Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn’s Disease and Ulcerative Colitis with Non-Inflammatory Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection and Preparation
2.3. Statistical Analysis
3. Results
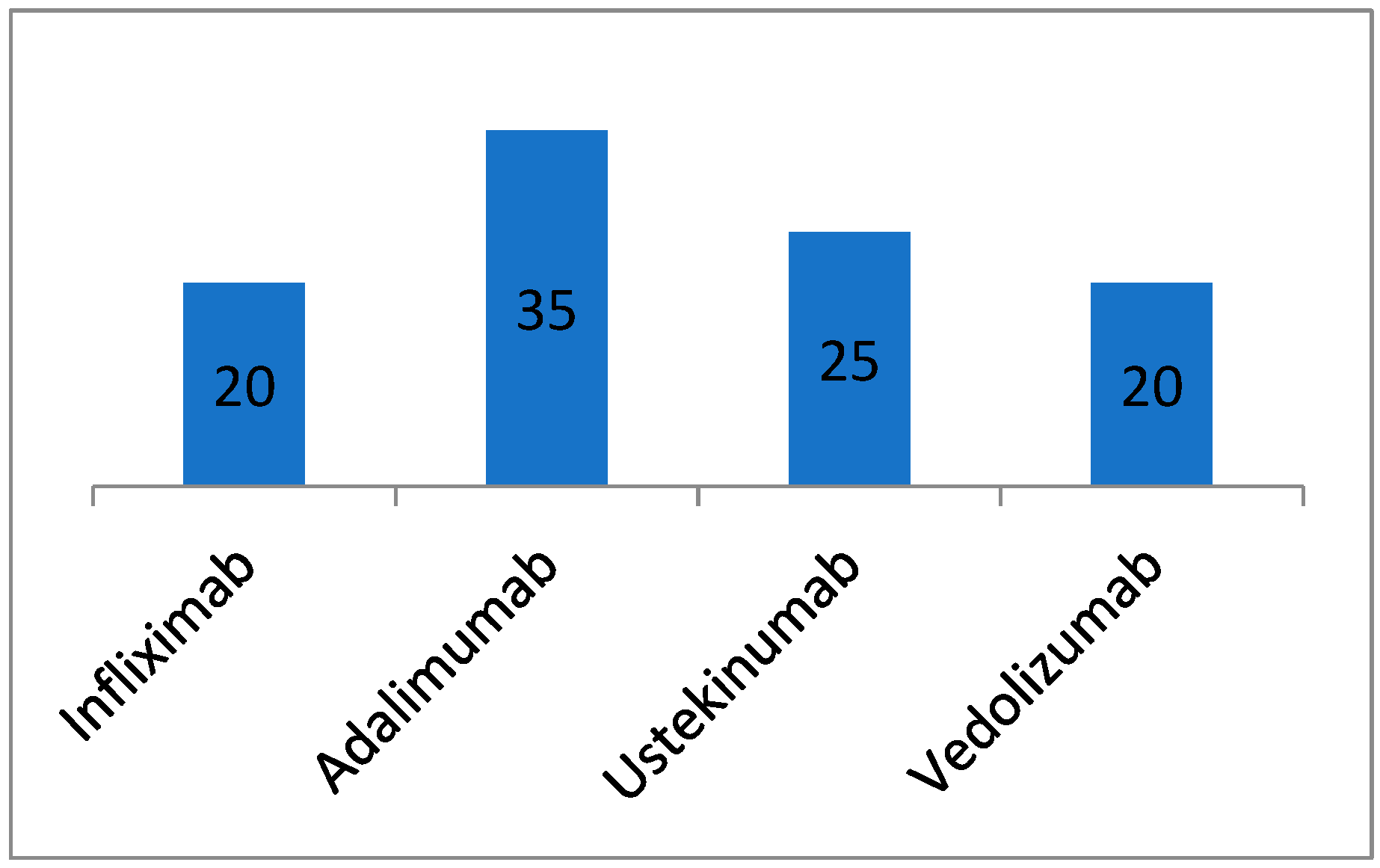
3.1. Demographic and Clinical Characteristics
3.2. GDF-15
3.3. Conventional Markers of Inflammation
4. Discussion
5. Study Limitations
- This was a small, single-center, cross-sectional study designed to compare the plasma GDF-15 levels between patients with CD and UC receiving biologic therapy and a control group.
- The study primarily focused on patients with severe IBD undergoing biologic therapy, which may limit the generalizability of the findings to patients with less severe forms of IBD or those not receiving biologic treatment.
- While the study identified associations between plasma GDF-15 levels and severe IBD, the clinical significance of these findings in terms of diagnosis, prognosis, or treatment remains to be fully elucidated.
6. Conclusions
- Patients with severe IBD, including CD and UC, undergoing biologic therapy, exhibited significantly higher levels of GDF-15 in their plasma compared to individuals without chronic inflammatory diseases.
- There was a positive correlation between elevated GDF-15 levels and other markers of inflammation, such as age, fibrinogen, and IL-6, indicating that GDF-15 may serve as a complementary marker in assessing the disease status in IBD patients.
- Further research is warranted to uncover the specific mechanisms underlying GDF-15 dysregulation in IBD and to explore its potential as a biomarker for disease activity and the treatment response.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a Novel Macrophage Inhibitory Cytokine, Is a Divergent Member of the TGF-β Superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Lawton, L.N.; Bonaldo, M.D.F.; Jelenc, P.C.; Qiu, L.; Baumes, S.A.; Marcelino, R.A.; De Jesus, G.M.; Wellington, S.; Knowles, J.A.; Warburton, D.; et al. Identification of a Novel Member of the TGF-Beta Superfamily Highly Expressed in Human Placenta. Gene 1997, 203, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hromas, R.; Hufford, M.; Sutton, J.; Xu, D.; Li, Y.; Lu, L. PLAB, a Novel Placental Bone Morphogenetic Protein. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1997, 1354, 40–44. [Google Scholar] [CrossRef]
- Baek, S.J.; Horowitz, J.M.; Eling, T.E. Molecular Cloning and Characterization of Human Nonsteroidal Anti-Inflammatory Drug-Activated Gene Promoter. J. Biol. Chem. 2001, 276, 33384–33392. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.J.; Blobe, G.C. Role of Transforming Growth Factor-β Superfamily Signaling Pathways in Human Disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2008, 1782, 197–228. [Google Scholar] [CrossRef] [PubMed]
- Santibañez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β/TGF-β Receptor System and Its Role in Physiological and Pathological Conditions. Clin. Sci. 2011, 121, 233–251. [Google Scholar] [CrossRef] [PubMed]
- De Caestecker, M. The Transforming Growth Factor-β Superfamily of Receptors. Cytokine Growth Factor Rev. 2004, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massagué, J. Cytostatic and Apoptotic Actions of TGF-β in Homeostasis and Cancer. Nat. Rev. Cancer 2003, 3, 807–820. [Google Scholar] [CrossRef]
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F.; Ross, R. TGF-β Induces Bimodal Proliferation of Connective Tissue Cells via Complex Control of an Autocrine PDGF Loop. Cell 1990, 63, 515–524. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming Growth Factor-β in Stem Cells and Tissue Homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an Update of the Physiological and Pathological Roles It Plays: A Review. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging Biology and Therapeutic Applications for Obesity and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Böttner, M.; Laaff, M.; Schechinger, B.; Rappold, G.; Unsicker, K.; Suter-Crazzolara, C. Characterization of the Rat, Mouse, and Human Genes of Growth/Differentiation Factor-15/Macrophage Inhibiting Cytokine-1 (GDF-15/MIC-1). Gene 1999, 237, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Divergent Molecular Mechanisms Underlying the Pleiotropic Functions of Macrophage Inhibitory Cytokine-1 in Cancer. J. Cell. Physiol. 2010, 224, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Koopmann, J.; Buckhaults, P.; Brown, D.A.; Zahurak, M.L.; Sato, N.; Fukushima, N.; Sokoll, L.J.; Chan, D.W.; Yeo, C.J.; Hruban, R.H.; et al. Serum Macrophage Inhibitory Cytokine 1 as a Marker of Pancreatic and Other Periampullary Cancers. Clin. Cancer Res. 2004, 10, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Strelau, J.; Strzelczyk, A.; Rusu, P.; Bendner, G.; Wiese, S.; Diella, F.; Altick, A.L.; Von Bartheld, C.S.; Klein, R.; Sendtner, M.; et al. Progressive Postnatal Motoneuron Loss in Mice Lacking GDF-15. J. Neurosci. 2009, 29, 13640–13648. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.R.; Kelly, J.A.; Shim, M.; Huffer, W.E.; Nordeen, S.K.; Baek, S.J.; Eling, T.E.; Lucia, M.S. Prostate Derived Factor in Human Prostate Cancer Cells: Gene Induction by Vitamin D via a P53-dependent Mechanism and Inhibition of Prostate Cancer Cell Growth. J. Cell. Physiol. 2006, 208, 566–574. [Google Scholar] [CrossRef]
- Yokoyama-Kobayashi, M.; Saeki, M.; Sekine, S.; Kato, S. Human cDNA Encoding a Novel TGF- Superfamily Protein Highly Expressed in Placenta. J. Biochem. 1997, 122, 622–626. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.H.; Han, D.H.; Jo, Y.S.; Lee, Y.; Lee, M.-S. Growth Differentiation Factor 15 Ameliorates Nonalcoholic Steatohepatitis and Related Metabolic Disorders in Mice. Sci. Rep. 2018, 8, 6789. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; Kiens, B.; Richter, E.A. Exercise Increases Circulating GDF15 in Humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Tsai, V.W.-W.; Macia, L.; Feinle-Bisset, C.; Manandhar, R.; Astrup, A.; Raben, A.; Lorenzen, J.K.; Schmidt, P.T.; Wiklund, F.; Pedersen, N.L.; et al. Serum Levels of Human MIC-1/GDF15 Vary in a Diurnal Pattern, Do Not Display a Profile Suggestive of a Satiety Factor and Are Related to BMI. PLoS ONE 2015, 10, e0133362. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Adler, J.; Chachu, K.A.; Nguyen, N.H.; Siddique, S.M.; Weiss, J.M.; Sultan, S.; Velayos, F.S.; Cohen, B.L.; Singh, S. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease. Gastroenterology 2023, 165, 1367–1399. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Pare, G.; Hess, S.; Ford, R.J.; Sjaarda, J.; Raman, K.; McQueen, M.; Lee, S.; Haenel, H.; Steinberg, G.R. Growth Differentiation Factor 15 as a Novel Biomarker for Metformin. Diabetes Care 2017, 40, 280–283. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Papadakis, M.; Nadwa, E.H.; Albogami, S.M.; Alorabi, M.; Saad, H.M.; Batiha, G.E. Metformin and Growth Differentiation Factor 15 (GDF15) in Type 2 Diabetes Mellitus: A Hidden Treasure. J. Diabetes 2022, 14, 806–814. [Google Scholar] [CrossRef]
- Welsh, J.B.; Sapinoso, L.M.; Kern, S.G.; Brown, D.A.; Liu, T.; Bauskin, A.R.; Ward, R.L.; Hawkins, N.J.; Quinn, D.I.; Russell, P.J.; et al. Large-Scale Delineation of Secreted Protein Biomarkers Overexpressed in Cancer Tissue and Serum. Proc. Natl. Acad. Sci. USA 2003, 100, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.-W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-Induced Anorexia and Weight Loss Are Mediated by the TGF-β Superfamily Cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef]
- He, Y.-W.; He, C.-S. Association of Growth and Differentiation Factor 15 in Rheumatoid Arthritis. J. Inflamm. Res. 2022, 15, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Talaee, R.; Zaker, S.F.; Nikoueinejad, H. Investigating the Correlation between Growth Differentiation Factor 15 Serum Level and Its Gene Expression with Psoriasis and Its Severity. Iran. J. Allergy Asthma Immunol. 2021, 20, 593–599. [Google Scholar] [CrossRef]
- Elbarky, E.M.; Hussien, M.I.; Elgazzar, N.M.; Mabrouk, M.M.; Elsaadany, H.M. Serum Growth Differentiation Factor-15 (GDF-15) Level in Behcet’s Disease Patients: Relation to Clinical Characteristics, Musculoskeletal Ultrasound Findings and Disease Activity. Egypt. Rheumatol. 2021, 43, 261–266. [Google Scholar] [CrossRef]
- Fuchs, T.; Trollor, J.N.; Crawford, J.; Brown, D.A.; Baune, B.T.; Samaras, K.; Campbell, L.; Breit, S.N.; Brodaty, H.; Sachdev, P.; et al. Macrophage Inhibitory Cytokine-1 Is Associated with Cognitive Impairment and Predicts Cognitive Decline—The Sydney Memory and Aging Study. Aging Cell 2013, 12, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.L.; Hilal, S.; Chong, J.P.C.; Ng, Y.X.; Liew, O.W.; Xu, X.; Ikram, M.K.; Venketasubramanian, N.; Richards, A.M.; Lai, M.K.P.; et al. Growth Differentiation Factor-15 and White Matter Hyperintensities in Cognitive Impairment and Dementia. Medicine 2016, 95, e4566. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallaoui, N.; Claggett, B.; Zile, M.R.; McMurray, J.J.V.; O’Meara, E.; Packer, M.; Prescott, M.F.; Swedberg, K.; Solomon, S.D.; Rouleau, J.L.; et al. Growth Differentiation Factor-15 Is Not Modified by Sacubitril/Valsartan and Is an Independent Marker of Risk in Patients with Heart Failure and Reduced Ejection Fraction: The PARADIGM-HF Trial. Eur. J. Heart Fail. 2018, 20, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; Bjorklund, E.; Olofsson, S.; Lindahl, B.; Allhoff, T.; Peter, T.; Tongers, J.; Wollert, K.C.; Wallentin, L. Growth-Differentiation Factor-15 Improves Risk Stratification in ST-Segment Elevation Myocardial Infarction. Eur. Heart J. 2007, 28, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Peter, T.; Olofsson, S.; James, S.; Johnston, N.; Lindahl, B.; Horn-Wichmann, R.; Brabant, G.; Simoons, M.L.; et al. Prognostic Value of Growth-Differentiation Factor-15 in Patients With Non–ST-Elevation Acute Coronary Syndrome. Circulation 2007, 115, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, D.; James, S.K.; Gabrysch, K.; Storey, R.F.; Himmelmann, A.; Cannon, C.P.; Mahaffey, K.W.; Steg, P.G.; Held, C.; Siegbahn, A.; et al. Association of Multiple Biomarkers With Risk of All-Cause and Cause-Specific Mortality After Acute Coronary Syndromes: A Secondary Analysis of the PLATO Biomarker Study. JAMA Cardiol. 2018, 3, 1160. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Cai, Y.-L.; Li, H.-Y.; Hao, B.-C.; Chen, J.-Q.; Liu, H.-B. Growth Differentiation Factor-15 Is Associated with Cardiovascular Outcomes in Patients with Coronary Artery Disease. Cardiovasc. Diabetol. 2020, 19, 120. [Google Scholar] [CrossRef]
- Sharma, A.; Hijazi, Z.; Andersson, U.; Al-Khatib, S.M.; Lopes, R.D.; Alexander, J.H.; Held, C.; Hylek, E.M.; Leonardi, S.; Hanna, M.; et al. Use of Biomarkers to Predict Specific Causes of Death in Patients With Atrial Fibrillation: Insights From the ARISTOTLE Trial. Circulation 2018, 138, 1666–1676. [Google Scholar] [CrossRef]
- Xu, J.; Kimball, T.R.; Lorenz, J.N.; Brown, D.A.; Bauskin, A.R.; Klevitsky, R.; Hewett, T.E.; Breit, S.N.; Molkentin, J.D. GDF15/MIC-1 Functions As a Protective and Antihypertrophic Factor Released From the Myocardium in Association With SMAD Protein Activation. Circ. Res. 2006, 98, 342–350. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Zügel, S.; Thogersen, J.; Walter, S.A.; Haberkorn, U.; Strelau, J.; Kinscherf, R. Growth Differentiation Factor-15 Deficiency Inhibits Atherosclerosis Progression by Regulating Interleukin-6–Dependent Inflammatory Response to Vascular Injury. J. Am. Heart Assoc. 2012, 1, e002550. [Google Scholar] [CrossRef]
- Preusch, M.R.; Baeuerle, M.; Albrecht, C.; Blessing, E.; Bischof, M.; Katus, H.A.; Bea, F. GDF-15 Protects from Macrophage Accumulation in a Mousemodel of Advanced Atherosclerosis. Eur. J. Med. Res. 2013, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Johnen, H.; Kuffner, T.; Brown, D.A.; Wu, B.J.; Stocker, R.; Breit, S.N. Increased Expression of the TGF-b Superfamily Cytokine MIC-1/GDF15 Protects ApoE−/− Mice from the Development of Atherosclerosis. Cardiovasc. Pathol. 2012, 21, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Robinson-Cohen, C.; Smith, M.R.; Bellovich, K.A.; Bhat, Z.Y.; Bobadilla, M.; Brosius, F.; De Boer, I.H.; Essioux, L.; Formentini, I.; et al. Growth Differentiation Factor–15 and Risk of CKD Progression. J. Am. Soc. Nephrol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Shin, H.-W.; Chun, Y.-S.; Park, J.-W. CST3 and GDF15 Ameliorate Renal Fibrosis by Inhibiting Fibroblast Growth and Activation. Biochem. Biophys. Res. Commun. 2018, 500, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The Metabolic Effects of GDF15 Are Mediated by the Orphan Receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.-N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL Is the Receptor for GDF15 and the Ligand Promotes Weight Loss in Mice and Nonhuman Primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.-C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL Is the Receptor for GDF15 and Is Required for the Anti-Obesity Effects of the Ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Bauskin, A.R.; Brown, D.A.; Kuffner, T.; Johnen, H.; Luo, X.W.; Hunter, M.; Breit, S.N. Role of Macrophage Inhibitory Cytokine-1 in Tumorigenesis and Diagnosis of Cancer. Cancer Res. 2006, 66, 4983–4986. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Pothuraju, R.; Khan, P.; Sharma, G.; Muniyan, S.; Seshacharyulu, P.; Jain, M.; Nasser, M.W.; Batra, S.K. Pathophysiological Role of Growth Differentiation Factor 15 (GDF15) in Obesity, Cancer, and Cachexia. Cytokine Growth Factor Rev. 2022, 64, 71–83. [Google Scholar] [CrossRef]
- Corre, J.; Hébraud, B.; Bourin, P. Concise Review: Growth Differentiation Factor 15 in Pathology: A Clinical Role? Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef]
- Wallin, U.; Glimelius, B.; Jirström, K.; Darmanis, S.; Nong, R.Y.; Pontén, F.; Johansson, C.; Påhlman, L.; Birgisson, H. Growth Differentiation Factor 15: A Prognostic Marker for Recurrence in Colorectal Cancer. Br. J. Cancer 2011, 104, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Vocka, M.; Langer, D.; Fryba, V.; Petrtyl, J.; Hanus, T.; Kalousova, M.; Zima, T.; Petruzelka, L. Growth/Differentiation Factor 15 (GDF-15) as New Potential Serum Marker in Patients with Metastatic Colorectal Cancer. Cancer Biomark. 2018, 21, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Esalatmanesh, K.; Fayyazi, H.; Esalatmanesh, R.; Khabbazi, A. The Association between Serum Levels of Growth Differentiation Factor-15 and Rheumatoid Arthritis Activity. Int. J. Clin. Pract. 2020, 74, e13564. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.E.; Elhanafy, M.S.; Eigela, S.E.; Nasr, H.E.; ELgendy, M.E. Serum GDF-15 Level in Rheumatoid Arthritis and Relation to Disease Activity and Severity. Benha J. Appl. Sci. 2020, 5, 131–134. [Google Scholar] [CrossRef]
- Taşolar, M.K.; Erfan, G.; Raimoğlu, O.; Albayrak, H.; Yanık, M.E. Role of GDF-15 as an Inflammatory Marker in Patients with Psoriasis Vulgaris. Arch. Turk. Dermatol. Venerol. 2021, 55, 184–188. [Google Scholar] [CrossRef]
- Sarıyıldız, M.A.; Yazmalar, L.; Batmaz, İ.; Alpaycı, M.; Burkan, Y.K.; Sula, B.; Kaplan, İ.; Yıldız, M.; Akar, Z.A.; Bozkurt, M. Serum GDF -15 Level in Behçet’s Disease: Relationships between Disease Activity and Clinical Parameters. Int. J. Dermatol. 2016, 55, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Meadows, C.A.; Risbano, M.G.; Zhang, L.; Geraci, M.W.; Tuder, R.M.; Collier, D.H.; Bull, T.M. Increased Expression of Growth Differentiation Factor-15 in Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. Chest 2011, 139, 994–1002. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum Biomarkers for Inflammatory Bowel Disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef]
- Con, D.; Andrew, B.; Nicolaides, S.; Van Langenberg, D.R.; Vasudevan, A. Biomarker Dynamics during Infliximab Salvage for Acute Severe Ulcerative Colitis: C-Reactive Protein (CRP)-Lymphocyte Ratio and CRP-Albumin Ratio Are Useful in Predicting Colectomy. Intestig. Res. 2022, 20, 101–113. [Google Scholar] [CrossRef]

| Characteristics | IBD Patients | Controls | p-Value | Test |
|---|---|---|---|---|
| n = 100 | n = 50 | |||
| Age (y) | 43 (25–67) | 43 (18–61) | 0.829 | U-test |
| Sex (male) | 60 (60%) | 21 (42%) | 0.037 | χ2 |
| Sex (female) | 40 (40%) | 29 (58%) | 0.037 | χ2 |
| Current smoker | 18 (18%) | 4 (8%) | 0.103 | χ2 |
| Body mass index | 25 | |||
| Total protein (g/L) | 73.6 | 71 | 0.003 | t-test |
| Albumin (g/L) | 44.2 | 44.6 | 0.449 | t-test |
| Abdominal surgery | 71 (71%) | |||
| Disease duration (y) | 13.4 |
| CD | n = 77 |
|---|---|
| inflammatory | 38 |
| stricturing | 10 |
| fistulizing | 29 |
| UC | n = 23 |
| extensive colitis | 17 |
| left-sided colitis | 5 |
| Type of Treatment | Number of Patients |
|---|---|
| biologic therapy | 100 (100%) |
| 5-aminosalicylic acid (5-ASA) | 63 (63%) |
| azathioprine | 8 (8%) |
| methotrexate | 1 (1%) |
| corticoids | 2 (2%) |
| Concomitant chronic inflammatory diseases | Number of patients |
| autoimmune thyroiditis | 2 |
| celiac disease | 1 |
| psoriasis | 5 |
| hidradenitis suppurativa | 2 |
| atopic eczema | 2 |
| antiphospholipid syndrome | 1 |
| Median | Mean | Std. Deviation | Minimum | Maximum | ||
|---|---|---|---|---|---|---|
| GDF-15 (ng/L) | Control | 527 | 569.34 | 188.655 | 400 | 1171 |
| IBD | 751 | 943.23 | 608.579 | 400 | 3406 | |
| Leukocytes (×109/L) | Control | 6.095 | 6.338 | 1.703 | 3.44 | 11.58 |
| IBD | 6.84 | 7.109 | 1.876 | 3 | 13.17 | |
| hsCRP (µg/L) | Control | 817.55 | 2113.322 | 2611.358 | 25 | 9461 |
| IBD | 1511.7 | 2977.522 | 3811.365 | 87.6 | 24,497 | |
| IL-6 (ng/L) | Control | 1.5 | 1.776 | 0.617 | 1.5 | 4.3 |
| IBD | 1.6 | 2.751 | 2.556 | 1.5 | 18 | |
| Fibrinogen (g/L) | Control | 2.62 | 2.668 | 0.483 | 1.77 | 4.04 |
| IBD | 2.82 | 2.861 | 0.474 | 1.8 | 4.11 |
| Mann-Whitney U Test. | ||
|---|---|---|
| W | p | |
| GDF-15 | 1263.5 | <0.001 |
| Leukocytes | 1867.5 | 0.012 |
| hsCRP | 1970 | 0.035 |
| IL-6 | 1825.5 | 0.003 |
| Fibrinogen | 1902.5 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučerka, O.; Blahutová, M.; Kosek, V.; Mináriková, P.; Horáček, J.M.; Urbánek, P.; Malý, M. Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn’s Disease and Ulcerative Colitis with Non-Inflammatory Controls. Metabolites 2024, 14, 185. https://doi.org/10.3390/metabo14040185
Kučerka O, Blahutová M, Kosek V, Mináriková P, Horáček JM, Urbánek P, Malý M. Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn’s Disease and Ulcerative Colitis with Non-Inflammatory Controls. Metabolites. 2024; 14(4):185. https://doi.org/10.3390/metabo14040185
Chicago/Turabian StyleKučerka, Ondřej, Marie Blahutová, Vít Kosek, Petra Mináriková, Jan M. Horáček, Petr Urbánek, and Martin Malý. 2024. "Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn’s Disease and Ulcerative Colitis with Non-Inflammatory Controls" Metabolites 14, no. 4: 185. https://doi.org/10.3390/metabo14040185
APA StyleKučerka, O., Blahutová, M., Kosek, V., Mináriková, P., Horáček, J. M., Urbánek, P., & Malý, M. (2024). Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn’s Disease and Ulcerative Colitis with Non-Inflammatory Controls. Metabolites, 14(4), 185. https://doi.org/10.3390/metabo14040185










