Metformin and the Liver: Unlocking the Full Therapeutic Potential
Abstract
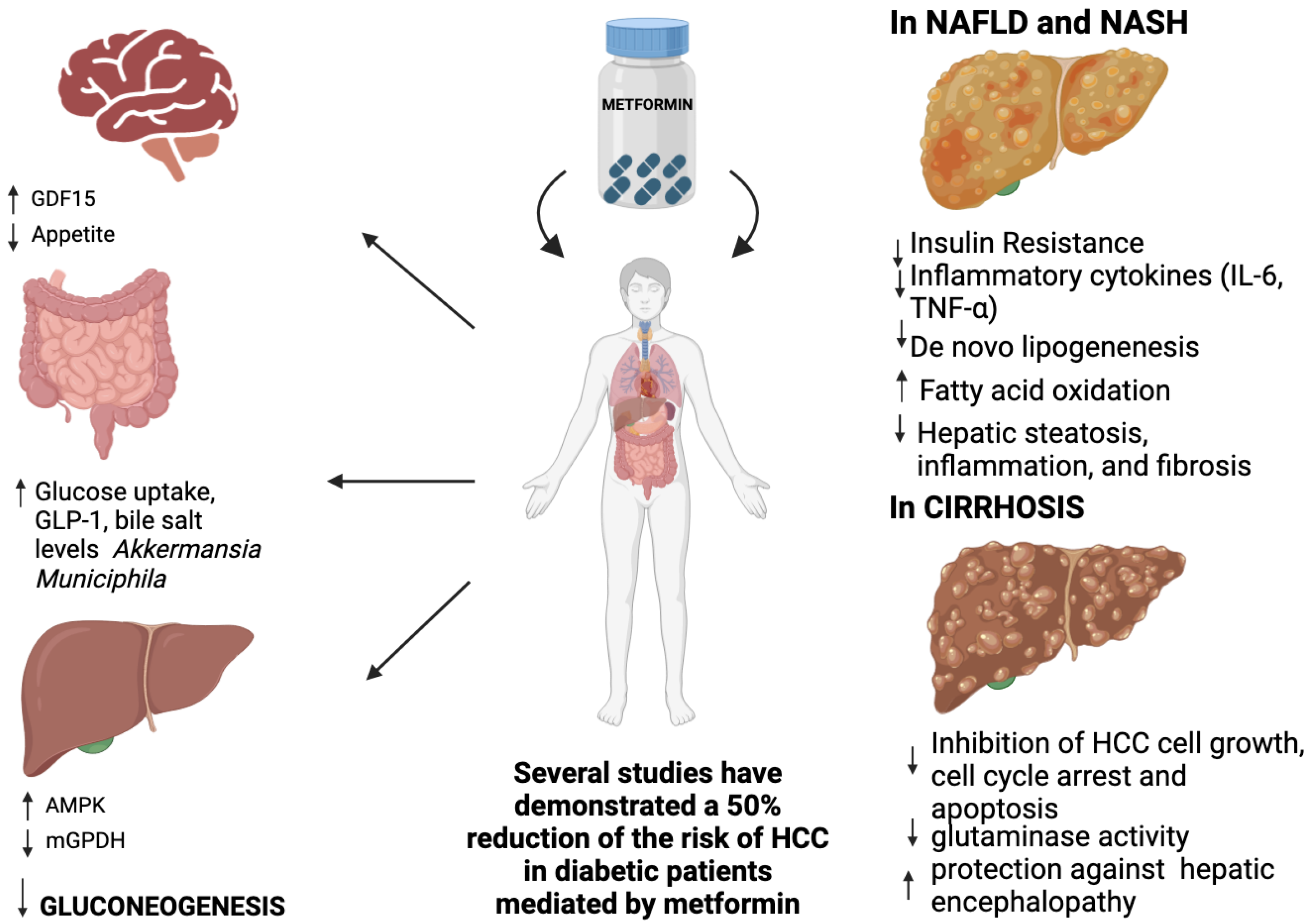
1. Introduction
2. Metformin’s Mechanism of Action and Role in Liver Disease
2.1. Historical Backgrounds and Absorption
2.2. Mechanism of Action of Metformin
2.2.1. Regulation of Hepatic Gluconeogenesis
2.2.2. Metformin and Glucagon-Like Peptide 1
2.2.3. Metformin and Skeletal Muscle
2.2.4. Gut Glucose Uptake and Intestinal Microbiota Modification
2.2.5. Metformin and Peptide Hormone Growth/Differentiation Factor 15 (GDF15)
2.2.6. Metformin and Platelet-Derived Growth Factor (PDGF)
2.2.7. Metformin and Mitochondria
2.2.8. Regulation of Lipid Metabolism
3. Metformin in Clinical Practice
3.1. Efficacy of Metformin in Liver Diseases
3.1.1. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
3.1.2. Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Fibrotic-MASH
3.1.3. Advanced Chronic Liver Disease (Cirrhosis) and Complications
Portal Hypertension-Related Complications
Hepatic Encephalopathy
Hepatocellular Carcinoma
3.2. Potential Preventive Effects of Metformin in High-Risk Populations
3.3. Drug-Induced Liver Injury (DILI) and Chemical-Induced Liver Injury
4. Adverse Effects and Safety Profile of Metformin in Liver Disease Management
4.1. Common Side Effects of Metformin
4.1.1. Gastrointestinal Side Effects
4.1.2. Metformin Associated Lactic Acidosis (MALA)
4.2. Safety of Metformin in Patients with Liver Disease
4.3. Monitoring Guidelines for Patients on Metformin Therapy
4.3.1. Serum Creatinine Level Monitoring
4.3.2. Vitamin B12 Monitoring
5. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Wei, S.; Wang, L.; Evans, P.C.; Xu, S. NAFLD and NASH: Etiology, Targets and Emerging Therapies. Drug Discov. Today 2024, 29, 103910. [Google Scholar] [CrossRef]
- Colosimo, S.; Marchesini, G. Editorial: Should NAFLD Be Included in the Definition of Metabolic Syndrome? Aliment. Pharmacol. Ther. 2023, 57, 1151–1152. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in Children: New Genes, New Diagnostic Modalities and New Drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Mazzotti, A.; Caletti, M.T.; Brodosi, L.; Di Domizio, S.; Forchielli, M.L.; Petta, S.; Bugianesi, E.; Bianchi, G.; Marchesini, G. An Internet-Based Approach for Lifestyle Changes in Patients with NAFLD: Two-Year Effects on Weight Loss and Surrogate Markers. J. Hepatol. 2018, 69, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Armandi, A.; Bugianesi, E. Dietary and Pharmacological Treatment in Patients with Metabolic-Dysfunction Associated Steatotic Liver Disease. Eur. J. Intern. Med. 2024. ahead of print. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A Meta-Analysis of Randomized Trials for the Treatment of Nonalcoholic Fatty Liver Disease. Hepatology 2010, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Crowley, M.J.; Wang, Y.; Moylan, C.A.; Guy, C.D.; Henao, R.; Piercy, D.L.; Seymour, K.A.; Sudan, R.; Portenier, D.D.; et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2021, 74, 1220–1233. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Targher, G. Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Metabolites 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Zoli, M.; Melchionda, N. Metformin in Non-Alcoholic Steatohepatitis. Lancet 2001, 358, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Taubmann, G. Die Wirkung des Biguanids und seiner Derivate auf den Zuckerstoffwechsel. Arch. Exp. Pathol. Pharmakol. 1929, 142, 290–308. [Google Scholar] [CrossRef]
- Slotta, K.H.; Tschesche, R. Über Biguanide, II.: Die Blutzucker-senkende Wirkung Der Biguanide. Berichte Dtsch. Chem. Ges. A/B 1929, 62, 1398–1405. [Google Scholar] [CrossRef]
- Sterne, J. Du Nouveau dans les Antidiabetiques. La NN Dimethylamine Guanyl Guanide (NNDG). Available online: https://scholar.google.com/scholar_lookup?journal=Maroc+Med&title=Du+nouveau+dans+les+antidiab%C3%A9tiques.+La+NN+dimethylamine+guanyl+guanide+(N.N.D.G.)&author=J+Sterne&volume=36&publication_year=1957&pages=1295-1296& (accessed on 4 February 2024).
- Sterne, J. Blood sugar-lowering effect of 1,1-dimethylbiguanide. Therapie 1958, 13, 650–659. [Google Scholar]
- Sterne, J. Treatment of diabetes mellitus with N,N-dimethylguanylguanidine (LA. 6023, glucophage). Therapie 1959, 14, 625–630. [Google Scholar]
- Sterne, J. Report on 5-years’ experience with dimethylbiguanide (metformin, glucophage) in diabetic therapy. Wien. Med. Wochenschr. 1963, 113, 599–602. [Google Scholar] [PubMed]
- Sterne, J. Mechanism of Action of Antidiabetic Biguanides. Presse Med. 1964, 72, 17–19. [Google Scholar]
- American Diabetes Association. Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin. Diabetes 2023, 41, 4–31. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on Mechanisms of Action and Repurposing Potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2020, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the Gastrointestinal Tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, K.; Landau, B.R.; Wajngot, A.; Chandramouli, V.; Efendic, S.; Brunengraber, H.; Wahren, J. Contributions by Kidney and Liver to Glucose Production in the Postabsorptive State and after 60 h of Fasting. Diabetes 1999, 48, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Kim, E.; Liu, N.-C.; Yu, I.-C.; Lin, H.-Y.; Lee, Y.-F.; Sparks, J.D.; Chen, L.-M.; Chang, C. Metformin Inhibits Nuclear Receptor TR4–Mediated Hepatic Stearoyl-CoA Desaturase 1 Gene Expression With Altered Insulin Sensitivity. Diabetes 2011, 60, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, H.; Xia, M.; Chang, X.; Xu, X.; Wang, L.; Sun, X.; Lu, Y.; Bian, H.; Li, X.; et al. Metformin Attenuates Triglyceride Accumulation in HepG2 Cells through Decreasing Stearyl-Coenzyme A Desaturase 1 Expression. Lipids Health Dis. 2018, 17, 114. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology 2012, 153, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Mietlicki-Baase, E.G.; Ortinski, P.I.; Rupprecht, L.E.; Olivos, D.R.; Alhadeff, A.L.; Pierce, R.C.; Hayes, M.R. The Food Intake-Suppressive Effects of Glucagon-like Peptide-1 Receptor Signaling in the Ventral Tegmental Area Are Mediated by AMPA/Kainate Receptors. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1367–E1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Liu, J.-J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, N.; Sharma, D.; Ashok Bidve, P.; Akhilesh; Chouhan, D.; Allani, M.; Kumar Patel, S.; Ghosh Chowdhury, M.; Shard, A.; Tiwari, V. Glucose Regulation by Newly Synthesized Boronic Acid Functionalized Molecules as Dipeptidyl Peptidase IV Inhibitor: A Potential Compound for Therapeutic Intervention in Hyperglycaemia. J. Biomol. Struct. Dyn. 2023, 42, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Marette, A.; Burdett, E.; Douen, A.; Vranic, M.; Klip, A. Insulin Induces the Translocation of GLUT4 from a Unique Intracellular Organelle to Transverse Tubules in Rat Skeletal Muscle. Diabetes 1992, 41, 1562–1569. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.-W.; Huang, X.; Song, B.-L.; Wang, Y.; Wang, Y. Regulation of Glucose and Lipid Metabolism in Health and Disease. Sci. China Life Sci. 2019, 62, 1420–1458. [Google Scholar] [CrossRef]
- Ahlborg, G.; Felig, P.; Hagenfeldt, L.; Hendler, R.; Wahren, J. Substrate Turnover during Prolonged Exercise in Man: Splanchnic and LEG Metabolism of Glucose, free fatty acids, and amino acids. J. Clin. Investig. 1974, 53, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The Effect of Insulin on the Disposal of Intravenous Glucose: Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.C.; Warram, J.H.; Krolewski, A.S.; Soeldner, J.S.; Kahn, C.R.; Martin, B.C.; Bergman, R.N. Role of Glucose and Insulin Resistance in Development of Type 2 Diabetes Mellitus: Results of a 25-Year Follow-up Study. Lancet 1992, 340, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Miettinen, H.; Gaskill, S.P.; Stern, M.P. Decreased Insulin Secretion and Increased Insulin Resistance Are Independently Related to the 7-Year Risk of NIDDM in Mexican-Americans. Diabetes 1995, 44, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of β-Cell Dysfunction and Insulin Resistance to the Pathogenesis of Impaired Glucose Tolerance and Impaired Fasting Glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Minamii, T.; Nogami, M.; Ogawa, W. Mechanisms of Metformin Action: In and out of the Gut. J. Diabetes Investig. 2018, 9, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Nogami, M.; Morita, Y.; Sakaguchi, K.; Komada, H.; Hirota, Y.; Sugawara, K.; Tamori, Y.; Zeng, F.; Murakami, T.; et al. Dose-Dependent Accumulation of Glucose in the Intestinal Wall and Lumen Induced by Metformin as Revealed by 18F-Labelled Fluorodeoxyglucose Positron Emission Tomography-MRI. Diabetes Obes. Metab. 2021, 23, 692–699. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Carter, D.; Howlett, H.C.S.; Wiernsperger, N.F.; Bailey, C.J. Differential Effects of Metformin on Bile Salt Absorption from the Jejunum and Ileum. Diabetes Obes. Metab. 2003, 5, 120–125. [Google Scholar] [CrossRef]
- Napolitano, A.; Miller, S.; Nicholls, A.W.; Baker, D.; Horn, S.V.; Thomas, E.; Rajpal, D.; Spivak, A.; Brown, J.R.; Nunez, D.J. Novel Gut-Based Pharmacology of Metformin in Patients with Type 2 Diabetes Mellitus. PLoS ONE 2014, 9, e100778. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging Biology and Therapeutic Applications for Obesity and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.-N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL Is the Receptor for GDF15 and the Ligand Promotes Weight Loss in Mice and Nonhuman Primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Hsu, J.-Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Non-Homeostatic Body Weight Regulation through a Brainstem-Restricted Receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.-C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL Is the Receptor for GDF15 and Is Required for the Anti-Obesity Effects of the Ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Macia, L.; Tsai, V.W.-W.; Nguyen, A.D.; Johnen, H.; Kuffner, T.; Shi, Y.-C.; Lin, S.; Herzog, H.; Brown, D.A.; Breit, S.N.; et al. Macrophage Inhibitory Cytokine 1 (MIC-1/GDF15) Decreases Food Intake, Body Weight and Improves Glucose Tolerance in Mice on Normal & Obesogenic Diets. PLoS ONE 2012, 7, e34868. [Google Scholar] [CrossRef]
- Xie, B.; Murali, A.; Vandevender, A.M.; Chen, J.; Silva, A.G.; Bello, F.M.; Chuan, B.; Bahudhanapati, H.; Sipula, I.; Dedousis, N.; et al. Hepatocyte-Derived GDF15 Suppresses Feeding and Improves Insulin Sensitivity in Obese Mice. iScience 2022, 25, 105569. [Google Scholar] [CrossRef]
- Kleinert, M.; Bojsen-Møller, K.N.; Jørgensen, N.B.; Svane, M.S.; Martinussen, C.; Kiens, B.; Wojtaszewski, J.F.P.; Madsbad, S.; Richter, E.A.; Clemmensen, C. Effect of Bariatric Surgery on Plasma GDF15 in Humans. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E615–E621. [Google Scholar] [CrossRef]
- Sjøberg, K.A.; Sigvardsen, C.M.; Alvarado-Diaz, A.; Andersen, N.R.; Larance, M.; Seeley, R.J.; Schjerling, P.; Knudsen, J.G.; Katzilieris-Petras, G.; Clemmensen, C.; et al. GDF15 Increases Insulin Action in the Liver and Adipose Tissue via a β-Adrenergic Receptor-Mediated Mechanism. Cell Metab. 2023, 35, 1327–1340.e5. [Google Scholar] [CrossRef]
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 Mediates the Effects of Metformin on Body Weight and Energy Balance. Nature 2020, 578, 444–448. [Google Scholar] [CrossRef]
- de Zegher, F.; Díaz, M.; Villarroya, J.; Cairó, M.; López-Bermejo, A.; Villarroya, F.; Ibáñez, L. The Relative Deficit of GDF15 in Adolescent Girls with PCOS Can Be Changed into an Abundance That Reduces Liver Fat. Sci. Rep. 2021, 11, 7018. [Google Scholar] [CrossRef]
- Carreras-Badosa, G.; Gómez-Vilarrubla, A.; Mas-Parés, B.; Martínez-Calcerrada, J.-M.; Xargay-Torrent, S.; Prats-Puig, A.; Puerto-Carranza, E.; Díaz-Roldán, F.; de Zegher, F.; Ibañez, L.; et al. A 24-Month Metformin Treatment Study of Children with Obesity: Changes in Circulating GDF-15 and Associations with Changes in Body Weight and Visceral Fat. Pediatr. Obes. 2022, 17, e12845. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Wang, Y.; Lu, J.; Lu, W.; Zhou, J.; Yin, J.; Ma, X. Growth Differentiation Factor 15 Is Not Associated with Glycemic Control in Patients with Type 2 Diabetes Mellitus Treated with Metformin: A Post-Hoc Analysis of AIM Study. BMC Endocr. Disord. 2022, 22, 256. [Google Scholar] [CrossRef]
- Ruan, G.; Wu, F.; Shi, D.; Sun, H.; Wang, F.; Xu, C. Metformin: Update on Mechanisms of Action on Liver Diseases. Front. Nutr. 2023, 10, 1327814. [Google Scholar] [CrossRef]
- Li, Z.; Ding, Q.; Ling, L.-P.; Wu, Y.; Meng, D.-X.; Li, X.; Zhang, C.-Q. Metformin Attenuates Motility, Contraction, and Fibrogenic Response of Hepatic Stellate Cells in Vivo and in Vitro by Activating AMP-Activated Protein Kinase. World J. Gastroenterol. 2018, 24, 819–832. [Google Scholar] [CrossRef]
- Caligiuri, A.; Bertolani, C.; Guerra, C.T.; Aleffi, S.; Galastri, S.; Trappoliere, M.; Vizzutti, F.; Gelmini, S.; Laffi, G.; Pinzani, M.; et al. Adenosine Monophosphate-Activated Protein Kinase Modulates the Activated Phenotype of Hepatic Stellate Cells. Hepatology 2008, 47, 668–676. [Google Scholar] [CrossRef]
- Adachi, M.; Brenner, D.A. High Molecular Weight Adiponectin Inhibits Proliferation of Hepatic Stellate Cells via Activation of Adenosine Monophosphate–Activated Protein Kinase. Hepatology 2008, 47, 677–685. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired Mitochondrial Activity in the Insulin-Resistant Offspring of Patients with Type 2 Diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Castelló, R.; Falcón, R.; Gómez, M.; Rocha, M.; Hernández-Mijares, A. Effects of Metformin on Mitochondrial Function of Leukocytes from Polycystic Ovary Syndrome Patients with Insulin Resistance. Eur. J. Endocrinol. 2015, 173, 683–691. [Google Scholar] [CrossRef]
- Larsen, S.; Rabøl, R.; Hansen, C.N.; Madsbad, S.; Helge, J.W.; Dela, F. Metformin-Treated Patients with Type 2 Diabetes Have Normal Mitochondrial Complex I Respiration. Diabetologia 2012, 55, 443–449. [Google Scholar] [CrossRef]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin Improves Healthspan and Lifespan in Mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523.e5. [Google Scholar] [CrossRef]
- Alshawi, A.; Agius, L. Low Metformin Causes a More Oxidized Mitochondrial NADH/NAD Redox State in Hepatocytes and Inhibits Gluconeogenesis by a Redox-Independent Mechanism. J. Biol. Chem. 2019, 294, 2839–2853. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-Activated Protein Kinase Mediates Mitochondrial Fission in Response to Energy Stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef]
- Duez, H.; Lamarche, B.; Uffelman, K.D.; Valero, R.; Cohn, J.S.; Lewis, G.F. Hyperinsulinemia Is Associated with Increased Production Rate of Intestinal Apolipoprotein B-48-Containing Lipoproteins in Humans. Arter. Thromb. Vasc. Biol. 2006, 26, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Dash, S.; Morgantini, C.; Adeli, K.; Lewis, G.F. Gut Peptides Are Novel Regulators of Intestinal Lipoprotein Secretion: Experimental and Pharmacological Manipulation of Lipoprotein Metabolism. Diabetes 2015, 64, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Repiso, C.; Rodriguez-Pacheco, F.; Garcia-Arnes, J.; Valdes, S.; Gonzalo, M.; Soriguer, F.; Moreno-Ruiz, F.J.; Rodriguez-Cañete, A.; Gallego-Perales, J.L.; Alcain-Martinez, G.; et al. The Expression of Genes Involved in Jejunal Lipogenesis and Lipoprotein Synthesis Is Altered in Morbidly Obese Subjects with Insulin Resistance. Lab. Investig. 2015, 95, 1409–1417. [Google Scholar] [CrossRef]
- Jeppesen, J.; Zhou, M.Y.; Chen, Y.D.; Reaven, G.M. Effect of Metformin on Postprandial Lipemia in Patients with Fairly to Poorly Controlled NIDDM. Diabetes Care 1994, 17, 1093–1099. [Google Scholar] [CrossRef]
- Field, F.J.; Born, E.; Murthy, S.; Mathur, S.N. Gene Expression of Sterol Regulatory Element-Binding Proteins in Hamster Small Intestine. J. Lipid Res. 2001, 42, 1–8. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, Regulated by the Insulin and AMPK Signaling Pathways, Plays a Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-Activated Protein Kinase: An Emerging Drug Target to Regulate Imbalances in Lipid and Carbohydrate Metabolism to Treat Cardio-Metabolic Diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef]
- Lutz, T.A.; Osto, E. Glucagon-like Peptide-1, Glucagon-like Peptide-2, and Lipid Metabolism. Curr. Opin. Lipidol. 2016, 27, 257–263. [Google Scholar] [CrossRef]
- Dash, S.; Xiao, C.; Morgantini, C.; Lewis, G.F. New Insights into the Regulation of Chylomicron Production. Annu. Rev. Nutr. 2015, 35, 265–294. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hébrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-Activated Protein Kinase in the Regulation of Hepatic Energy Metabolism: From Physiology to Therapeutic Perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef]
- Madsen, A.; Bozickovic, O.; Bjune, J.-I.; Mellgren, G.; Sagen, J.V. Metformin Inhibits Hepatocellular Glucose, Lipid and Cholesterol Biosynthetic Pathways by Transcriptionally Suppressing Steroid Receptor Coactivator 2 (SRC-2). Sci. Rep. 2015, 5, 16430. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Song, X.-M.; Chen, Q.-T.; Liu, T.; Teng, J.-T.; Zhou, K.; Luo, D.-Q. Effect of Metformin on Global Gene Expression in Liver of KKAy Mice. Pharmacol. Rep. 2016, 68, 1332–1338. [Google Scholar] [CrossRef]
- Mazière, J.-C.; Mazière, C.; Mora, L.; Gardette, J.; Salmon, S.; Auclair, M.; Polonovski, J. The Antidiabetic Drug Metformin Decreases Cholesterol Metabolism in Cultured Human Fibroblasts. Atherosclerosis 1988, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Tomkin, G.H. Changes in Hepatic and Intestinal Cholesterol Regulatory Enzymes: The Influence of Metformin. Biochem. Pharmacol. 1983, 32, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Sonne, D.P.; Knop, F.K. Comment on Xu et al. Effects of Metformin on Metabolite Profiles and LDL Cholesterol in Patients With Type 2 Diabetes. Diabetes Care 2015;38:1858-1867. Diabetes Care 2015, 38, e215. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R. Key Discoveries in Bile Acid Chemistry and Biology and Their Clinical Applications: History of the Last Eight Decades. J. Lipid Res. 2014, 55, 1553–1595. [Google Scholar] [CrossRef] [PubMed]
- van Stee, M.F.; de Graaf, A.A.; Groen, A.K. Actions of Metformin and Statins on Lipid and Glucose Metabolism and Possible Benefit of Combination Therapy. Cardiovasc. Diabetol. 2018, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.S.; Goodarzi, G.; Panahi, G.; Zamani-Garmsiri, F.; Meshkani, R. The Combination of Metformin with Morin Alleviates Hepatic Steatosis via Modulating Hepatic Lipid Metabolism, Hepatic Inflammation, Brown Adipose Tissue Thermogenesis, and White Adipose Tissue Browning in High-Fat Diet-Fed Mice. Life Sci. 2023, 323, 121706. [Google Scholar] [CrossRef]
- Lin, H.Z.; Yang, S.Q.; Chuckaree, C.; Kuhajda, F.; Ronnet, G.; Diehl, A.M. Metformin Reverses Fatty Liver Disease in Obese, Leptin-Deficient Mice. Nat. Med. 2000, 6, 998–1003. [Google Scholar] [CrossRef]
- Resuli, B.; Demiraj, V.; Babameto, A.; Sema, K.; Malaj, V. Metformin Superior to Low-fat Diet for the Treatment of Patients with Nonalcoholic Fatty Liver Disease and/or Steatohepatitis. Pol. Arch. Intern. Med. 2012, 122, 68–71. [Google Scholar] [CrossRef]
- Shargorodsky, M.; Omelchenko, E.; Matas, Z.; Boaz, M.; Gavish, D. Relation between Augmentation Index and Adiponectin during One-Year Metformin Treatment for Nonalcoholic Steatohepatosis: Effects beyond Glucose Lowering? Cardiovasc. Diabetol. 2012, 11, 61. [Google Scholar] [CrossRef]
- Torres, D.M.; Jones, F.J.; Shaw, J.C.; Williams, C.D.; Ward, J.A.; Harrison, S.A. Rosiglitazone versus Rosiglitazone and Metformin versus Rosiglitazone and Losartan in the Treatment of Nonalcoholic Steatohepatitis in Humans: A 12-Month Randomized, Prospective, Open- Label Trial. Hepatology 2011, 54, 1631–1639. [Google Scholar] [CrossRef]
- Sofer, E.; Boaz, M.; Matas, Z.; Mashavi, M.; Shargorodsky, M. Treatment with Insulin Sensitizer Metformin Improves Arterial Properties, Metabolic Parameters, and Liver Function in Patients with Nonalcoholic Fatty Liver Disease: A Randomized, Placebo-Controlled Trial. Metabolism 2011, 60, 1278–1284. [Google Scholar] [CrossRef]
- Krakoff, J.; Clark, J.M.; Crandall, J.P.; Wilson, C.; Molitch, M.E.; Brancati, F.L.; Edelstein, S.L.; Knowler, W.C.; Diabetes Prevention Program Research Group. Effects of Metformin and Weight Loss on Serum Alanine Aminotransferase Activity in the Diabetes Prevention Program. Obesity 2010, 18, 1762–1767. [Google Scholar] [CrossRef]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A Randomized Controlled Trial of Metformin versus Vitamin E or Prescriptive Diet in Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2005, 100, 1082–1090. [Google Scholar] [CrossRef]
- Omer, Z.; Cetinkalp, S.; Akyildiz, M.; Yilmaz, F.; Batur, Y.; Yilmaz, C.; Akarca, U. Efficacy of Insulin-Sensitizing Agents in Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2010, 22, 18–23. [Google Scholar] [CrossRef]
- Loomba, R.; Lutchman, G.; Kleiner, D.E.; Ricks, M.; Feld, J.J.; Borg, B.B.; Modi, A.; Nagabhyru, P.; Sumner, A.E.; Liang, T.J.; et al. Clinical Trial: Pilot Study of Metformin for the Treatment of Non-Alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2009, 29, 172–182. [Google Scholar] [CrossRef]
- Preiss, D.; Sattar, N.; Harborne, L.; Norman, J.; Fleming, R. The Effects of 8 Months of Metformin on Circulating GGT and ALT Levels in Obese Women with Polycystic Ovarian Syndrome. Int. J. Clin. Pract. 2008, 62, 1337–1343. [Google Scholar] [CrossRef]
- Idilman, R.; Mizrak, D.; Corapcioglu, D.; Bektas, M.; Doganay, B.; Sayki, M.; Coban, S.; Erden, E.; Soykan, I.; Emral, R.; et al. Clinical Trial: Insulin-Sensitizing Agents May Reduce Consequences of Insulin Resistance in Individuals with Non-Alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2008, 28, 200–208. [Google Scholar] [CrossRef]
- Nair, S.; Diehl, A.M.; Wiseman, M.; Farr, G.H.; Perrillo, R.P. Metformin in the Treatment of Non-Alcoholic Steatohepatitis: A Pilot Open Label Trial. Aliment. Pharmacol. Ther. 2004, 20, 23–28. [Google Scholar] [CrossRef]
- Khoshbaten, M.; Beheshtirouy, S.; Shayanrad, S.; Gharekhani, A.; Rezaee, H. Comparison of the Efficacy of Pioglitazone and Metformin on Ultrasound Grade and Liver Enzymes Level in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. Drug Res. 2023, 73, 232–237. [Google Scholar] [CrossRef]
- Feng, W.; Bi, Y.; Li, P.; Yin, T.; Gao, C.; Shen, S.; Gao, L.; Yang, D.; Zhu, D. Effects of Liraglutide, Metformin and Gliclazide on Body Composition in Patients with Both Type 2 Diabetes and Non-alcoholic Fatty Liver Disease: A Randomized Trial. J. Diabetes Investig. 2019, 10, 399–407. [Google Scholar] [CrossRef]
- Garinis, G.A.; Fruci, B.; Mazza, A.; De Siena, M.; Abenavoli, S.; Gulletta, E.; Ventura, V.; Greco, M.; Abenavoli, L.; Belfiore, A. Metformin versus Dietary Treatment in Nonalcoholic Hepatic Steatosis: A Randomized Study. Int. J. Obes. 2010, 34, 1255–1264. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, V.; Salas-Romero, R.; Del Villar-Morales, A.; Martínez-Coria, E.; Pegueros-Pérez, A.; Franco-Sánchez, J.G. Decrease of liver fat content by aerobic exercise or metformin therapy in overweight or obese women. Rev. Investig. Clin. 2013, 65, 307–317. [Google Scholar]
- Handzlik, G.; Holecki, M.; Kozaczka, J.; Kukla, M.; Wyskida, K.; Kędzierski, L.; Pawlicki, K.; Duława, J. Evaluation of Metformin Therapy Using Controlled Attenuation Parameter and Transient Elastography in Patients with Non-Alcoholic Fatty Liver Disease. Pharmacol. Rep. 2019, 71, 183–188. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Yan, C.; Li, C.; Zhang, L.; Zhang, L.; Liang, E.; Liu, T.; Mao, J. Effect of Metformin on Nonalcoholic Fatty Liver Based on Meta-Analysis and Network Pharmacology. Medicine 2022, 101, e31437. [Google Scholar] [CrossRef]
- Lian, J.; Fu, J. Efficacy of Various Hypoglycemic Agents in the Treatment of Patients With Nonalcoholic Liver Disease With or Without Diabetes: A Network Meta-Analysis. Front. Endocrinol. 2021, 12, 649018. [Google Scholar] [CrossRef]
- Sawangjit, R.; Chongmelaxme, B.; Phisalprapa, P.; Saokaew, S.; Thakkinstian, A.; Kowdley, K.V.; Chaiyakunapruk, N. Comparative Efficacy of Interventions on Nonalcoholic Fatty Liver Disease (NAFLD): A PRISMA-Compliant Systematic Review and Network Meta-Analysis. Medicine 2016, 95, e4529. [Google Scholar] [CrossRef]
- Haukeland, J.W.; Konopski, Z.; Eggesbø, H.B.; von Volkmann, H.L.; Raschpichler, G.; Bjøro, K.; Haaland, T.; Løberg, E.M.; Birkeland, K. Metformin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Controlled Trial. Scand. J. Gastroenterol. 2009, 44, 853–860. [Google Scholar] [CrossRef]
- Anushiravani, A.; Haddadi, N.; Pourfarmanbar, M.; Mohammadkarimi, V. Treatment Options for Nonalcoholic Fatty Liver Disease: A Double-Blinded Randomized Placebo-Controlled Trial. Eur. J. Gastroenterol. Hepatol. 2019, 31, 613. [Google Scholar] [CrossRef]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin Improves Liver Fat Deposition Compared to Metformin in Type 2 Diabetes Patients with Non-Alcoholic Fatty Liver Disease: A Prospective Randomized Controlled Pilot Study. Diabetes Obes. Metab. 2018, 20, 438–442. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Jianfang, F.; Wanxia, X.; Xiling, G.; Jing, X.; Wenjuan, Y.; Jianrong, L.; Qingzhen, H.; Kaiyan, M.; Jingxuan, L.; Taixiong, C.; et al. Effect and Safety of Pioglitazone-Metformin Tablets in the Treatment of Newly Diagnosed Type 2 Diabetes Patients with Nonalcoholic Fatty Liver Disease in Shaanxi Province: A Randomized, Double-Blinded, Double-Simulated Multicenter Study. J. Diabetes Res. 2023, 2023, 2044090. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of Vitamin E or Metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents: The TONIC Randomized Controlled Trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef]
- Rakoski, M.O.; Singal, A.G.; Rogers, M.A.; Conjeevaram, H. Meta-Analysis: Insulin Sensitizers for the Treatment of Non-Alcoholic Steatohepatitis. Aliment. Pharmacol. Ther. 2010, 32, 1211–1221. [Google Scholar] [CrossRef]
- Tang, W.; Xu, Q.; Hong, T.; Tong, G.; Feng, W.; Shen, S.; Bi, Y.; Zhu, D. Comparative Efficacy of Anti-Diabetic Agents on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized and Non-Randomized Studies. Diabetes Metab. Res. Rev. 2016, 32, 200–216. [Google Scholar] [CrossRef]
- Gkiourtzis, N.; Michou, P.; Moutafi, M.; Glava, A.; Cheirakis, K.; Christakopoulos, A.; Vouksinou, E.; Fotoulaki, M. The Benefit of Metformin in the Treatment of Pediatric Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Pediatr. 2023, 182, 4795–4806. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Cotrim, H.P.; Stefano, J.T.; Siqueira, A.C.G.; Salgado, A.L.A.; Parise, E.R. N-acetylcysteine and/or ursodeoxycholic acid associated with metformin in non-alcoholic steatohepatitis: An open-label multicenter randomized controlled trial. Arq. Gastroenterol. 2019, 56, 184–190. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, J.S.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, S.U. Evolution of Liver Fibrosis and Steatosis Markers in Patients with Type 2 Diabetes after Metformin Treatment for 2 years. J. Diabetes Complicat. 2021, 35, 107747. [Google Scholar] [CrossRef]
- Sturm, N.; Bronowicki, J.-P.; Maynard-Muet, M.; Tran, A.; Heluwaert, F.; Plages, A.; Zarski, J.-P. Metformin plus Pentoxifylline versus Prescriptive Diet in Non-Alcoholic Steatohepatitis (NASH): A Randomized Controlled Pilot Trial. Gastroentérol. Clin. Biol. 2009, 33, 984–986. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Amin, H.; Garg, R.; Chadalavada, P.; Al-Yaman, W.; Lopez, R.; Singh, A. Medications in Type-2 Diabetics and Their Association with Liver Fibrosis. World J. Gastroenterol. 2020, 26, 3249–3259. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Reyes, M.J.; Mishra, A.; Mehta, R.; Henry, L. Systematic Review with Meta-Analysis: Non-Alcoholic Steatohepatitis—A Case for Personalised Treatment Based on Pathogenic Targets. Aliment. Pharmacol. Ther. 2014, 39, 3–14. [Google Scholar] [CrossRef]
- Said, A.; Akhter, A. Meta-Analysis of Randomized Controlled Trials of Pharmacologic Agents in Non-Alcoholic Steatohepatitis. Ann. Hepatol. 2017, 16, 538–547. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Scorletti, E.; Mantzoros, C.S.; Targher, G. Efficacy and Safety of Anti-Hyperglycaemic Drugs in Patients with Non-Alcoholic Fatty Liver Disease with or without Diabetes: An Updated Systematic Review of Randomized Controlled Trials. Diabetes Metab. 2020, 46, 427–441. [Google Scholar] [CrossRef]
- Alam, S.; Mustafa, G.; Alam, M.; Ahmad, N. Insulin Resistance in Development and Progression of Nonalcoholic Fatty Liver Disease. World J. Gastrointest. Pathophysiol. 2016, 7, 211. [Google Scholar] [CrossRef]
- De Oliveira, S.; Houseright, R.A.; Graves, A.L.; Golenberg, N.; Korte, B.G.; Miskolci, V.; Huttenlocher, A. Metformin Modulates Innate Immune-Mediated Inflammation and Early Progression of NAFLD-Associated Hepatocellular Carcinoma in Zebrafish. J. Hepatol. 2019, 70, 710–721. [Google Scholar] [CrossRef]
- Güven Çetin, E. The Relationship between Insulin Resistance and Liver Damage in Non-Alcoholic Fatty Liver Patients. Med. Bull. Sisli Etfal Hosp. 2018, 54, 411–415. [Google Scholar] [CrossRef]
- García-Pagán, J.-C.; Gracia-Sancho, J.; Bosch, J. Functional Aspects on the Pathophysiology of Portal Hypertension in Cirrhosis. J. Hepatol. 2012, 57, 458–461. [Google Scholar] [CrossRef]
- Berzigotti, A.; Seijo, S.; Reverter, E.; Bosch, J. Assessing Portal Hypertension in Liver Diseases. Expert. Rev. Gastroenterol. Hepatol. 2013, 7, 141–155. [Google Scholar] [CrossRef]
- Tripathi, D.M.; Erice, E.; Lafoz, E.; García-Calderó, H.; Sarin, S.K.; Bosch, J.; Gracia-Sancho, J.; García-Pagán, J.C. Metformin Reduces Hepatic Resistance and Portal Pressure in Cirrhotic Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G301–G309. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, I.-C.; Sun, W.; Su, M.-I.; Hsu, P.-H.; Fu, Y.; Zhu, Y.; DeFea, K.; Pan, S.; Tsai, M.-D.; et al. AMP-Activated Protein Kinase Functionally Phosphorylates Endothelial Nitric Oxide Synthase Ser633. Circ. Res. 2009, 104, 496–505. [Google Scholar] [CrossRef]
- Chen, Z.P.; Mitchelhill, K.I.; Michell, B.J.; Stapleton, D.; Rodriguez-Crespo, I.; Witters, L.A.; Power, D.A.; Ortiz de Montellano, P.R.; Kemp, B.E. AMP-Activated Protein Kinase Phosphorylation of Endothelial NO Synthase. FEBS Lett. 1999, 443, 285–289. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, C.; Ceng, C.; Zhan, H.; Zheng, D.; Han, W. Metformin Enhances Nitric Oxide Production and Diminishes Rho Kinase Activity in Rats with Hyperlipidemia. Lipids Health Dis. 2014, 13, 115. [Google Scholar] [CrossRef]
- Rittig, N.; Aagaard, N.K.; Villadsen, G.E.; Sandahl, T.D.; Jessen, N.; Grønbæk, H.; George, J. Randomised Clinical Study: Acute Effects of Metformin versus Placebo on Portal Pressure in Patients with Cirrhosis and Portal Hypertension. Aliment. Pharmacol. Ther. 2021, 54, 320–328. [Google Scholar] [CrossRef]
- Jafaripour, L.; Esmaeilpour, K.; Maneshian, M.; Bashiri, H.; Rajizadeh, M.A.; Ahmadvand, H.; Asadi-Shekaari, M. The Effect of Gallic Acid on Memory and Anxiety-like Behaviors in Rats with Bile Duct Ligation-Induced Hepatic Encephalopathy: Role of AMPK Pathway. Avicenna J. Phytomed 2022, 12, 425–438. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Shahbazi, A.; Sahab Negah, S.; Joghataei, M.T.; Larsen, F.S. Drug-Induced-Acute Liver Failure: A Critical Appraisal of the Thioacetamide Model for the Study of Hepatic Encephalopathy. Toxicol. Rep. 2021, 8, 962–970. [Google Scholar] [CrossRef]
- Saleh, D.O.; Mansour, D.F.; Fayez, A.M. Thioacetamide-Induced Acute Hepatic Encephalopathy: Central vs Peripheral Effect of Allicin. Metab. Brain Dis. 2021, 36, 1331–1340. [Google Scholar] [CrossRef]
- Ampuero, J.; Ranchal, I.; Nuñez, D.; Díaz-Herrero, M.D.M.; Maraver, M.; Campo, J.A.D.; Rojas, Á.; Camacho, I.; Figueruela, B.; Bautista, J.D.; et al. Metformin Inhibits Glutaminase Activity and Protects against Hepatic Encephalopathy. PLoS ONE 2012, 7, e49279. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Onikanni, S.A.; Lawal, B.; Bakare, O.S.; Ajiboye, B.O.; Ojo, O.A.; Farasani, A.; Kabrah, S.M.; Batiha, G.E.-S.; Conte-Junior, C.A. Cancer of the Liver and Its Relationship with Diabetes Mellitus. Technol. Cancer Res. Treat. 2022, 21, 1–9. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease–Free Survival in Patients With Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- Kong, W.; Liu, Z.; Zhang, N.; Wu, X.; Zhao, X.; Yan, L. A Prospective Cohort Study of Metformin as an Adjuvant Therapy for Infertile Women With Endometrial Complex Hyperplasia/Complex Atypical Hyperplasia and Their Subsequent Assisted Reproductive Technology Outcomes. Front. Endocrinol. 2022, 13, 849794. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, W.; Wei, L.; Zhu, S. Current Status and Frontier Tracking of Clinical Trials on Metformin for Cancer Treatment. J. Cancer Res. Clin. Oncol. 2023, 149, 16931–16946. [Google Scholar] [CrossRef]
- Bilusic, M.; Toney, N.J.; Donahue, R.N.; Wroblewski, S.; Zibelman, M.; Ghatalia, P.; Ross, E.A.; Karzai, F.; Madan, R.A.; Dahut, W.L.; et al. A Randomized Phase 2 Study of Bicalutamide with or without Metformin for Biochemical Recurrence in Overweight or Obese Prostate Cancer Patients (BIMET-1). Prostate Cancer Prostatic Dis. 2022, 25, 735–740. [Google Scholar] [CrossRef]
- Brown, J.C.; Zhang, S.; Ligibel, J.A.; Irwin, M.L.; Jones, L.W.; Campbell, N.; Pollak, M.N.; Sorrentino, A.; Cartmel, B.; Harrigan, M.; et al. Effect of Exercise or Metformin on Biomarkers of Inflammation in Breast and Colorectal Cancer: A Randomized Trial. Cancer Prev. Res. 2020, 13, 1055–1062. [Google Scholar] [CrossRef]
- Obara, A.; Fujita, Y.; Abudukadier, A.; Fukushima, T.; Oguri, Y.; Ogura, M.; Harashima, S.; Hosokawa, M.; Inagaki, N. DEPTOR-Related mTOR Suppression Is Involved in Metformin’s Anti-Cancer Action in Human Liver Cancer Cells. Biochem. Biophys. Res. Commun. 2015, 460, 1047–1052. [Google Scholar] [CrossRef]
- Ludwig, H.; Khayat, D.; Giaccone, G.; Facon, T. Proteasome Inhibition and Its Clinical Prospects in the Treatment of Hematologic and Solid Malignancies. Cancer 2005, 104, 1794–1807. [Google Scholar] [CrossRef]
- Guo, N.; Peng, Z. MG132, a Proteasome Inhibitor, Induces Apoptosis in Tumor Cells. Asia-Pac. J. Clin. Oncol. 2013, 9, 6–11. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Chen, Y.; Fang, L.; Guan, C.; Bai, F.; Ma, M.; Lyu, J.; Meng, Q.H. Metformin Inhibits Tumorigenesis and Tumor Growth of Breast Cancer Cells by Upregulating miR-200c but Downregulating AKT2 Expression. J. Cancer 2017, 8, 1849–1864. [Google Scholar] [CrossRef]
- Miyoshi, H.; Kato, K.; Iwama, H.; Maeda, E.; Sakamoto, T.; Fujita, K.; Toyota, Y.; Tani, J.; Nomura, T.; Mimura, S.; et al. Effect of the Anti-Diabetic Drug Metformin in Hepatocellular Carcinoma in Vitro and in Vivo. Int. J. Oncol. 2014, 45, 322–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xiao, H. Metformin Actions on the Liver: Protection Mechanisms Emerging in Hepatocytes and Immune Cells against NASH-Related HCC. Int. J. Mol. Sci. 2021, 22, 5016. [Google Scholar] [CrossRef]
- Kato, K.; Iwama, H.; Yamashita, T.; Kobayashi, K.; Fujihara, S.; Fujimori, T.; Kamada, H.; Kobara, H.; Masaki, T. The Anti-Diabetic Drug Metformin Inhibits Pancreatic Cancer Cell Proliferation in Vitro and in Vivo: Study of the microRNAs Associated with the Antitumor Effect of Metformin. Oncol. Rep. 2016, 35, 1582–1592. [Google Scholar] [CrossRef]
- Sekino, N.; Kano, M.; Matsumoto, Y.; Sakata, H.; Murakami, K.; Toyozumi, T.; Otsuka, R.; Yokoyama, M.; Shiraishi, T.; Okada, K.; et al. The Antitumor Effects of Metformin on Gastric Cancer In Vitro and on Peritoneal Metastasis. Anticancer. Res. 2018, 38, 6263–6269. [Google Scholar] [CrossRef]
- Kaplan, D.E.; Serper, M.; John, B.V.; Tessiatore, K.M.; Lerer, R.; Mehta, R.; Fox, R.; Aytaman, A.; Baytarian, M.; Hunt, K.; et al. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients With Diabetes and Cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 2148–2160.e14. [Google Scholar] [CrossRef]
- You, J.H.; Song, S.O.; Kang, M.J.; Cho, Y.Y.; Kim, S.W.; Suh, S.H.; Lee, S.; Lee, Y.; Lee, B.-W. Metformin and Gastrointestinal Cancer Development in Newly Diagnosed Type 2 Diabetes: A Population-Based Study in Korea. Clin. Transl. Gastroenterol. 2020, 11, e00254. [Google Scholar] [CrossRef]
- Li, Q.; Xu, H.; Sui, C.; Zhang, H. Impact of Metformin Use on Risk and Mortality of Hepatocellular Carcinoma in Diabetes Mellitus. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101781. [Google Scholar] [CrossRef]
- Zeng, R.W.; Yong, J.N.; Tan, D.J.H.; Fu, C.E.; Lim, W.H.; Xiao, J.; Chan, K.E.; Tan, C.; Goh, X.L.; Chee, D.; et al. Meta-Analysis: Chemoprevention of Hepatocellular Carcinoma with Statins, Aspirin and Metformin. Aliment. Pharmacol. Ther. 2023, 57, 600–609. [Google Scholar] [CrossRef]
- Zhou, J.; Ke, Y.; Lei, X.; Wu, T.; Li, Y.; Bao, T.; Tang, H.; Zhang, C.; Wu, X.; Wang, G.; et al. Meta-Analysis: The Efficacy of Metformin and Other Anti-Hyperglycemic Agents in Prolonging the Survival of Hepatocellular Carcinoma Patients with Type 2 Diabetes. Ann. Hepatol. 2020, 19, 320–328. [Google Scholar] [CrossRef]
- Yuan, B.; Ma, J.; Wang, J.; Hao, J. The Effect of Metformin Usage on Survival Outcomes for Hepatocellular Carcinoma Patients with Type 2 Diabetes Mellitus after Curative Therapy. Front. Endocrinol. 2022, 13, 1060768. [Google Scholar] [CrossRef]
- Papadakos, S.P.; Ferraro, D.; Carbone, G.; Frampton, A.E.; Vennarecci, G.; Kykalos, S.; Schizas, D.; Theocharis, S.; Machairas, N. The Emerging Role of Metformin in the Treatment of Hepatocellular Carcinoma: Is There Any Value in Repurposing Metformin for HCC Immunotherapy? Cancers 2023, 15, 3161. [Google Scholar] [CrossRef]
- Gangale, M.F.; Miele, L.; Lanzone, A.; Sagnella, F.; Martinez, D.; Tropea, A.; Moro, F.; Morciano, A.; Ciardulli, A.; Palla, C.; et al. Long-Term Metformin Treatment Is Able to Reduce the Prevalence of Metabolic Syndrome and Its Hepatic Involvement in Young Hyperinsulinaemic Overweight Patients with Polycystic Ovarian Syndrome. Clin. Endocrinol. 2011, 75, 520–527. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of Current Treatments on Liver Disease, Glucose Metabolism and Cardiovascular Risk in Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Randomised Trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Metformin in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Biomed. Rep. 2013, 1, 57–64. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Yang, G.-Y.; Wang, C.; Chen, X.-Y.; Zhang, L.-L. Effectiveness of Drug Interventions in Nonalcoholic Fatty Liver Disease: A Network Meta-Analysis. World J. Diabetes 2021, 12, 1576–1586. [Google Scholar] [CrossRef]
- Yu, Y.; Mao, Y.; Chen, C.; Chen, J.; Chen, J.; Cong, W.; Ding, Y.; Duan, Z.; Fu, Q.; Guo, X.; et al. CSH Guidelines for the Diagnosis and Treatment of Drug-Induced Liver Injury. Hepatol. Int. 2017, 11, 221–241. [Google Scholar] [CrossRef]
- Katarey, D.; Verma, S. Drug-Induced Liver Injury. Clin. Med. 2016, 16, s104–s109. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Acetaminophen Hepatotoxicity. Semin. Liver Dis. 2019, 39, 221–234. [Google Scholar] [CrossRef]
- Seki, E.; Brenner, D.A.; Karin, M. A Liver Full of JNK: Signaling in Regulation of Cell Function and Disease Pathogenesis, and Clinical Approaches. Gastroenterology 2012, 143, 307–320. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hwang, J.H.; Kim, K.-S.; Noh, J.-R.; Choi, D.-H.; Kim, D.-K.; Tadi, S.; Yim, Y.-H.; Choi, H.-S.; Lee, C.-H. Metformin Ameliorates Acetaminophen Hepatotoxicity via Gadd45β-Dependent Regulation of JNK Signaling in Mice. J. Hepatol. 2015, 63, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; Weemhoff, J.L.; Chavan, H.; Xie, Y.; Krishnamurthy, P.; Jaeschke, H. Editor’s Highlight: Metformin Protects Against Acetaminophen Hepatotoxicity by Attenuation of Mitochondrial Oxidant Stress and Dysfunction. Toxicol. Sci. 2016, 154, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hu, K.; Lin, L.; Ai, Q.; Ge, P.; Liu, Y.; Dai, J.; Ye, B.; Zhang, L. AMPK Dependent Protective Effects of Metformin on Tumor Necrosis Factor-Induced Apoptotic Liver Injury. Biochem. Biophys. Res. Commun. 2015, 465, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, L.; Zheng, W.; Wan, J.; Ge, P.; Li, H.; Zhang, L. Antidiabetic Drug Metformin Alleviates Endotoxin-Induced Fulminant Liver Injury in Mice. Int. Immunopharmacol. 2012, 12, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.K.T.; Chiu, P.-Y.; Mak, D.H.F.; Ko, K.-M. Metformin Protects Against Carbon Tetrachloride Hepatotoxicity in Mice. J. Pharmacol. Sci. 2003, 93, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Dailey, G.E.; Jabbour, S.A.; Reasner, C.A.; Mills, D.J. Gastrointestinal Tolerability of Extended-Release Metformin Tablets Compared to Immediate-Release Metformin Tablets: Results of a Retrospective Cohort Study. Curr. Med. Res. Opin. 2004, 20, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Scheen, A. Understanding and Overcoming Metformin Gastrointestinal Intolerance. Diabetes Obes. Metab. 2017, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Dujic, T.; Zhou, K.; Donnelly, L.A.; Tavendale, R.; Palmer, C.N.A.; Pearson, E.R. Association of Organic Cation Transporter 1 With Intolerance to Metformin in Type 2 Diabetes: A GoDARTS Study. Diabetes 2015, 64, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Han, T.K.; Proctor, W.R.; Costales, C.L.; Cai, H.; Everett, R.S.; Thakker, D.R. Four Cation-Selective Transporters Contribute to Apical Uptake and Accumulation of Metformin in Caco-2 Cell Monolayers. J. Pharmacol. Exp. Ther. 2015, 352, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Duan, H.; Hebert, M.F.; Liang, C.J.; Rice, K.M.; Wang, J. Taste of a Pill: Organic Cation Transporter-3 (OCT3) Mediates Metformin Accumulation and Secretion in Salivary Glands. J. Biol. Chem. 2014, 289, 27055–27064. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signalling in the Gut—Functions, Dysfunctions and Therapeutic Targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Scarpello, J.H.B.; Hodgson, E.; Howlett, H.C.S. Effect of Metformin on Bile Salt Circulation and Intestinal Motility in Type 2 Diabetes Mellitus. Diabet. Med. 1998, 15, 651–656. [Google Scholar] [CrossRef]
- Rodriguez, J.; Hiel, S.; Delzenne, N.M. Metformin: Old Friend, New Ways of Action-Implication of the Gut Microbiome? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Sadeeqa, S.; Fatima, M.; Latif, S.; Afzal, H.; Nazir, S.U.R.; Saeed, H. Prevelance of Metformin-Induced Gastrointestinal Problems. Acta Pol. Pharm.–Drug Res. 2019, 76, 1073–1077. [Google Scholar] [CrossRef]
- Schommers, P.; Thurau, A.; Bultmann-Mellin, I.; Guschlbauer, M.; Klatt, A.R.; Rozman, J.; Klingenspor, M.; de Angelis, M.H.; Alber, J.; Gründemann, D.; et al. Metformin Causes a Futile Intestinal–Hepatic Cycle Which Increases Energy Expenditure and Slows down Development of a Type 2 Diabetes-like State. Mol. Metab. 2017, 6, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Dyatlova, N.; Tobarran, N.V.; Kannan, L.; North, R.; Wills, B.K. Metformin-Associated Lactic Acidosis (MALA). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Di Mauro, S.; Filippello, A.; Scamporrino, A.; Purrello, F.; Piro, S.; Malaguarnera, R. Metformin: When Should We Fear Lactic Acidosis? Int. J. Mol. Sci. 2022, 23, 8320. [Google Scholar] [CrossRef] [PubMed]
- Duong, J.K.; Furlong, T.J.; Roberts, D.M.; Graham, G.G.; Greenfield, J.R.; Williams, K.M.; Day, R.O. The Role of Metformin in Metformin-Associated Lactic Acidosis (MALA): Case Series and Formulation of a Model of Pathogenesis. Drug Saf. 2013, 36, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence That Metformin Exerts Its Anti-Diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- Blough, B.; Moreland, A.; Mora, A. Metformin-Induced Lactic Acidosis with Emphasis on the Anion Gap. Bayl. Univ. Med. Cent. Proc. 2015, 28, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Lalau, J.D.; Lacroix, C.; Compagnon, P.; de Cagny, B.; Rigaud, J.P.; Bleichner, G.; Chauveau, P.; Dulbecco, P.; Guérin, C.; Haegy, J.M. Role of Metformin Accumulation in Metformin-Associated Lactic Acidosis. Diabetes Care 1995, 18, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Sekiguchi, N.; Hirano, A.; Oshima, A.; Imai, T. Metformin-Associated Lactic Acidosis Induced by Excessive Alcohol Consumption. Intern. Med. 2023. [Google Scholar] [CrossRef]
- Smith, F.C.; Stocker, S.L.; Danta, M.; Carland, J.E.; Kumar, S.S.; Liu, Z.; Greenfield, J.R.; Braithwaite, H.E.; Cheng, T.S.; Graham, G.G.; et al. The Safety and Pharmacokinetics of Metformin in Patients with Chronic Liver Disease. Aliment. Pharmacol. Ther. 2020, 51, 565–575. [Google Scholar] [CrossRef]
- Jeppesen, J.B.; Mortensen, C.; Bendtsen, F.; Møller, S. Lactate Metabolism in Chronic Liver Disease. Scand. J. Clin. Lab. Investig. 2013, 73, 293–299. [Google Scholar] [CrossRef]
- Woll, P.J.; Record, C.O. Lactate Elimination in Man: Effects of Lactate Concentration and Hepatic Dysfunction. Eur. J. Clin. Investig. 1979, 9, 397–404. [Google Scholar] [CrossRef]
- Tucker, G.; Casey, C.; Phillips, P.; Connor, H.; Ward, J.; Woods, H. Metformin Kinetics in Healthy Subjects and in Patients with Diabetes Mellitus. Brit. J. Clin. Pharma. 1981, 12, 235–246. [Google Scholar] [CrossRef]
- Lalau, J.-D.; Lemaire-Hurtel, A.-S.; Lacroix, C. Establishment of a Database of Metformin Plasma Concentrations and Erythrocyte Levels in Normal and Emergency Situations. Clin. Drug Investig. 2011, 31, 435–438. [Google Scholar] [CrossRef]
- Duong, J.K.; Roberts, D.M.; Furlong, T.J.; Kumar, S.S.; Greenfield, J.R.; Kirkpatrick, C.M.; Graham, G.G.; Williams, K.M.; Day, R.O. Metformin Therapy in Patients with Chronic Kidney Disease. Diabetes Obes. Metab. 2012, 14, 963–965. [Google Scholar] [CrossRef]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020357 (accessed on 27 July 2023).
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Heine, R.J.; Holman, R.R.; Sherwin, R.; Zinman, B. Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy: A Consensus Statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006, 29, 1963–1972. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2017 Executive Summary. Endocr. Pract. 2017, 23, 207–238. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Emslie-Smith, A.M.; Boyle, D.I.; Evans, J.M.; Sullivan, F.; Morris, A.D.; DARTS/MEMO Collaboration. Contraindications to Metformin Therapy in Patients with Type 2 Diabetes—A Population-Based Study of Adherence to Prescribing Guidelines. Diabet. Med. 2001, 18, 483–488. [Google Scholar] [CrossRef]
- Ekström, N.; Schiöler, L.; Svensson, A.-M.; Eeg-Olofsson, K.; Jonasson, J.M.; Zethelius, B.; Cederholm, J.; Eliasson, B.; Gudbjörnsdottir, S. Effectiveness and Safety of Metformin in 51 675 Patients with Type 2 Diabetes and Different Levels of Renal Function: A Cohort Study from the Swedish National Diabetes Register. BMJ Open 2012, 2, e001076. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.J.; Diamantidis, C.J.; McDuffie, J.R.; Cameron, B.; Stanifer, J.; Mock, C.K.; Kosinski, A.; Wang, X.; Tang, S.; Williams, J.W., Jr. FDA Safety Announcements for Metformin. In Metformin Use in Patients with Historical Contraindications or Precautions; Department of Veterans Affairs: Washington, DC, USA, 2016. [Google Scholar]
- FDA. FDA Drug Safety Communication: FDA Revises Warnings Regarding Use of the Diabetes Medicine Metformin in Certain Patients with Reduced Kidney Function. 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain (accessed on 27 January 2024).
- Tomkin, G.H.; Hadden, D.R.; Weaver, J.A.; Montgomery, D.A. Vitamin-B12 Status of Patients on Long-Term Metformin Therapy. Br. Med. J. 1971, 2, 685–687. [Google Scholar] [CrossRef]
- Stowers, J.M.; Smith, O.A. Vitamin B 12 and Metformin. Br. Med. J. 1971, 3, 246–247. [Google Scholar] [CrossRef]
- Haeusler, S.; Parry-Strong, A.; Krebs, J.D. The Prevalence of Low Vitamin B12 Status in People with Type 2 Diabetes Receiving Metformin Therapy in New Zealand—A Clinical Audit. N. Z. Med. J. 2014, 127, 8–16. [Google Scholar]
- Akinlade, K.S.; Agbebaku, S.O.; Rahamon, S.K.; Balogun, W.O. Vitamin B12 Levels in Patients with Type 2 Diabetes Mellitus on Metformin. Ann. Ib. Postgrad. Med. 2015, 13, 79–83. [Google Scholar]
- Rehman, H. Vitamin B12 Deficiency Causing Night Sweats. Scott. Med. J. 2014, 59, e8–e11. [Google Scholar] [CrossRef]
- Sulkin, T.V.; Bosman, D.; Krentz, A.J. Contraindications to Metformin Therapy in Patients With NIDDM. Diabetes Care 1997, 20, 925–928. [Google Scholar] [CrossRef]
- Vidal-Alaball, J.; Butler, C.C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral Vitamin B12 versus Intramuscular Vitamin B12 for Vitamin B12 Deficiency. Cochrane Database Syst. Rev. 2005, CD004655. [Google Scholar] [CrossRef]
- Langan, R.C.; Zawistoski, K.J. Update on Vitamin B12 Deficiency. Am. Fam. Physician 2011, 83, 1425–1430. [Google Scholar]
- Mansourian, M.; Sadeghi, H.; Doustimotlagh, A.H. Activation of the Glutathione Peroxidase by Metformin in the Bile-Duct Ligation Induced Liver Injury: In Vivo Combined with Molecular Docking Studies. Curr. Pharm. Des. 2018, 24, 3256–3263. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Ganeshpandian, M.; Loganathan, R.; Bhuvanesh, N.S.P.; Sabina, X.J.; Karthikeyan, J. Water Soluble Ru(II)–Arene Complexes of the Antidiabetic Drug Metformin: DNA and Protein Binding, Molecular Docking, Cytotoxicity and Apoptosis-Inducing Activity. RSC Adv. 2017, 7, 37706–37719. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D’Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F.; et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14, 186. https://doi.org/10.3390/metabo14040186
Perazza F, Leoni L, Colosimo S, Musio A, Bocedi G, D’Avino M, Agnelli G, Nicastri A, Rossetti C, Sacilotto F, et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites. 2024; 14(4):186. https://doi.org/10.3390/metabo14040186
Chicago/Turabian StylePerazza, Federica, Laura Leoni, Santo Colosimo, Alessandra Musio, Giulia Bocedi, Michela D’Avino, Giulio Agnelli, Alba Nicastri, Chiara Rossetti, Federica Sacilotto, and et al. 2024. "Metformin and the Liver: Unlocking the Full Therapeutic Potential" Metabolites 14, no. 4: 186. https://doi.org/10.3390/metabo14040186
APA StylePerazza, F., Leoni, L., Colosimo, S., Musio, A., Bocedi, G., D’Avino, M., Agnelli, G., Nicastri, A., Rossetti, C., Sacilotto, F., Marchesini, G., Petroni, M. L., & Ravaioli, F. (2024). Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites, 14(4), 186. https://doi.org/10.3390/metabo14040186






