S-Adenosylhomocysteine with Hepatocellular Carcinoma" Metabolites 15, no. 11: 740. https://doi.org/10.3390/metabo15110740
APA StyleYalley, N. N., Armasu, S. M., Fan, W. Z., Yan, I. K., Ahmed, F. Y., Stål, P., Roberts, L. R., Patel, T., & Antwi, S. O. (2025). Associations of S-Adenosylmethionine and S-Adenosylhomocysteine with Hepatocellular Carcinoma. Metabolites, 15(11), 740. https://doi.org/10.3390/metabo15110740






