Poly(ADP-Ribose) Polymerases-Inhibitor Talazoparib Inhibits Muscle Atrophy and Fatty Infiltration in a Tendon Release Infraspinatus Sheep Model: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Surgical Technique
2.3. Anesthesia and Euthanasia
2.4. Pharmacological Treatment
2.5. Radiological Assessment of Structural Muscular Changes
2.6. Histological Assessment
2.7. Statistics
3. Results
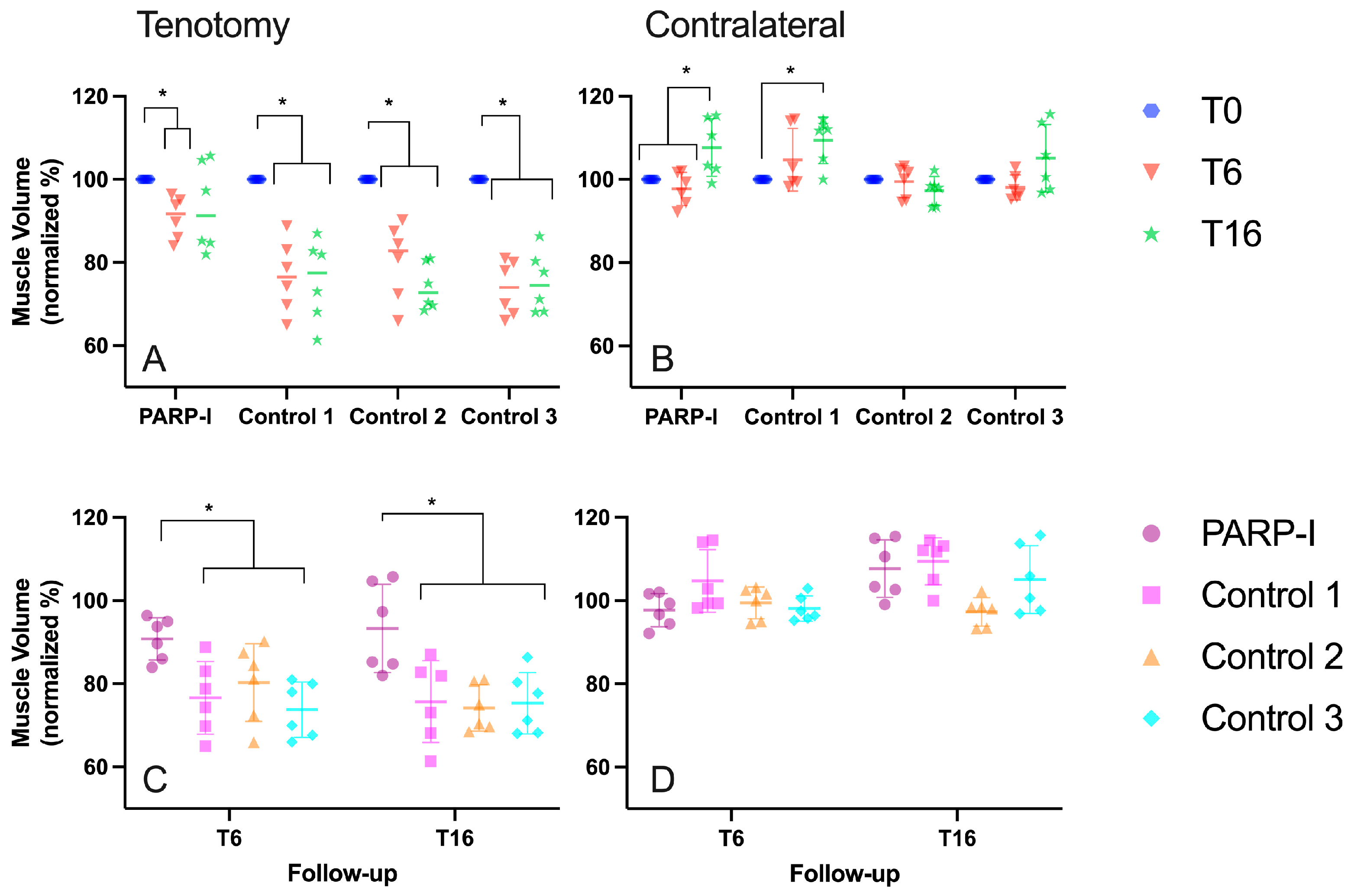
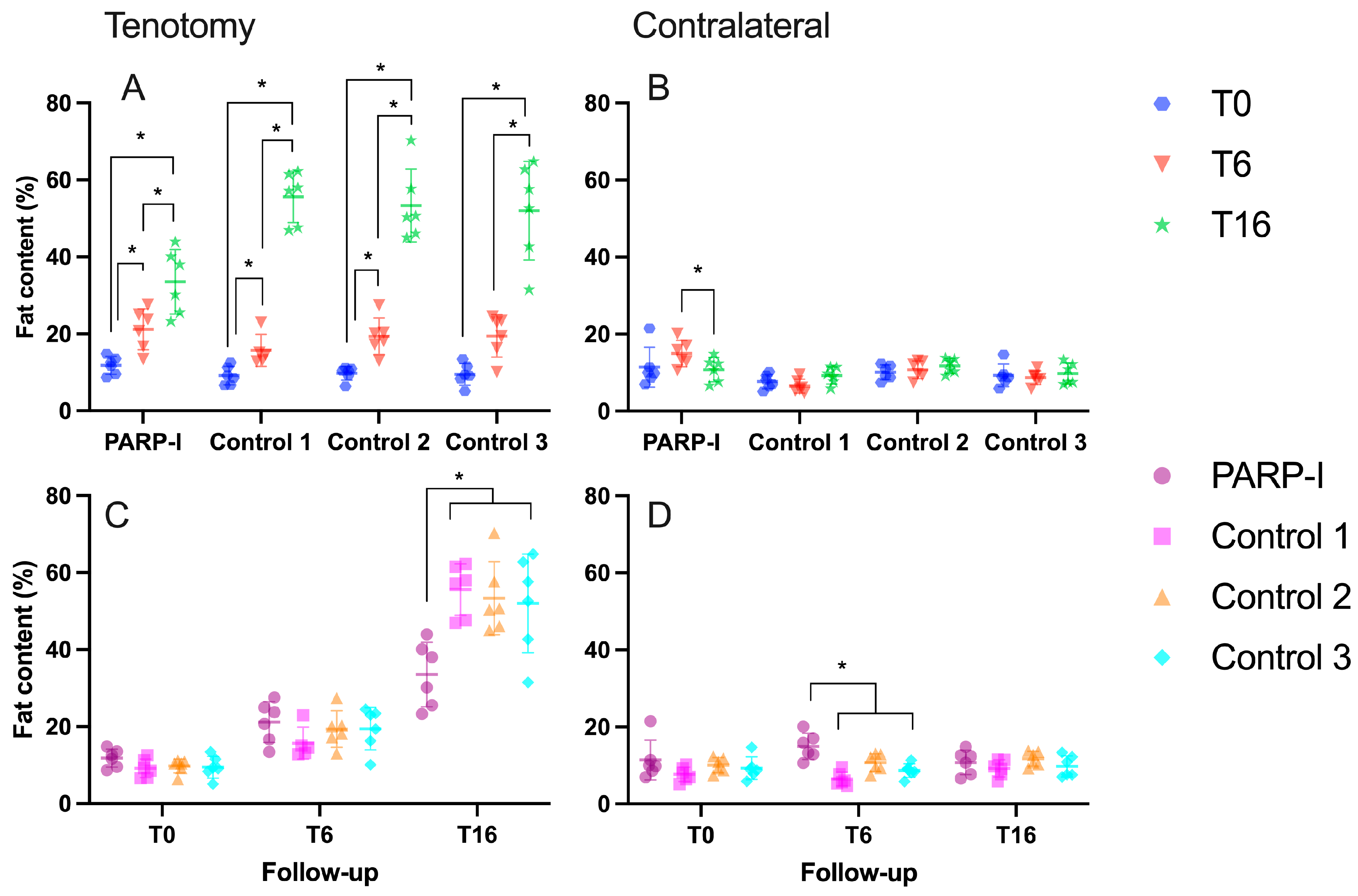
3.1. Radiological Measurements
3.1.1. Muscle Volume
3.1.2. Fatty Infiltration
3.1.3. Muscle Architecture
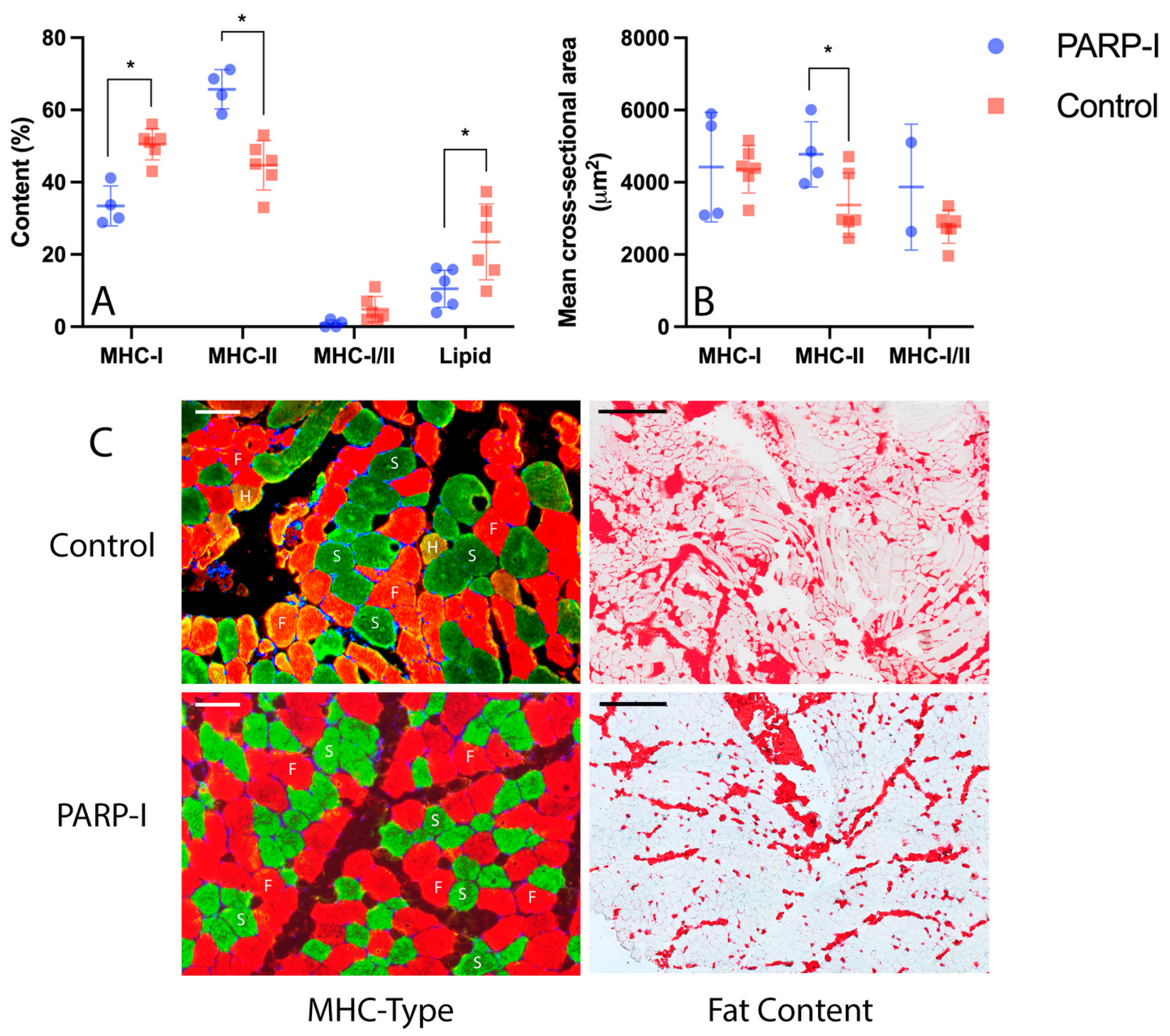
3.2. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, A.; Takagishi, K.; Osawa, T.; Yanagawa, T.; Nakajima, D.; Shitara, H.; Kobayashi, T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elb. Surg. 2010, 19, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Jt. Surg. Am. 2004, 86, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.; Meyer, D.C.; Schneeberger, A.G.; Hoppeler, H.; von Rechenberg, B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: An experimental study in sheep. J. Bone Jt. Surg. Am. 2004, 86, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Goutallier, D.; Postel, J.-M.; Gleyze, P.; Leguilloux, P.; Van Driessche, S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J. Shoulder Elb. Surg. 2003, 12, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Zhang, Y.; Pruznak, A.; Kim, H.M. Effect of tamoxifen on fatty degeneration and atrophy of rotator cuff muscles in chronic rotator cuff tear: An animal model study. J. Orthop. Res. 2015, 33, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, H.; Matsumura, N.; Shimoda, M.; Oki, S.; Yoda, M.; Tohmonda, T.; Kanai, Y.; Matsumoto, M.; Nakamura, M.; Horiuchi, K. Inhibition of PDGFR signaling prevents muscular fatty infiltration after rotator cuff tear in mice. Sci. Rep. 2017, 7, srep41552. [Google Scholar] [CrossRef]
- Gerber, C.; Meyer, D.C.; Fluck, M.; Benn, M.C.; von Rechenberg, B.; Wieser, K. Anabolic Steroids Reduce Muscle Degeneration Associated With Rotator Cuff Tendon Release in Sheep. Am. J. Sports Med. 2015, 43, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Brunyánszki, A.; Kiss, B.; Nagy, L.; Gergely, P.; Virág, L.; Bai, P. Poly(ADP-ribose) polymerase-2: Emerging transcriptional roles of a DNA-repair protein. Cell. Mol. Life Sci. 2012, 69, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Virág, L.; Szabó, C. The Therapeutic Potential of Poly(ADP-Ribose) Polymerase Inhibitors. Pharmacol. Rev. 2002, 54, 375–429. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bennici, E.; Novelli, F.; Pioli, C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology 2013, 139, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Sishi, B.J.; Engelbrecht, A.M. Tumor necrosis factor alpha (TNF-alpha) inactivates the PI3-kinase/PKB pathway and induces atrophy and apoptosis in L6 myotubes. Cytokine 2011, 54, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; Rovesta, C.; Ferretti, M. Striated muscle fiber apoptosis after experimental tendon lesion in a rat model. J. Anat. 2012, 221, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.L.; Hueber, A.J.; Reilly, J.H.; Xu, Y.; Fazzi, U.G.; Murrell, G.A.C.; McInnes, I.B. Inflammation is present in early human tendinopathy. Am. J. Sports Med. 2010, 38, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Malhotra, S.; Kumar, A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 2008, 86, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Szántó, M.; Brunyánszki, A.; Márton, J.; Vámosi, G.; Nagy, L.; Fodor, T.; Kiss, B.; Virág, L.; Gergely, P.; Bai, P. Deletion of PARP-2 induces hepatic cholesterol accumulation and decrease in HDL levels. Biochim. Biophys. Acta 2014, 1842, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Canto, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012, 16, 290–295. [Google Scholar] [CrossRef]
- Kuenzler, M.B.; Nuss, K.; Karol, A.; Schär, M.O.; Hottiger, M.; Raniga, S.; Kenkel, D.; von Rechenberg, B.; Zumstein, M.A. Neer Award 2016: Reduced muscle degeneration and decreased fatty infiltration after rotator cuff tear in a poly(ADP-ribose) polymerase 1 (PARP-1) knock-out mouse model. J. Shoulder Elb. Surg. 2017, 26, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Pirinen, E.; Cantó, C.; Jo, Y.S.; Morato, L.; Zhang, H.; Menzies, K.J.; Williams, E.G.; Mouchiroud, L.; Moullan, N.; Hagberg, C.; et al. Pharmacological Inhibition of Poly(ADP-Ribose) Polymerases Improves Fitness and Mitochondrial Function in Skeletal Muscle. Cell Metab. 2014, 19, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, I.; Graziani, G. Role of BRCA Mutations in Cancer Treatment with Poly(ADP-ribose) Polymerase (PARP) Inhibitors. Cancers 2018, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.-Y.N.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Morris, J.; Teicher, B.; Doroshow, J.H.; Pommier, Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014, 13, 433–443. [Google Scholar] [CrossRef]
- Meyer, D.C.; Gerber, C.; Von Rechenberg, B.; Wirth, S.H.; Farshad, M. Amplitude and strength of muscle contraction are reduced in experimental tears of the rotator cuff. Am. J. Sports Med. 2011, 39, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.; Meyer, D.C.; Frey, E.; von Rechenberg, B.; Hoppeler, H.; Frigg, R.; Jost, B.; Zumstein, M.A. Neer Award 2007: Reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J. Shoulder Elb. Surg. 2009, 18, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.; Meyer, D.C.; Flück, M.; Valdivieso, P.; von Rechenberg, B.; Benn, M.C.; Wieser, K. Muscle Degeneration Associated with Rotator Cuff Tendon Release and/or Denervation in Sheep. Am. J. Sports Med. 2017, 45, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.C.; Hoppeler, H.; von Rechenberg, B.; Gerber, C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J. Orthop. Res. 2004, 22, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Flück, M.; Valdivieso, P.; Ruoss, S.; von Rechenberg, B.; Benn, M.C.; Meyer, D.C.; Wieser, K.; Gerber, C. Neurectomy preserves fast fibers when combined with tenotomy of infraspinatus muscle via upregulation of myogenesis. Muscle Nerve 2019, 59, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.R.; Liu, X.; Lee, L.; Laron, D.; Ning, A.Y.; Kim, H.T.; Feeley, B.T. TGF-β small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS ONE 2016, 11, e0155486. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms Modulating Skeletal Muscle Phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [PubMed]
- Beeler, S.; Ek, E.T.H.; Gerber, C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J. Shoulder Elb. Surg. 2013, 22, 1537–1546. [Google Scholar] [CrossRef]
- Gerber, C.; Meyer, D.C.; Nuss, K.M.; Farshad, M. Anabolic steroids reduce muscle damage caused by rotator cuff tendon release in an experimental study in rabbits. J. Bone Jt. Surg. Am. 2011, 93, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.C.; Lidov, H.G.; Shoelson, S.E. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Dawson, T.M.; Dawson, V.L. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci. 2004, 25, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Brocca, L.; Toniolo, L.; Reggiani, C.; Bottinelli, R.; Sandri, M.; Pellegrino, M.A. FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension. J. Physiol. 2017, 595, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.-H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin–proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef] [PubMed]
- O’neill, B.T.; Bhardwaj, G.; Penniman, C.M.; Krumpoch, M.T.; Beltran, P.A.S.; Klaus, K.; Poro, K.; Li, M.; Pan, H.; Dreyfuss, J.M.; et al. FoxO Transcription Factors Are Critical Regulators of Diabetes-Related Muscle Atrophy. Diabetes 2019, 68, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Perdiguero, E.; Serrano, A.L.; Sousa-Victor, P.; Ortet, L.; Jardí, M.; Budanov, A.V.; Garcia-Prat, L.; Sandri, M.; Thomson, D.M.; et al. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nat. Commun. 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, J.-I.; Daitoku, H.; Yoshimochi, K.; Miwa, M.; Fukamizu, A. Regulation of FOXO1-mediated transcription and cell proliferation by PARP-1. Biochem. Biophys. Res. Commun. 2009, 382, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Pallafacchina, G.; Calabria, E.; Serrano, A.L.; Kalhovde, J.M.; Schiaffino, S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. USA 2002, 99, 9213–9218. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Serrano, A.L.; Calabria, E.; Pallafacchina, G.; Lomo, T.; Schiaffino, S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat. Cell Biol. 2000, 2, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Canato, M.; Agatea, L.; Toniolo, L.; Mammucari, C.; Masiero, E.; Abraham, R.; Sandri, M.; Schiaffino, S.; Reggiani, C. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J. 2009, 23, 3896–3905. [Google Scholar] [CrossRef] [PubMed]
- Tapodi, A.; Bognar, Z.; Szabo, C.; Gallyas, F.; Sumegi, B.; Hocsak, E. PARP inhibition induces Akt-mediated cytoprotective effects through the formation of a mitochondria-targeted phospho-ATM-NEMO-Akt-mTOR signalosome. Biochem. Pharmacol. 2019, 162, 98–108. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Cell Physiol. 2009, 296, C1248-57. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Weeks, K.L.; Thomson, R.E.; Sepulveda, P.V.; Beyer, C.; Qian, H.; Chen, J.L.; Allen, J.M.; Lancaster, G.I.; Febbraio, M.A.; et al. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J. Cell Biol. 2012, 197, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Lönn, P.; van der Heide, L.P.; Dahl, M.; Hellman, U.; Heldin, C.-H.; Moustakas, A. PARP-1 Attenuates Smad-Mediated Transcription. Mol. Cell 2010, 40, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.A.; Gibbons, M.C.; Sato, E.; Lane, J.G.; Ward, S.R.; Engler, A.J. Epimuscular Fat in the Human Rotator Cuff Is a Novel Beige Depot. Stem Cells Transl. Med. 2015, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.R.; Gupta, R. Mechanisms of fatty degeneration in massive rotator cuff tears. J. Shoulder Elb. Surg. 2012, 21, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, J.Y.; Oh, K.S.; Chung, S.W. Fatty acid-binding protein 4 regulates fatty infiltration after rotator cuff tear by hypoxia-inducible factor 1 in mice. J. Cachexia Sarcopenia Muscle 2017, 8, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Erener, S.; Hesse, M.; Kostadinova, R.; Hottiger, M.O. Poly(ADP-Ribose)Polymerase-1 (PARP1) Controls Adipogenic Gene Expression and Adipocyte Function. Mol. Endocrinol. 2012, 26, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Erener, S.; Mirsaidi, A.; Hesse, M.; Tiaden, A.N.; Ellingsgaard, H.; Kostadinova, R.; Donath, M.Y.; Richards, P.J.; Hottiger, M.O. ARTD1 deletion causes increased hepatic lipid accumulation in mice fed a high-fat diet and impairs adipocyte function and differentiation. FASEB J. 2012, 26, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Hottiger, M.O. A Role of Poly (ADP-Ribose) Polymerase in NF-B Transcriptional Activation. Biol. Chem. 1999, 380, 953–959. [Google Scholar] [CrossRef]
- Hunter, R.B.; Kandarian, S.C. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J. Clin. Investig. 2004, 114, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Beelen, M.; Savelberg, H.H.; Meijer, K.; Kuipers, H.; van Loon, L.J. Characteristics of Muscle Fiber Type Are Predictive of Skeletal Muscle Mass and Strength in Elderly Men. J. Am. Geriatr. Soc. 2010, 58, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.G.; Flück, M.; Borbas, P.; Valdivieso, P.; Toigo, M.; Egli, F.; Joshy, J.; Filli, L.; Snedeker, J.G.; Gerber, C.; et al. Structural Musculotendinous Parameters That Predict Failed Tendon Healing After Rotator Cuff Repair. Orthop. J. Sports Med. 2023, 11, 23259671231196875. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olthof, M.G.L.; Hasler, A.; Valdivieso, P.; Flück, M.; Gerber, C.; Gehrke, R.; Klein, K.; von Rechenberg, B.; Snedeker, J.G.; Wieser, K. Poly(ADP-Ribose) Polymerases-Inhibitor Talazoparib Inhibits Muscle Atrophy and Fatty Infiltration in a Tendon Release Infraspinatus Sheep Model: A Pilot Study. Metabolites 2024, 14, 187. https://doi.org/10.3390/metabo14040187
Olthof MGL, Hasler A, Valdivieso P, Flück M, Gerber C, Gehrke R, Klein K, von Rechenberg B, Snedeker JG, Wieser K. Poly(ADP-Ribose) Polymerases-Inhibitor Talazoparib Inhibits Muscle Atrophy and Fatty Infiltration in a Tendon Release Infraspinatus Sheep Model: A Pilot Study. Metabolites. 2024; 14(4):187. https://doi.org/10.3390/metabo14040187
Chicago/Turabian StyleOlthof, Maurits G. L., Anita Hasler, Paola Valdivieso, Martin Flück, Christian Gerber, Rieke Gehrke, Karina Klein, Brigitte von Rechenberg, Jess G. Snedeker, and Karl Wieser. 2024. "Poly(ADP-Ribose) Polymerases-Inhibitor Talazoparib Inhibits Muscle Atrophy and Fatty Infiltration in a Tendon Release Infraspinatus Sheep Model: A Pilot Study" Metabolites 14, no. 4: 187. https://doi.org/10.3390/metabo14040187
APA StyleOlthof, M. G. L., Hasler, A., Valdivieso, P., Flück, M., Gerber, C., Gehrke, R., Klein, K., von Rechenberg, B., Snedeker, J. G., & Wieser, K. (2024). Poly(ADP-Ribose) Polymerases-Inhibitor Talazoparib Inhibits Muscle Atrophy and Fatty Infiltration in a Tendon Release Infraspinatus Sheep Model: A Pilot Study. Metabolites, 14(4), 187. https://doi.org/10.3390/metabo14040187





