Dendrobium officinale Polysaccharide Relieves the DSS-Induced Chronic Colitis in C57BL/6J Mice and Regulates Colonic Microflora Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of DOP
2.2. Animals
2.3. DSS-Induced Chronic Colitis Model
2.4. Disease Activity Index (DAI) Measurement
2.5. Hematoxylin and Eosin (H&E), Alcian Blue, and Immunohistochemical Staining
2.6. Evaluation of Inflammation
2.7. Colonic Microflora Analysis
2.8. Non-Targeted Metabolomics Analysis
2.9. Statistical Analysis
3. Results
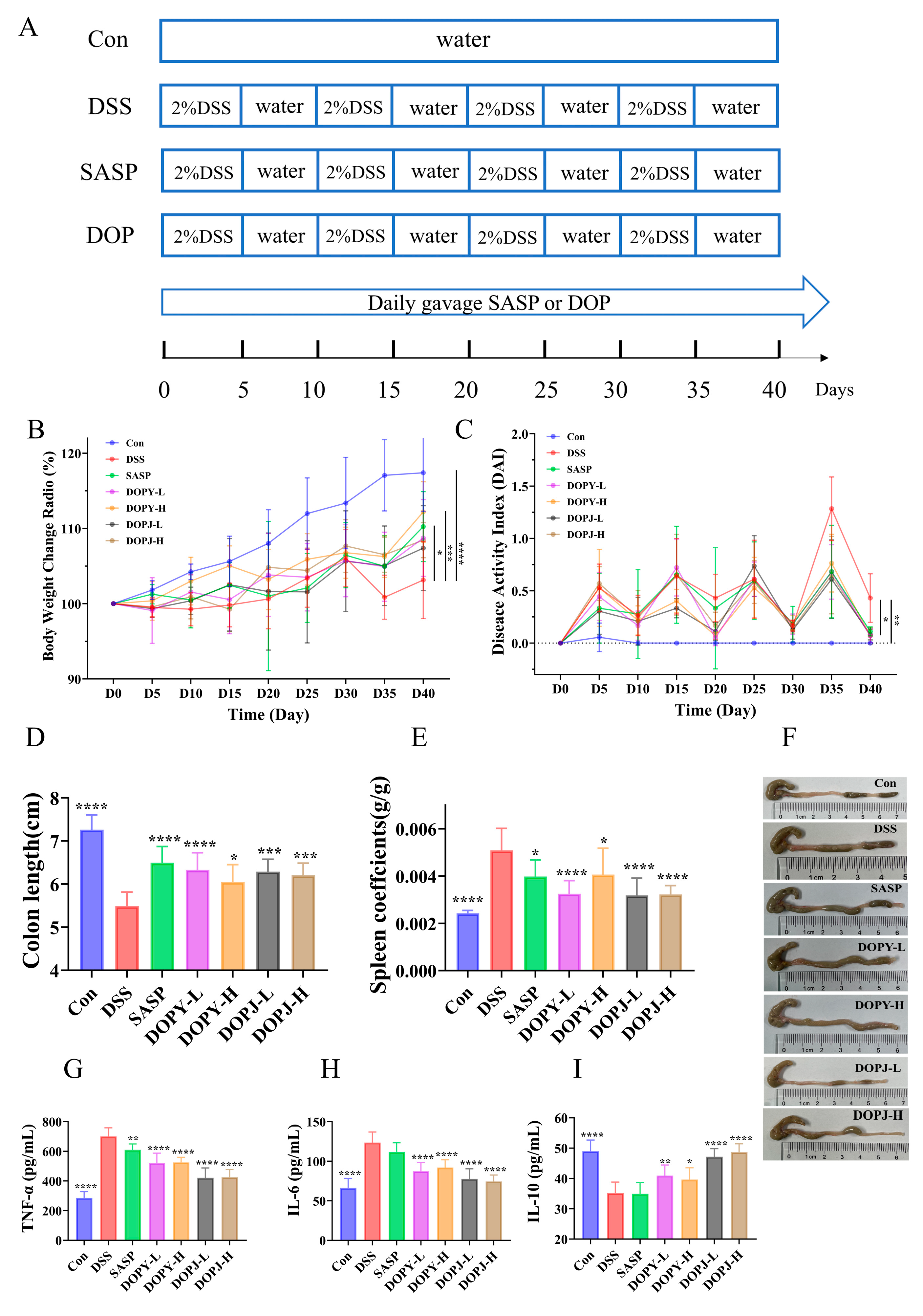
3.1. DOP Alleviates Colitis Symptoms in Chronic Colitis Mice
3.2. Interventional Therapy with DOP Attenuates DSS-Induced Colon Tissue Injury
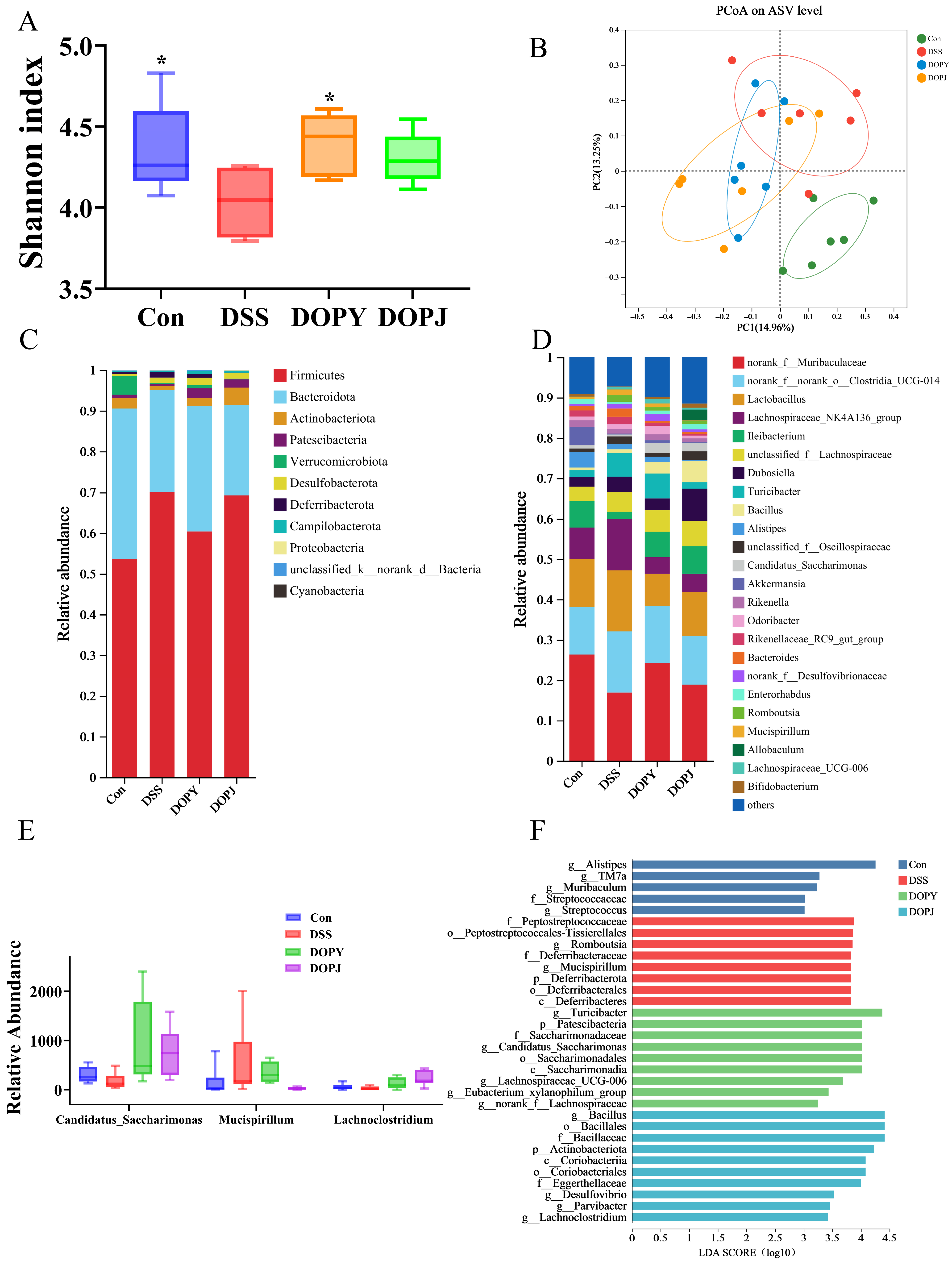
3.3. DOP Modulates the Gut Microbiota
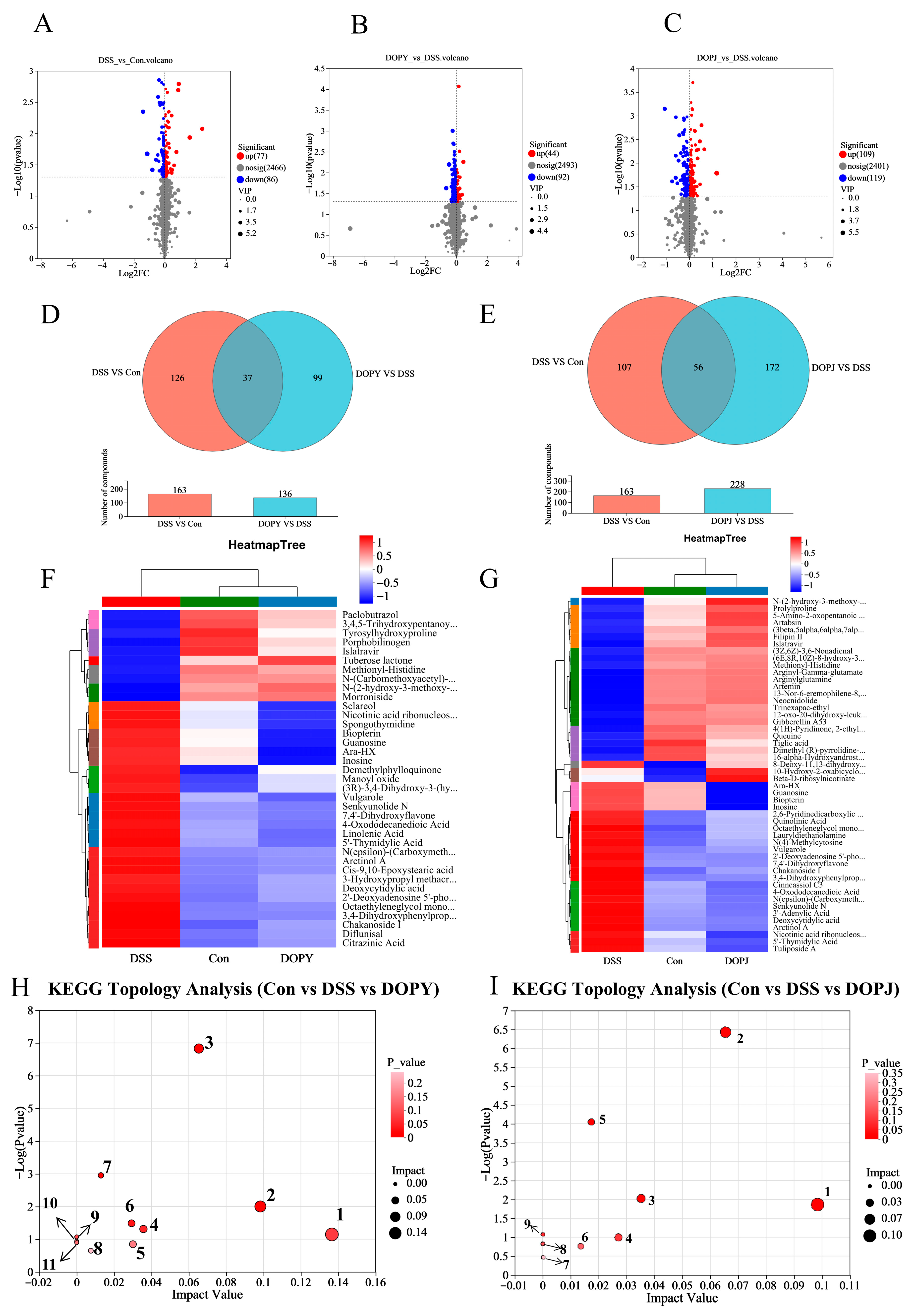
3.4. DOP Regulates the Function of Metabolic Pathways in Chronic Colitis Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOP | Dendrobium officinale polysaccharides |
| DSS | Dextran sulfate sodium |
| DAI | Disease Activity Index |
| TNF-α | Tumor Necrosis Factor-alpha |
| ZO-1 | Zonula occludens-1 |
| DO | Dendrobium officinale |
| DOPY | polysaccharides from Dendrobium officinale leaves |
| DOPJ | polysaccharides from Dendrobium officinale stems |
| HPGPC | High-performance gel permeation chromatography |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| SASP | Sulfasalazine |
| DOPY-L | low-dose polysaccharides from Dendrobium officinale leaves |
| DOPY-H | high-dose polysaccharides from Dendrobium officinale leaves |
| DOPJ-L | low-dose polysaccharides from Dendrobium officinale stems |
| DOPJ-H | high-dose polysaccharides from Dendrobium officinale stems |
| H&E | Hematoxylin and Eosin |
| PBS | Phosphate-buffered saline |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| ELISA | Enzyme-linked immunosorbent assay |
| ANOVA | Analysis of variance |
| UC | Ulcerative colitis |
| UQ | Ubiquinone |
References
- Tungalag, T.; Park, J.Y.; Park, K.W.; Yang, D.K. Sesame cake extract attenuates dextran sulfate sodium-induced colitis through inhibition of oxidative stress in mice. Food Sci. Biotechnol. 2024, 33, 699–709. [Google Scholar] [CrossRef]
- Soliman, N.A.; Keshk, W.A.; Rizk, F.H.; Ibrahim, M.A. The possible ameliorative effect of simvastatin versus sulfasalazine on acetic acid induced ulcerative colitis in adult rats. Chem. Biol. Interact. 2019, 298, 57–65. [Google Scholar]
- Beiranvand, M. A review of the biological and pharmacological activities of mesalazine or 5-aminosalicylic acid (5-ASA): An anti-ulcer and anti-oxidant drug. Inflammopharmacology 2021, 29, 1279–1290. [Google Scholar] [CrossRef]
- Ashton, J.J.; Green, Z.; Kolimarala, V.; Beattie, R.M. Inflammatory bowel disease: Long-term therapeutic challenges. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1049–1063. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Luo, Y.; Fu, S.; Liu, Y.; Kong, S.; Liao, Q.; Lin, L.; Li, H. Banxia Xiexin decoction modulates gut microbiota and gut microbiota metabolism to alleviate DSS-induced ulcerative colitis. J. Ethnopharmacol. 2024, 326, 117990. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Jia, H.; Cai, W.; Cao, R.; Xue, C.; Dong, N. Rehmannia glutinosa polysaccharides attenuates colitis via reshaping gut microbiota and short-chain fatty acid production. J. Sci. Food Agric. 2023, 103, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Noh, J.Y.; Farhataziz, N.; Kinter, M.T.; Yan, X.; Sun, Y. Colonic Dysregulation of Major Metabolic Pathways in Experimental Ulcerative Colitis. Metabolites 2024, 14, 194. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhu, X.; Hua, Y. Chemical Constituents, Bioactivities, and Pharmacological Mechanisms of Dendrobium officinale: A Review of the Past Decade. J. Agric. Food Chem. 2023, 71, 14870–14889. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.F.; Teleb, M.; El-Kersh, D.M.; El Gendy, A.N.G.; Elshamy, A.; Farag, M.A. Natural acylated flavonoids: Their chemistry and biological merits in context to molecular docking studies. Phytochem. Rev. 2023, 22, 1469–1508. [Google Scholar]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef]
- Li, J.R.; Tao, W.Y.; Yang, Y.; Zhou, W.Y.; Lu, S.M.; Wang, Y. Comparative studies of polysaccharides of three Dendrobium species from Zhejiang Province and exploration of their physiological functions. Acta Agric. Zhejiangensis 2023, 35, 1888–1895. [Google Scholar]
- Li, Y.H.; Adam, R.; Colombel, J.F.; Bian, Z.X. A characterization of pro-inflammatory cytokines in dextran sulfate sodium-induced chronic relapsing colitis mice model. Int. Immunopharmacol. 2018, 60, 194–201. [Google Scholar]
- Yao, L.; Fang, J.; Zhao, J.W.; Yu, J.; Zhang, X.Q.; Chen, W.D.; Han, L.; Peng, D.Y.; Chen, Y.N. Dendrobium huoshanense in the treatment of ulcerative colitis: Network pharmacology and experimental validation. J. Ethnopharmacol. 2024, 323, 117729. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef]
- Rodrigues, V.F.; Camelo, G.M.A.; de Rezende, M.C.; Maggi, L.; Silva, J.; Rodrigues, J.G.M.; Araújo, M.S.S.; Martins-Filho, O.A.; Negrão-Corrêa, D. Infection by Strongyloides venezuelensis attenuates chronic colitis induced by Dextran Sodium Sulfate ingestion in BALB/c mice. Immunobiology 2021, 226, 152129. [Google Scholar] [CrossRef]
- Tian, B.M.; Huang, P.J.; Pan, Y.Z.; Gu, H.; Yang, K.; Wei, Z.X.; Zhang, X.C. Tea Polyphenols Reduced Obesity by Modulating Gut Microbiota-SCFAs-Barrier and Inflammation in High-Fat Diet-Induced Mice. Mol. Nutr. Food Res. 2024, 68, e2400685. [Google Scholar] [CrossRef]
- Rangel, I.; Sundin, J.; Fuentes, S.; Repsilber, D.; de Vos, W.M.; Brummer, R.J. The relationship between faecal-associated and mucosal-associated microbiota in irritable bowel syndrome patients and healthy subjects. Aliment. Pharmacol. Ther. 2015, 42, 1211–1221. [Google Scholar] [PubMed]
- Li, J.; Tao, W.; Zhou, W.; Xing, J.; Luo, M.; Lu, S.; Yang, Y. Dendrobium officinale polysaccharides alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate purine metabolism. J. Funct. Foods 2024, 119, 106336. [Google Scholar] [CrossRef]
- Yu, R.Y.; Zhou, Q.L.Y.; Liu, T.L.; Liu, P.; Li, H.; Bian, Y.F.; Liu, Z.J. Kaempferol relieves the DSS-induced chronic colitis in C57BL/6J mice, alleviates intestinal angiogenesis, and regulates colonic microflora structure. J. Funct. Foods 2023, 107, 105646. [Google Scholar] [CrossRef]
- Liang, J.; Chen, S.; Chen, J.; Lin, J.; Xiong, Q.; Yang, Y.; Yuan, J.; Zhou, L.; He, L.; Hou, S.; et al. Therapeutic roles of polysaccharides from Dendrobium officinaleon colitis and its underlying mechanisms. Carbohydr. Polym. 2018, 185, 159–168. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. Hormesis provides a generalized quantitative estimate of biological plasticity. J. Cell Commun. Signal 2011, 5, 25–38. [Google Scholar] [PubMed]
- Peng, S.; Gu, P.F.; Mao, N.N.; Yu, L.; Zhu, T.Y.; He, J.; Yang, Y.; Liu, Z.G.; Wang, D.Y. Structural Characterization and In Vitro Anti-Inflammatory Activity of Polysaccharides Isolated from the Fruits of Rosa laevigata. Int. J. Mol. Sci. 2024, 25, 2133. [Google Scholar] [CrossRef]
- Su, Y.T.; Huang, M.T.; Chen, Q.C.; He, J.Y.; Li, S.Q.; Wang, M.F. Harnessing β-glucan conjugated quercetin nanocomplex to function as a promising anti-inflammatory agent via macrophage-targeted delivery. Carbohydr. Polym. 2025, 349, 122952. [Google Scholar]
- Irwin, S.; Chupina Estrada, A.; Nelson, B.; Bullock, A.; Limketkai, B.; Ho, W.; Acton, S.; Chesnel, L.; Koon, H.W. ADS024, a single-strain live biotherapeutic product of Bacillus velezensis alleviates dextran sulfate-mediated colitis in mice, protects human colonic epithelial cells against apoptosis, and maintains epithelial barrier function. Front. Microbiol. 2024, 14, 1284083. [Google Scholar] [CrossRef]
- Li, Y.Y.; He, Y.X.; Wu, Y.Q.; Liu, C.; Ren, L.Z.; Lu, X.Y.; Wang, Y.M.; Yu, Y. Compatibility between cold-natured medicine CP and hot-natured medicine AZ synergistically mitigates colitis mice through attenuating inflammation and restoring gut barrier. J. Ethnopharmacol. 2023, 303, 115902. [Google Scholar] [PubMed]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, 2000745. [Google Scholar]
- Zhou, R.; Huang, K.; Chen, S.; Wang, M.; Liu, F.; Liu, F.; Lin, C.; Zhu, C. Zhilining Formula alleviates DSS-induced colitis through suppressing inflammation and gut barrier dysfunction via the AHR/NF-κBp65 axis. Phytomedicine 2024, 129, 155571. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Alvarez, M.D.; Herranz, B.; Moreno-Arribas, M.V.; Laguna, L. Physical effects of dietary fibre on simulated luminal flow, studied by in vitro dynamic gastrointestinal digestion and fermentation. Food Funct. 2019, 10, 3452–3465. [Google Scholar]
- Wang, J.L.; Han, X.; Li, J.X.; Shi, R.; Liu, L.L.; Wang, K.; Liao, Y.T.; Jiang, H.; Zhang, Y.; Hu, J.C.; et al. Differential analysis of intestinal microbiota and metabolites in mice with dextran sulfate sodium-induced colitis. World J. Gastroenterol. 2022, 28, 6109–6130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Lin, L.H.; Liang, H.J.; Li, Y.Q.; Zhao, F.Q.; Sun, T.Y.; Liu, Z.Y.; Zhu, J.Y.; Gu, F.; Xu, J.N.; et al. Lycium barbarum polysaccharide alleviates DSS-induced chronic ulcerative colitis by restoring intestinal barrier function and modulating gut microbiota. Ann. Med. 2023, 55, 2290213. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.; Osme, A.; Ghannoum, M.; Cominelli, F. A Novel Probiotic Combination Ameliorates Crohn’s Disease-Like Ileitis by Increasing Short-Chain Fatty Acid Production and Modulating Essential Adaptive Immune Pathways. Inflamm. Bowel Dis. 2023, 29, 1105–1117. [Google Scholar]
- Liu, M.; Xie, W.; Wan, X.; Deng, T. Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int. Immunopharmacol. 2020, 88, 106862. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wu, Y.; Xie, F.; Du, K.; Wang, Y.; Shi, L.; Ji, L.; Liu, T.; Ma, X. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget 2017, 8, 44625. [Google Scholar] [CrossRef]
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314. [Google Scholar] [CrossRef]
- Wu, J.; Rong, Y.; Li, T.; Wilson, C.M.; He, Y.Z.; Chen, D.Q.; Han, J.; Zhang, X.M. Editorial: Targeting nucleotide metabolism for enhancing antitumor immunity. Front. Immunol. 2024, 15, 1412057. [Google Scholar] [CrossRef]
- Yanamadala, V. Nucleotide Metabolism. In Essential Medical Biochemistry and Metabolic Disease: A Pocket Guide for Medical Students and Residents; Yanamadala, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2024; Volume 4, pp. 125–143. [Google Scholar]
- Zhu, L.; Wang, Z.; Gao, L.; Chen, X. Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications. Nutrients 2024, 16, 2760. [Google Scholar] [CrossRef]
- Smyth, M.; Lunken, G.; Jacobson, K. Insights Into Inflammatory Bowel Disease and Effects of Dietary Fatty Acid Intake with a Focus on Polyunsaturated Fatty Acids Using Preclinical Models. J. Can. Assoc. Gastroenterol. 2024, 7, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Erimban, S.; Daschakraborty, S. Extreme makeover: The incredible cell membrane adaptations of extremophiles to harsh environments. Chem. Commun. 2024, 60, 10280–10294. [Google Scholar] [CrossRef]
- Wang, Y.; Lilienfeldt, N.; Hekimi, S. Understanding coenzyme Q. Physiol. Rev. 2024, 104, 1533–1610. [Google Scholar] [CrossRef]
- Aglago, E.K.; Qu, C.; Harlid, S.; Phipps, A.I.; Steinfelder, R.S.; Ogino, S.; Thomas, C.E.; Hsu, L.; Toland, A.E.; Brenner, H.; et al. Folate intake and colorectal cancer risk according to genetic subtypes defined by targeted tumor sequencing. Am. J. Clin. Nutr. 2024, 120, 664–673. [Google Scholar] [CrossRef]
- Prasad, S.K.; Acharjee, A.; Singh, V.V.; Trigun, S.K.; Acharjee, P. Modulation of brain energy metabolism in hepatic encephalopathy: Impact of glucose metabolic dysfunction. Metab. Brain Dis. 2024, 39, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Wang, R.; Wang, Q.; Chen, F.; Ye, Z.; Li, Y. A fast and efficient liquid chromatography–tandem mass spectrometry method for measuring l- and d-amino acids in the urine of patients with immunoglobulin A nephropathy. Biomed. Chromatogr. 2024, 38, e5866. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kobayashi, T.; Hanamatsu, H.; Yokota, I.; Teranishi, Y.; Iwamoto, A.; Kitagawa, M.; Ashida, S.; Sakurai, A.; Matsuo, S.; et al. Tolerable glycometabolic stress boosts cancer cell resilience through altered N-glycosylation and Notch signaling activation. Cell Death Dis. 2024, 15, 53. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Li, J.; Yuan, X.; Tao, W.; Zhou, W.; Xing, J.; Yang, Y.; Zhang, H. Dendrobium officinale Polysaccharide Relieves the DSS-Induced Chronic Colitis in C57BL/6J Mice and Regulates Colonic Microflora Structure. Metabolites 2025, 15, 708. https://doi.org/10.3390/metabo15110708
Ma Y, Li J, Yuan X, Tao W, Zhou W, Xing J, Yang Y, Zhang H. Dendrobium officinale Polysaccharide Relieves the DSS-Induced Chronic Colitis in C57BL/6J Mice and Regulates Colonic Microflora Structure. Metabolites. 2025; 15(11):708. https://doi.org/10.3390/metabo15110708
Chicago/Turabian StyleMa, Yangyu, Jingrui Li, Xianling Yuan, Wenyang Tao, Wanyi Zhou, Jianrong Xing, Ying Yang, and Haihua Zhang. 2025. "Dendrobium officinale Polysaccharide Relieves the DSS-Induced Chronic Colitis in C57BL/6J Mice and Regulates Colonic Microflora Structure" Metabolites 15, no. 11: 708. https://doi.org/10.3390/metabo15110708
APA StyleMa, Y., Li, J., Yuan, X., Tao, W., Zhou, W., Xing, J., Yang, Y., & Zhang, H. (2025). Dendrobium officinale Polysaccharide Relieves the DSS-Induced Chronic Colitis in C57BL/6J Mice and Regulates Colonic Microflora Structure. Metabolites, 15(11), 708. https://doi.org/10.3390/metabo15110708





