Uncoupling Protein 1 Promotes Nile Tilapia Resistance to Acute Cold Stress by Regulating Liver Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Molecular Identification of UCP1 in Nile Tilapia
2.3. Tissue Distribution of Nile Tilapia UCP1
2.4. Cold Stress Treatment of Nile Tilapia
2.5. Hepatocytes Isolation, Culture and Treatment
2.6. Quantitative Real-Time PCR
2.7. siRNA Electroporation in Nile Tilapia Hepatocytes
2.8. Western Blot
2.9. Mitochondrial Membrane Potential (MMP)
2.10. ATP Assay
2.11. BAM15 Injection and Fed Experiment
2.12. Physiological Stress Indicator Analysis
2.13. H&E Staining
2.14. Statistical Analysis
3. Results
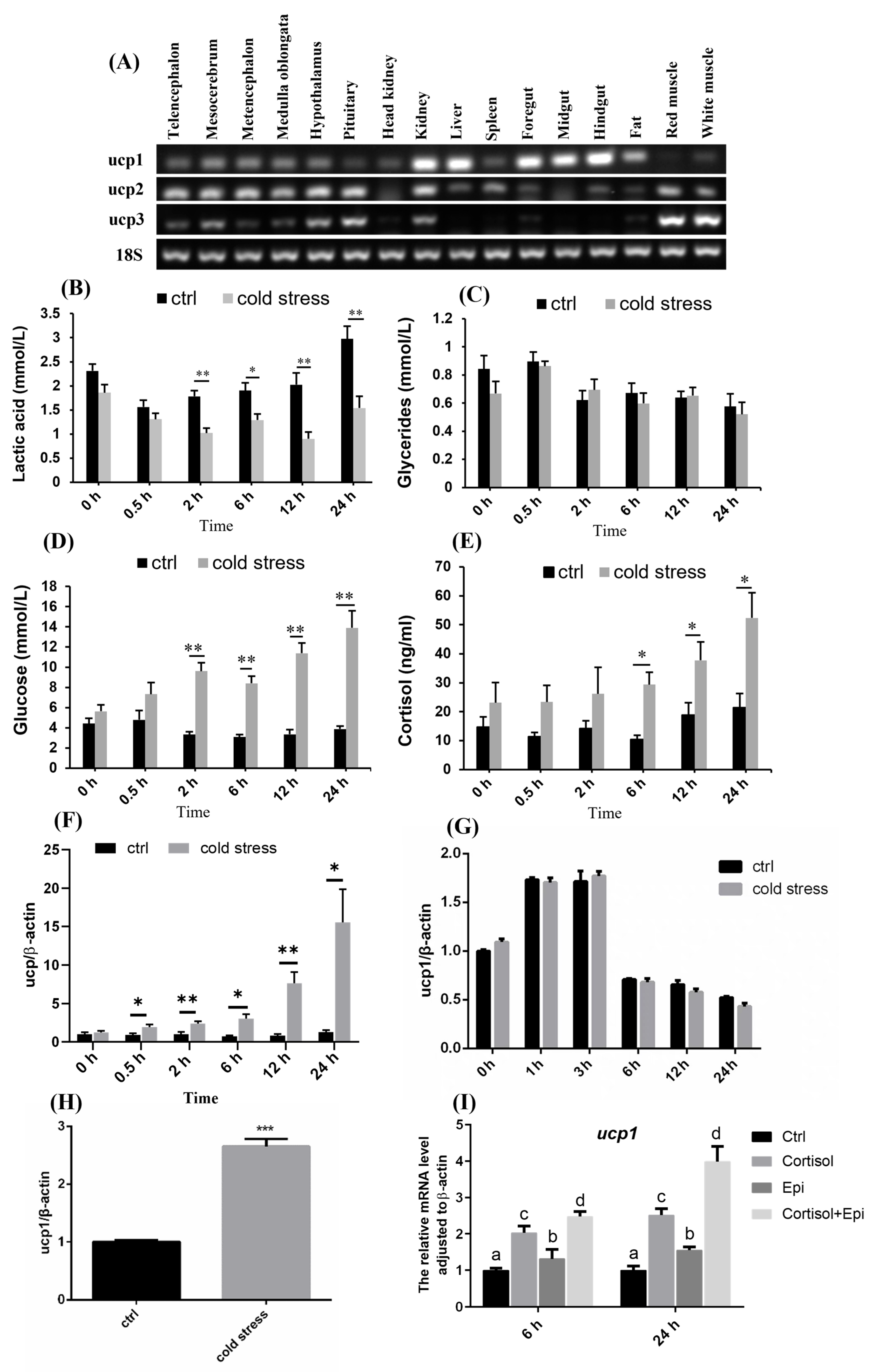
3.1. UCP1 Was Involved in Cold Stress Response in Nile Tilapia
3.2. UCP1 Acted on Cold Stress Response via Mitochondria Membrane Potential and Proteasome
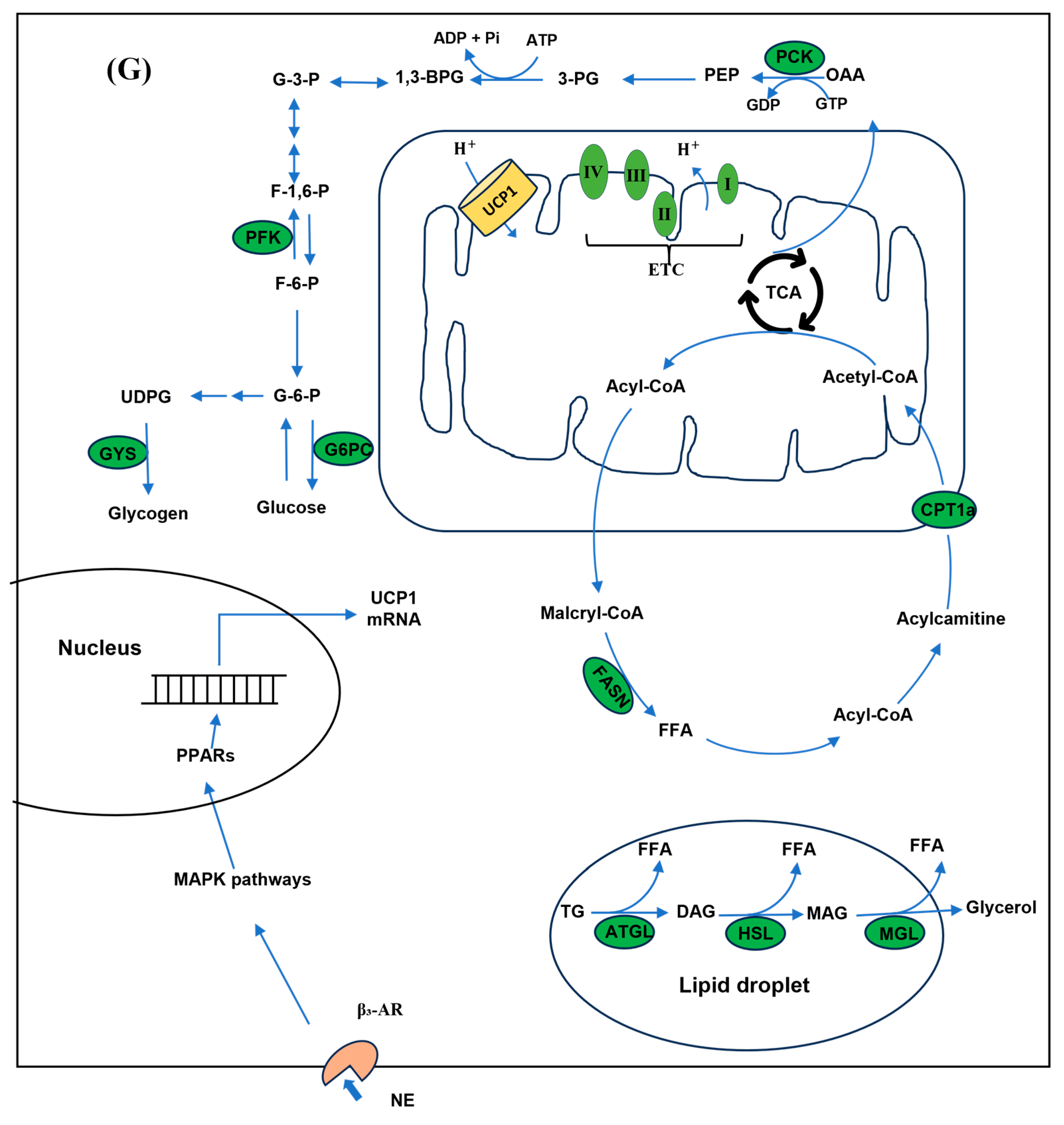
3.3. UCP1 Participated in the Regulation of Glucose and Lipid Metabolism Under Cold Stress
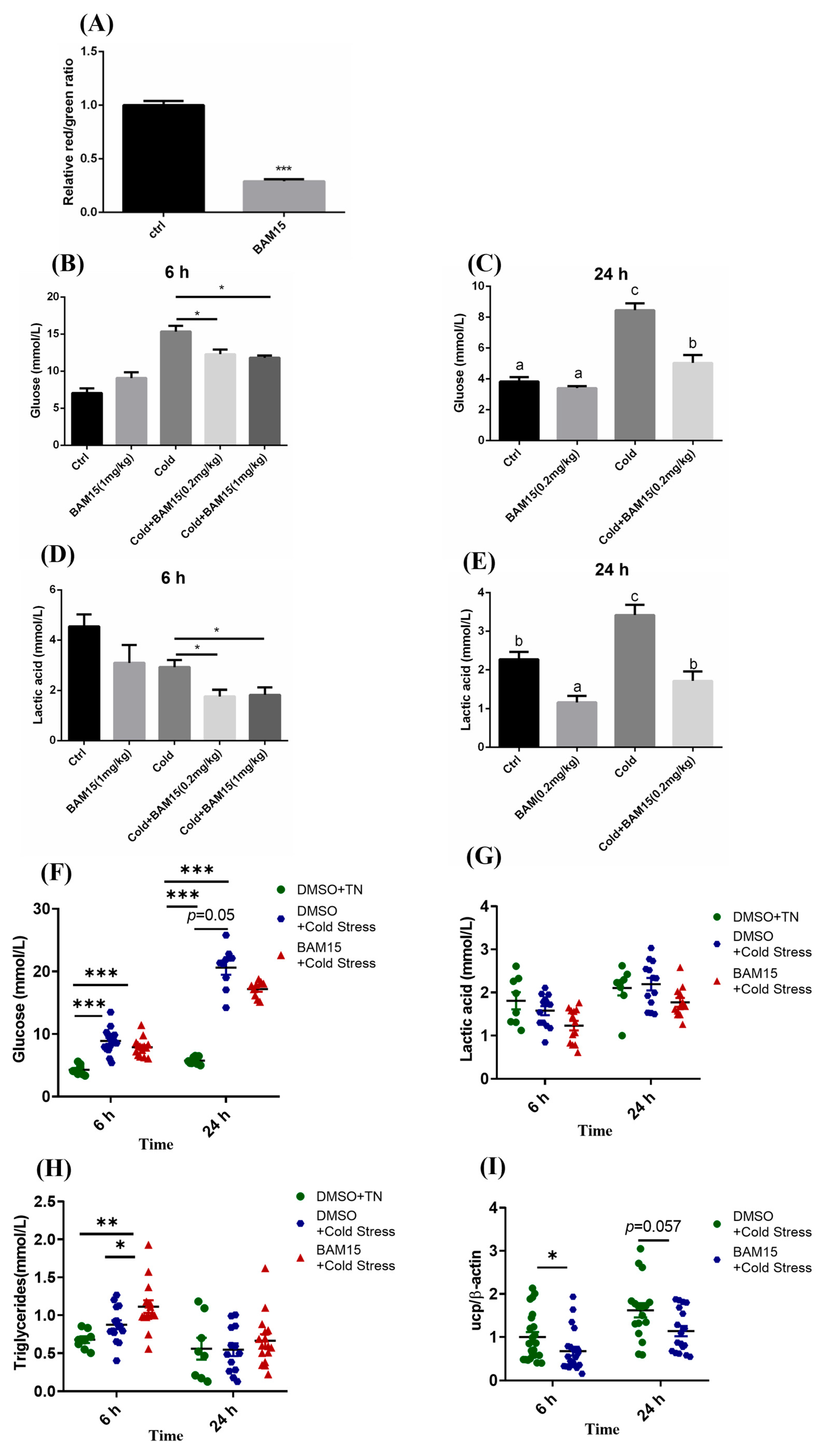
3.4. Mitochondrial Uncoupling Mediated by BAM15 Enhanced Acute Cold Stress Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ATP | adenosine triphosphate |
| FoxO | Forkhead box o |
| H&E | Hematoxylin and Eosin staining |
| MAPK | mitogen-activated protein kinase |
| ORF | open reading frame |
| qPCR | quantitative real-time PCR |
| ROS | reactive oxygen species |
| P/S | Penicillin-Streptomycin |
| TBST | Tris-buffered saline with 0.05% Tween20 |
| SDS-PAGE | sodium dodecyl sulphate |
| GDP | Guanosine diphosphate |
References
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Ndong, D.; Chen, Y.Y.; Lin, Y.H.; Vaseeharan, B.; Chen, J.C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Ma, Q.; Sun, S.X.; Zhang, H.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Reduced oxidative stress increases acute cold stress tolerance in zebrafish. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2019, 235, 166–173. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2024. Available online: https://openknowledge.fao.org/bitstreams/66538eba-9c85-4504-8438-c1cf0a0a3903/download (accessed on 20 September 2024).
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.F.C.; Niu, H.; Chen, L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019, 84, 1145–1156. [Google Scholar] [CrossRef]
- Yan, J.; Long, Y.; Zhou, T.; Ren, J.; Li, Q.; Song, G.; Cui, Z. Dynamic Phosphoproteome Profiling of Zebrafish Embryonic Fibroblasts during Cold Acclimation. Proteomics 2020, 20, e1900257. [Google Scholar] [CrossRef]
- Nitzan, T.; Kokou, F.; Doron-Faigenboim, A.; Slosman, T.; Biran, J.; Mizrahi, I.; Zak, T.; Benet, A.; Cnaani, A. Transcriptome Analysis Reveals Common and Differential Response to Low Temperature Exposure Between Tolerant and Sensitive Blue Tilapia (Oreochromis aureus). Front. Genet. 2019, 10, 100. [Google Scholar] [CrossRef]
- Qiang, J.; He, J.; Yang, H.; Wang, H.; Kpundeh, M.D.; Xu, P.; Zhu, Z.X. Temperature modulates hepatic carbohydrate metabolic enzyme activity and gene expression in juvenile GIFT tilapia (Oreochromis niloticus) fed a carbohydrate-enriched diet. J. Therm. Biol. 2014, 40, 25–31. [Google Scholar] [CrossRef]
- Qiang, J.; Wang, H.; Kpundeh, M.D.; He, J.; Xu, P. Effect of water temperature, salinity, and their interaction on growth, plasma osmolality, and gill Na+, K+-ATPase activity in juvenile GIFT tilapia Oreochromis niloticus (L.). J. Therm. Biol. 2013, 38, 331–338. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S.; Gentil, B.J.; Burelle, Y. Mitochondrial morphology transitions and functions: Implications for retrograde signaling? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R393–R406. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Thomas, S.A.; Palmiter, R.D. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 1997, 387, 94–97. [Google Scholar] [CrossRef]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial superoxide: Production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Liang, X.F.; He, S.; Li, L.; Shen, D.; Tao, Y.X. Molecular cloning and tissue expression of uncoupling protein 1, 2 and 3 genes in Chinese perch (Siniperca chuatsi). Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2015, 185, 24–33. [Google Scholar] [CrossRef]
- Jastroch, M.; Wuertz, S.; Kloas, W.; Klingenspor, M. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of mammalian nonshivering thermogenesis. Physiol. Genom. 2005, 22, 150–156. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Chen, R.D.; Lucassen, M.; Schmidt, M.M.; Dringen, R.; Abele, D.; Hwang, P.P. Exploring Uncoupling Proteins and Antioxidant Mechanisms under Acute Cold Exposure in Brains of Fish. PLoS ONE 2011, 6, e18180. [Google Scholar] [CrossRef]
- Qin, C.-J.; Wen, Z.-Y.; Wang, J.; He, Y.; Yuan, D.-Y.; Li, R. Uncoupling protein 1 in snakehead (Channa argus): Cloning, tissue distribution, and its expression in response to fasting and refeeding. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 225, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Ohi, M.; Ishikawa, S.; Shirai, M.; Horiguchi, H.; Nishino, Y.; Funaba, M. Adaptive expression of uncoupling protein 1 in the carp liver and kidney in response to changes in ambient temperature. Comp. Biochem. Physiol A Mol Integr. Physiol. 2015, 185, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Xin, Y.; Cui, L.L.; Jia, J.R.; Yuan, X.; Fu, S.W.; Zhang, J.H.; Sun, C.Y.; Miao, X.J.; Li, W.S. Effects of neuropeptide Y as a feed additive on stimulating the growth of tilapia (Oreochromis niloticus) fed low fish meal diets. Peptides 2021, 138, 170505. [Google Scholar] [CrossRef]
- Liu, B.; Wang, M.Y.; Xie, J.; Xu, P.; Ge, X.P.; He, Y.J.; Liao, L.H.; Pan, L.K. Effects of Cold Stress on Serum Biochemical Parameters and Hepatic HSP70 Gene Expression in GIFT Tilapia (Oreochromis niloticus). Acta Ecol. Sin. 2011, 17, 4866–4873. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.; Lin, W.; Yuan, X.; Jia, J.; Sun, C.; Li, W. Molecular identification, tissue distribution and functional analysis of somatostatin receptors (SSTRs) in red-spotted grouper (Epinephelus akaara). Gen. Comp. Endocrinol. 2019, 274, 87–96. [Google Scholar] [CrossRef]
- Kenwood, B.M.; Weaver, J.L.; Bajwa, A.; Poon, I.K.; Byrne, F.L.; Murrow, B.A.; Calderone, J.A.; Huang, L.; Divakaruni, A.S.; Tomsig, J.L.; et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol. Metab. 2014, 3, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, S.J.; Chen, S.Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef]
- Yu, Y.; Li, R.; Yu, X.; Hu, Y.; Liao, Z.; Li, W. Immuno-protective effect of neuropeptide Y immersion on the juvenile tilapia infected by Streptococcus agalactiae. Fish Shellfish Immunol. 2023, 141, 109072. [Google Scholar] [CrossRef]
- Nardocci, G.; Navarro, C.; Cortés, P.P.; Imarai, M.; Montoya, M.; Valenzuela, B.; Jara, P.; Acuña-Castillo, C.; Fernández, R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 2014, 40, 531–538. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Fraser, E.J.; Li, W.; Fang, Y.; Taylor, R.R.; Rogers, J.; Brass, A.; Cossins, A.R. Coping with cold: An integrative, multitissue analysis of the transcriptome of a poikilothermic vertebrate. Proc. Natl. Acad. Sci. USA 2004, 101, 16970–16975. [Google Scholar] [CrossRef]
- Lermen, C.L.; Lappe, R.; Crestani, M.; Vieira, V.P.; Gioda, C.R.; Schetinger, M.R.C.; Baldisserotto, B.; Moraes, G.; Morsch, V.M. Effect of different temperature regimes on metabolic and blood parameters of silver catfish Rhamdia quelen. Aquaculture 2004, 239, 497–507. [Google Scholar] [CrossRef]
- Kyprianou, T.-D.; Pörtner, H.O.; Anestis, A.; Kostoglou, B.; Feidantsis, K.; Michaelidis, B. Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: An analysis of mechanisms underlying the winter syndrome. J. Comp. Physiol. B 2010, 180, 1005–1018. [Google Scholar] [CrossRef]
- Axelrod, C.L.; King, W.T.; Davuluri, G.; Noland, R.C.; Hall, J.; Hull, M.; Dantas, W.S.; Zunica, E.R.M.; Alexopoulos, S.J.; Hoehn, K.L.; et al. BAM15-mediated mitochondrial uncoupling protects against obesity and improves glycemic control. EMBO Mol. Med. 2020, 12, e12088. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Harper, M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Santos, R.d.; Pecanha, F.L.; da-Silva, W.S. Functional characterization of an uncoupling protein in goldfish white skeletal muscle. J. Bioenerg. Biomembr. 2013, 45, 243–251. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Pan, C.; Liu, E.; Zhao, X.; Ling, Q. Alterations to transcriptomic profile, histopathology, and oxidative stress in liver of pikeperch (Sander lucioperca) under heat stress. Fish Shellfish Immunol. 2019, 95, 659–669. [Google Scholar] [CrossRef]
- Burka, J.F.; Briand, H.A.; Purcell, L.M.; Ireland, W.P. The effects of acute temperature change on smooth muscle contractility of rainbow trout (Oncorhynchus mykiss Walbaum) intestine. Fish Physiol. Biochem. 1993, 12, 53–60. [Google Scholar] [CrossRef]
- De Freitas Souza, C.; Baldissera, M.D.; Verdi, C.M.; Santos, R.C.V.; Da Rocha, M.; da Veiga, M.L.; da Silva, A.S.; Baldisserotto, B. Oxidative stress and antioxidant responses in Nile tilapia Oreochromis niloticus experimentally infected by Providencia rettgeri. Microb. Pathog. 2019, 131, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, T.; Li, E.; Chen, K.; Ding, Z.; Qin, J.G.; Chen, L.; Ye, J. Comparative transcriptome analysis reveals molecular strategies of oriental river prawn Macrobrachium nipponense in response to acute and chronic nitrite stress. Fish Shellfish Immunol. 2016, 48, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Klingenspor, M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp. Physiol. 2003, 88, 141–148. [Google Scholar] [CrossRef]
- Silva, J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef]
- Chen, W.H.; Sun, L.T.; Tsai, C.L.; Song, Y.L.; Chang, C.F. Cold-stress induced the modulation of catecholamines, cortisol, immunoglobulin M, and leukocyte phagocytosis in tilapia. Gen. Comp. Endocrinol. 2002, 126, 90–100. [Google Scholar] [CrossRef]
- Clarke, K.J.; Adams, A.E.; Manzke, L.H.; Pearson, T.W.; Borchers, C.H.; Porter, R.K. A role for ubiquitinylation and the cytosolic proteasome in turnover of mitochondrial uncoupling protein 1 (UCP1). Biochim. Biophys. Acta 2012, 1817, 1759–1767. [Google Scholar] [CrossRef]
- Rousset, S.; Mozo, J.; Dujardin, G.; Emre, Y.; Masscheleyn, S.; Ricquier, D.; Cassard-Doulcier, A.M. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007, 581, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Azzu, V.; Brand, M.D. Degradation of an intramitochondrial protein by the cytosolic proteasome. J. Cell Sci. 2010, 123, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Cao, W.; Robidoux, J. Learning new tricks from old dogs: Beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol. Endocrinol. 2004, 18, 2123–2131. [Google Scholar] [CrossRef]
- Locke, R.M.; Rial, E.; Scott, I.D.; Nicholls, D.G. Fatty acids as acute regulators of the proton conductance of hamster brown-fat mitochondria. Eur. J. Biochem. 1982, 129, 373–380. [Google Scholar] [CrossRef]
- Klingenberg, M. UCP1—A sophisticated energy valve. Biochimie 2017, 134, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Fromme, T.; Kleigrewe, K.; Dunkel, A.; Retzler, A.; Li, Y.; Maurer, S.; Fischer, N.; Diezko, R.; Kanzleiter, T.; Hirschberg, V.; et al. Degradation of brown adipocyte purine nucleotides regulates uncoupling protein 1 activity. Mol. Metab. 2018, 8, 77–85. [Google Scholar] [CrossRef]
- Jastroch, M.; Buckingham, J.A.; Helwig, M.; Klingenspor, M.; Brand, M.D. Functional characterisation of UCP1 in the common carp: Uncoupling activity in liver mitochondria and cold-induced expression in the brain. J. Comp. Physiol. B 2007, 177, 743–752. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Park, S.; Komatsu, T.; Kim, S.E.; Tanaka, K.; Hayashi, H.; Mori, R.; Shimokawa, I. Neuropeptide Y resists excess loss of fat by lipolysis in calorie-restricted mice: A trait potential for the life-extending effect of calorie restriction. Aging Cell 2017, 16, 339–348. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Xu, M.; Liu, Q.; Ye, H.; Zou, C.; Ye, C. Liver transcriptome analysis and de novo annotation of the orange-spotted groupers (Epinephelus coioides) under cold stress. Comp. Biochem. Physiol Part D Genom. Proteom. 2018, 29, 264–273. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Extreme winter cold-induced osmoregulatory, metabolic, and physiological responses in European seabass (Dicentrarchus labrax) acclimatized at different salinities. Sci. Total Environ. 2021, 771, 145202. [Google Scholar] [CrossRef] [PubMed]
- Pai-Po, L.; Lin, Y.H.; Chen, M.C.; Cheng, W.T. Dietary administration of sodium alginate ameliorated stress and promoted immune resistance of grouper Epinephelus coioides under cold stress. Fish. Shellfish. Immunol. 2017, 65, 127–135. [Google Scholar] [CrossRef]
- Ou, J.; Ball, J.M.; Luan, Y.; Zhao, T.; Miyagishima, K.J.; Xu, Y.; Zhou, H.; Chen, J.; Merriman, D.K.; Xie, Z.; et al. iPSCs from a Hibernator Provide a Platform for Studying Cold Adaptation and Its Potential Medical Applications. Cell 2018, 173, 851–863.e816. [Google Scholar] [CrossRef]
- Montoya, M.M.; Ansel, K.M. Small RNA Transfection in Primary Human Th17 Cells by Next Generation Electroporation. J. Vis. Exp. 2017, 122, e55546. [Google Scholar] [CrossRef]
- Ajit, A.; Santhosh Kumar, T.R.; Krishnan, L.K. Engineered Human Adipose-Derived Stem Cells Inducing Endothelial Lineage and Angiogenic Response. Tissue Eng. Part. C Methods 2019, 25, 148–159. [Google Scholar] [CrossRef]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient transfection method for primary cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, J.; Turza, N.; Volke, M.; Eckardt, K.U.; Hensel, M.; Steinkasserer, A.; Willam, C.; Prechtel, A.T. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J. Immunol. Methods 2008, 337, 71–77. [Google Scholar] [CrossRef] [PubMed]




| Name | Accession | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| ucp1-orf | XM_005456938 | ATGGTAGGACTTAAACCCTCAGA | TCAGTTAACCACTTCGATCCTCT |
| ucp1 | XM_005456938 | GTAGGACTTAAACCCTCAGACA | CCAGAGGGAATGTGACCATA |
| atgl | XM_005475579.4 | GGAGAATGTGCTCGTGTC | ATGCTGGTGTTGGTGAAA |
| fasn | XM_003454056.5 | CCAGACTTCAGAGACTCCATTC | TGCGTGAACTGTGTCTTCAA |
| hsl | XM_031726103.1 | GCCCTATCTAAAGAGTTGGTCC | CGATGTCGTCACACATAAGTTG |
| mgl | KX845691.1 | GCGTATGGAGAGGGAGAT | ATGGTGAAGAGCGTGGTA |
| pparα | NM_001290066.1 | GGCTGCTATTATCTGCTGTG | TGTCATCTGGGTGGTTGG |
| cpt1a | XM_003440552.5 | TTTCCAGGCCTCCTTACCCA | TTGTACTGCTCATTGTCCAGCAGA |
| pck | XM_003448375.3 | CCAGACCGCCGACAAATTATC | GTTGGTGATGCCCAGGATCA |
| g6pc | XM_013273429.2 | CGCCAGCATGAAGAAGTATTTC | CACCACTTCTGGGCTTTCTC |
| gys | ENSONIT00000002127 | TGACAAGGAGGCTGGTGAGAGG | ACTCGTGCATGGCTGAGAACTT |
| pfk | XM_003447353.4 | TGGCTAAGAAGCATTATAACTTGAA | TGGTCATTATGGCGTCAATTATC |
| 18S | XR_003218766.1 | AGAAACGGCTACCACATCC | CACCAGACTTGCCCTCCA |
| β-actin | XM_003443127 | ACCTTCTACAACGAGCTGAGAG | GCCTGGATGGCAACGTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Jia, J.; Liu, C.; Cai, R.; Yu, Y.; Yu, X.; Feng, W.; Sun, C.; Li, W. Uncoupling Protein 1 Promotes Nile Tilapia Resistance to Acute Cold Stress by Regulating Liver Metabolism. Metabolites 2025, 15, 668. https://doi.org/10.3390/metabo15100668
Li M, Jia J, Liu C, Cai R, Yu Y, Yu X, Feng W, Sun C, Li W. Uncoupling Protein 1 Promotes Nile Tilapia Resistance to Acute Cold Stress by Regulating Liver Metabolism. Metabolites. 2025; 15(10):668. https://doi.org/10.3390/metabo15100668
Chicago/Turabian StyleLi, Meiqing, Jirong Jia, Chenguang Liu, Ran Cai, Yang Yu, Xiaozheng Yu, Wei Feng, Caiyun Sun, and Wensheng Li. 2025. "Uncoupling Protein 1 Promotes Nile Tilapia Resistance to Acute Cold Stress by Regulating Liver Metabolism" Metabolites 15, no. 10: 668. https://doi.org/10.3390/metabo15100668
APA StyleLi, M., Jia, J., Liu, C., Cai, R., Yu, Y., Yu, X., Feng, W., Sun, C., & Li, W. (2025). Uncoupling Protein 1 Promotes Nile Tilapia Resistance to Acute Cold Stress by Regulating Liver Metabolism. Metabolites, 15(10), 668. https://doi.org/10.3390/metabo15100668







