Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures
Abstract
1. Introduction
2. Experimental Design
2.1. Plant Material
2.2. Extraction Protocols of Diverse Class of Compounds
2.2.1. Extraction of Alkaloids
2.2.2. Extraction of Cardiac Glycosides
2.2.3. Extraction of Pregnane Glycosides
2.2.4. Extraction of Terpenoids
2.2.5. Extraction of Flavonoids
2.2.6. Extraction of Phenolic Compounds
2.3. Characterization of Secondary Metabolites
2.3.1. Liquid Chromatography–Mass Spectrometry (LC-MS)
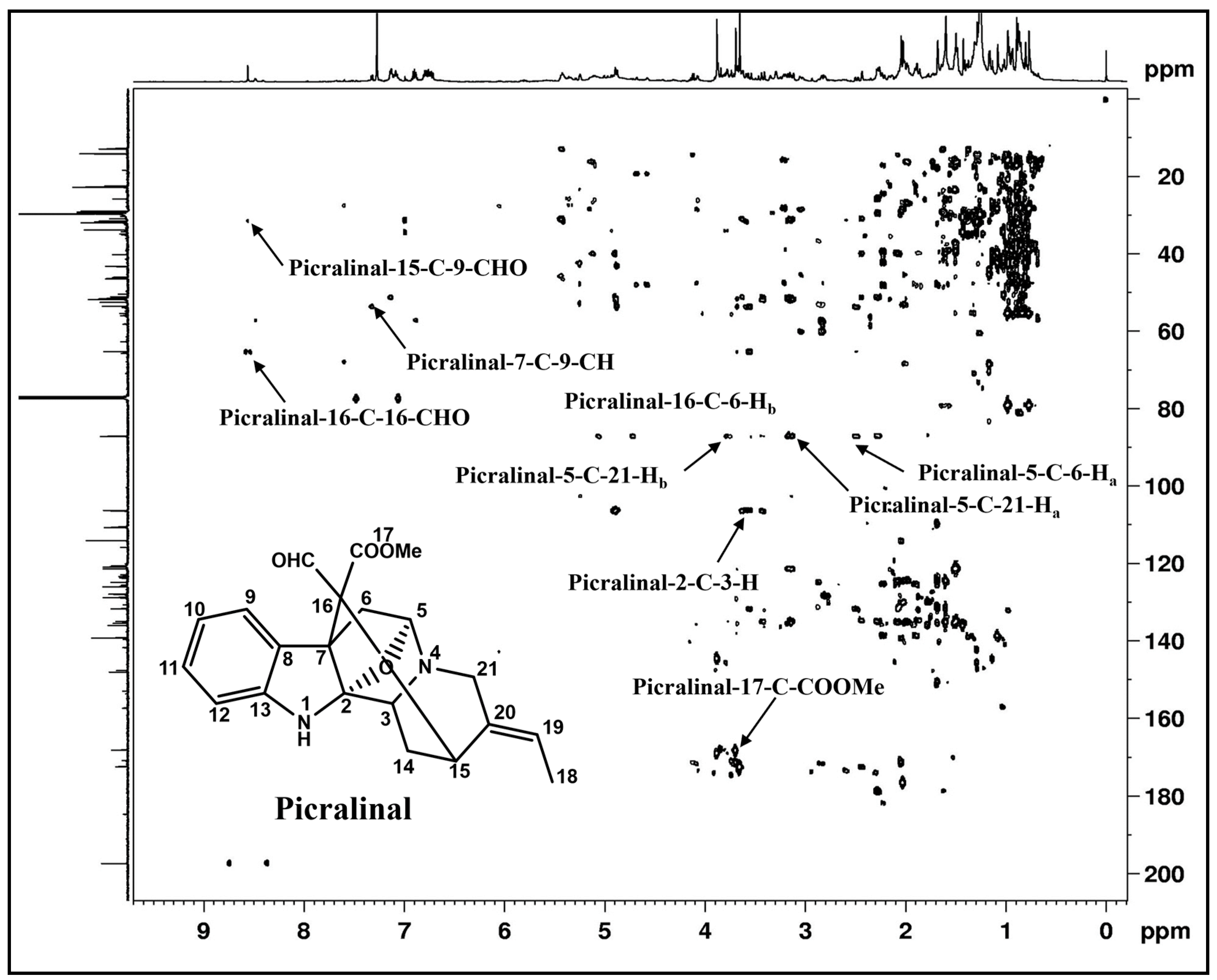
2.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.3. Gas Chromatography–Mass Spectrometry (GC-MS)
3. Results
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.U.; Abdullah; Khan, F.; Niaz, K. Introduction to Natural Products Analysis. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–15. ISBN 978-0-12-816455-6. [Google Scholar]
- Mendoza, N.; Silva, E.M.E. Introduction to Phytochemicals: Secondary Metabolites from Plants with Active Principles for Pharmacological Importance. In Phytochemicals—Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; InTech: London, UK, 2018; ISBN 978-1-78984-377-4. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Abdel-Nasser, A.; Hathout, A.S.; Badr, A.N.; Barakat, O.S.; Fathy, H.M. Extraction and Characterization of Bioactive Secondary Metabolites from Lactic Acid Bacteria and Evaluating Their Antifungal and Antiaflatoxigenic Activity. Biotechnol. Rep. 2023, 38, e00799. [Google Scholar] [CrossRef]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. ISBN 978-0-12-820876-2. [Google Scholar]
- Joshi, T.; Jain, T.; Mahar, R.; Singh, S.K.; Srivastava, P.; Shukla, S.K.; Mishra, D.K.; Bhatta, R.S.; Banerjee, D.; Kanojiya, S. Pyranocarbazoles from Murraya koenigii (L.) Spreng. as Antimicrobial Agents. Nat. Prod. Res. 2018, 32, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mahar, R.; Hasanain, M.; Shukla, S.K.; Sarkar, J.; Rameshkumar, K.B.; Kumar, B. Rapid Screening and Quantitative Determination of Bioactive Compounds from Fruit Extracts of Myristica Species and Their in Vitro Antiproliferative Activity. Food Chem. 2016, 211, 483–493. [Google Scholar] [CrossRef]
- Mahar, R.; Dixit, S.; Joshi, T.; Kanojiya, S.; Mishra, D.K.; Konwar, R.; Shukla, S.K. Bioactivity Guided Isolation of Oxypregnane-Oligoglycosides (Calotroposides) from the Root Bark of Calotropis Gigantea as Potent Anticancer Agents. RSC Adv. 2016, 6, 104215–104226. [Google Scholar] [CrossRef]
- Fotsing Yannick Stéphane, F.; Kezetas Jean Jules, B.; El-Saber Batiha, G.; Ali, I.; Ndjakou Bruno, L. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicinal Plants; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-275-8. [Google Scholar]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, E.; Salim, K.A.; Lim, L.B.L. Phytochemical Screening, Total Phenolics and Antioxidant Activities of Bark and Leaf Extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ.-Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef]
- Agidew, M.G. Phytochemical Analysis of Some Selected Traditional Medicinal Plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Ngwenya, N.; Mahlambi, P. Methods Optimization and Application: Solid Phase Extraction, Ultrasonic Extraction and Soxhlet Extraction for the Determination of Antiretroviral Drugs in River Water, Wastewater, Sludge, Soil and Sediment. J. Pharm. Biomed. Anal. 2023, 230, 115358. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.O.; Sanches, V.L.; Viganó, J.; De Souza Mesquita, L.M.; De Souza, M.C.; Da Silva, L.C.; Acunha, T.; Faccioli, L.H.; Rostagno, M.A. Integration of Pressurized Liquid Extraction and In-Line Solid-Phase Extraction to Simultaneously Extract and Concentrate Phenolic Compounds from Lemon Peel (Citrus limon L.). Food Res. Int. 2022, 157, 111252. [Google Scholar] [CrossRef]
- Akanda, M.J.H.; Sarker, M.Z.I.; Ferdosh, S.; Manap, M.Y.A.; Ab Rahman, N.N.N.; Ab Kadir, M.O. Applications of Supercritical Fluid Extraction (SFE) of Palm Oil and Oil from Natural Sources. Molecules 2012, 17, 1764–1794. [Google Scholar] [CrossRef]
- Saha, S.; Singh, A.K.; Keshari, A.K.; Raj, V.; Rai, A.; Maity, S. Modern Extraction Techniques for Drugs and Medicinal Agents. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2018; pp. 65–106. ISBN 978-0-12-811521-3. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Linares, G.; Rojas, M.L. Ultrasound-Assisted Extraction of Natural Pigments From Food Processing By-Products: A Review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef]
- Patel, K.; Panchal, N.; Ingle, P. Review of Extraction Techniques Extraction Methods: Microwave, Ultrasonic, Pressurized Fluid, Soxhlet Extraction, Etc. Int. J. Adv. Res. Chem. Sci. 2019, 6, 6–21. [Google Scholar] [CrossRef]
- Salem, M.A.; Perez de Souza, L.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the Context of Plant Natural Products Research: From Sample Preparation to Metabolite Analysis. Metabolites 2020, 10, E37. [Google Scholar] [CrossRef]
- Thomford, N.; Senthebane, D.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Mahar, R.; Manivel, N.; Kanojiya, S.; Mishra, D.K.; Shukla, S.K. Assessment of Tissue Specific Distribution and Seasonal Variation of Alkaloids in Alstonia Scholaris. Metabolites 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mahar, R.; Shukla, S.K.; Kant, R.; Chauhan, P.M.S. Potassium Carbonate Mediated Unusual Transformation of 2,3-Dihydroquinazolinone via Cascade Reaction. Tetrahedron Lett. 2013, 54, 6171–6177. [Google Scholar] [CrossRef]
- Grkovic, T.; Pouwer, R.H.; Vial, M.-L.; Gambini, L.; Noël, A.; Hooper, J.N.A.; Wood, S.A.; Mellick, G.D.; Quinn, R.J. NMR Fingerprints of the Drug-like Natural-Product Space Identify Iotrochotazine A: A Chemical Probe to Study Parkinson’s Disease. Angew. Chem. Int. Ed. 2014, 53, 6070–6074. [Google Scholar] [CrossRef]
- Cai, S.-S.; Short, L.C.; Syage, J.A.; Potvin, M.; Curtis, J.M. Liquid Chromatography–Atmospheric Pressure Photoionization-Mass Spectrometry Analysis of Triacylglycerol Lipids—Effects of Mobile Phases on Sensitivity. J. Chromatogr. A 2007, 1173, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Dighe, S.U.; Mahar, R.; Shukla, S.K.; Kant, R.; Srivastava, K.; Batra, S. Synthesis of S -(−)-5,6-Dihydrocanthin-4-Ones via a Triple Cooperative Catalysis-Mediated Domino Reaction. J. Org. Chem. 2016, 81, 4751–4761. [Google Scholar] [CrossRef]
- Reynolds, W.F. Natural Product Structure Elucidation by NMR Spectroscopy. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 567–596. ISBN 978-0-12-802104-0. [Google Scholar]
- Deschamps, J.R. X-Ray Crystallography of Chemical Compounds. Life Sci. 2010, 86, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Van Asten, A. The Importance of GC and GC-MS in Perfume Analysis. TrAC Trends Anal. Chem. 2002, 21, 698–708. [Google Scholar] [CrossRef]
- Mahar, R.; Ragavan, M.; Chang, M.C.; Hardiman, S.; Moussatche, N.; Behar, A.; Renne, R.; Merritt, M.E. Metabolic Signatures Associated with Oncolytic Myxoma Viral Infections. Sci. Rep. 2022, 12, 12599. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, S.C.; David, V. Derivatization Methods in GC and GC/MS. In Gas Chromatography—Derivatization, Sample Preparation, Application; Kusch, P., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83881-865-4. [Google Scholar]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy Effects of Herb Extracts: Pharmacokinetics and Pharmacodynamic Basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Nandan, S.; Singh, S.K.; Singh, P.; Bajpai, V.; Mishra, A.K.; Joshi, T.; Mahar, R.; Shukla, S.K.; Mishra, D.K.; Kanojiya, S. Quantitative Analysis of Bioactive Carbazole Alkaloids in Murraya koenigii (L.) from Six Different Climatic Zones of India Using UPLC/MS/MS and Their Principal Component Analysis. Chem. Biodivers. 2021, 18, e2100557. [Google Scholar] [CrossRef]
- Singh, Y.; Nimoriya, R.; Rawat, P.; Mishra, D.K.; Kanojiya, S. Structural Analysis of Diastereomeric Cardiac Glycosides and Their Genins Using Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 1205–1214. [Google Scholar] [CrossRef]
- Espejel-Nava, J.A.; Vega-Avila, E.; Alarcon-Aguilar, F.; Contreras-Ramos, A.; Díaz-Rosas, G.; Trejo-Aguilar, G.; Ortega-Camarillo, C. A Phenolic Fraction from Catharanthus roseus L. Stems Decreases Glycemia and Stimulates Insulin Secretion. Evid.-Based Complement. Altern. Med. 2018, 2018, 7191035. [Google Scholar] [CrossRef]
- Jiang, Z.; Kempinski, C.; Chappell, J. Extraction and Analysis of Terpenes/Terpenoids. Curr. Protoc. Plant Biol. 2016, 1, 345–358. [Google Scholar] [CrossRef]
- Feng, T.; Li, Y.; Cai, X.-H.; Gong, X.; Liu, Y.-P.; Zhang, R.-T.; Zhang, X.-Y.; Tan, Q.-G.; Luo, X.-D. Monoterpenoid Indole Alkaloids from Alstonia yunnanensis. J. Nat. Prod. 2009, 72, 1836–1841. [Google Scholar] [CrossRef]
- Cai, X.-H.; Du, Z.-Z.; Luo, X.-D. Unique Monoterpenoid Indole Alkaloids from Alstonia scholaris. Org. Lett. 2007, 9, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, C.L.; Schrader, K.K.; Mamonov, L.K.; Sitpaeva, G.T.; Kustova, T.S.; Dunbar, C.; Wedge, D.E. Isolation and Identification of Antifungal and Antialgal Alkaloids from Haplophyllum sieversii. J. Agric. Food Chem. 2005, 53, 7741–7748. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Osman, A.-M.M.; Almehdar, H.; Abdel-Sattar, E. Acylated Pregnane Glycosides from Caralluma Quadrangula. Phytochemistry 2013, 88, 54–60. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Xiang, C.; Qin, Y.; He, J.; Li, B.-C.; Li, P. Cytotoxicity of Pregnane Glycosides of Cynanchum Otophyllum. Steroids 2015, 104, 49–60. [Google Scholar] [CrossRef]
- Carro, M.D.; Ianni, C.; Magi, E. Determination of Terpenoids in Plant Leaves by GC-MS: Development of the Method and Application to Ocimum basilicum and Nicotiana langsdorffii. Anal. Lett. 2013, 46, 630–639. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.; Windisch, W. Essential Oils and Aromatic Plants in Animal Feeding—A European Perspective. A Review. Flavour Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Therapeutic and Biomedical Potentialities of Terpenoids—A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Fagbemi, K.O.; Aina, D.A.; Olajuyigbe, O.O. Soxhlet Extraction versus Hydrodistillation Using the Clevenger Apparatus: A Comparative Study on the Extraction of a Volatile Compound from Tamarindus Indica Seeds. Sci. World J. 2021, 2021, 5961586. [Google Scholar] [CrossRef]
- Majumder, S.; Ghosh, A.; Bhattacharya, M. Natural Anti-Inflammatory Terpenoids in Camellia Japonica Leaf and Probable Biosynthesis Pathways of the Metabolome. Bull. Natl. Res. Cent. 2020, 44, 141. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Shi, B. Microwave-Assisted Extraction of Flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Sati, P.; Dhyani, P.; Bhatt, I.D.; Pandey, A. Ginkgo Biloba Flavonoid Glycosides in Antimicrobial Perspective with Reference to Extraction Method. J. Tradit. Complement. Med. 2019, 9, 15–23. [Google Scholar] [CrossRef]
- Mackėla, I.; Andriekus, T.; Venskutonis, P.R. Biorefining of Buckwheat (Fagopyrum esculentum) Hulls by Using Supercritical Fluid, Soxhlet, Pressurized Liquid and Enzyme-Assisted Extraction Methods. J. Food Eng. 2017, 213, 38–46. [Google Scholar] [CrossRef]
- Yedhu Krishnan, R.; Neelesh Chandran, M.; Vadivel, V.; Rajan, K.S. Insights on the Influence of Microwave Irradiation on the Extraction of Flavonoids from Terminalia Chebula. Sep. Purif. Technol. 2016, 170, 224–233. [Google Scholar] [CrossRef]
- Dassoff, E.S.; Li, Y.O. Mechanisms and Effects of Ultrasound-Assisted Supercritical CO2 Extraction. Trends Food Sci. Technol. 2019, 86, 492–501. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Lei, J.; Mahar, R.; Chang, M.C.; Collins, J.; Merritt, M.E.; Garrett, T.J.; Yost, R.A. Segmented Flow Strategies for Integrating Liquid Chromatography–Mass Spectrometry with Nuclear Magnetic Resonance for Lipidomics. Anal. Chem. 2023, 95, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bi, T.; Jiang, H.; Liu, H. Review on NMR as a Tool to Analyse Natural Products Extract Directly: Molecular Structure Elucidation and Biological Activity Analysis. Phytochem. Anal. 2024, 35, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Elyashberg, M.; Argyropoulos, D. Computer Assisted Structure Elucidation (CASE): Current and Future Perspectives. Magn. Reson. Chem. 2021, 59, 669–690. [Google Scholar] [CrossRef]
- Kupče, Ē.; Claridge, T.D.W. NOAH: NMR Supersequences for Small Molecule Analysis and Structure Elucidation. Angew. Chem. Int. Ed. 2017, 56, 11779–11783. [Google Scholar] [CrossRef]
- Yong, J.R.J.; Kupče, E.; Claridge, T.D.W. Modular Pulse Program Generation for NMR Supersequences. Anal. Chem. 2022, 94, 2271–2278. [Google Scholar] [CrossRef]
- Balayssac, S.; Trefi, S.; Gilard, V.; Malet-Martino, M.; Martino, R.; Delsuc, M.-A. 2D and 3D DOSY 1H NMR, a Useful Tool for Analysis of Complex Mixtures: Application to Herbal Drugs or Dietary Supplements for Erectile Dysfunction. J. Pharm. Biomed. Anal. 2009, 50, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Monakhova, Y.B.; Diehl, B.W.K.; Do, T.X.; Schulze, M.; Witzleben, S. Novel Method for the Determination of Average Molecular Weight of Natural Polymers Based on 2D DOSY NMR and Chemometrics: Example of Heparin. J. Pharm. Biomed. Anal. 2018, 149, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Mahar, R.; McLeod, M.A.; Giacalone, A.G.; Huang, X.; Boothman, D.A.; Merritt, M.E. Synergistic Effect of β-Lapachone and Aminooxyacetic Acid on Central Metabolism in Breast Cancer. Nutrients 2022, 14, 3020. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, A.; Beleggia, R.; Schauer, N.; Isaacson, T.; Leuendorf, J.E.; Hellmann, H.; Rose, J.K.; Fernie, A.R. Application of GC-MS for the Detection of Lipophilic Compounds in Diverse Plant Tissues. Plant Methods 2009, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Düchting, P.; Weiler, E. A Multiplex GC-MS/MS Technique for the Sensitive and Quantitative Single-Run Analysis of Acidic Phytohormones and Related Compounds, and Its Application to Arabidopsis Thaliana. Planta 2002, 216, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Zarrinmehr, M.J.; Daneshvar, E.; Nigam, S.; Gopinath, K.P.; Biswas, J.K.; Kwon, E.E.; Wang, H.; Farhadian, O.; Bhatnagar, A. The Effect of Solvents Polarity and Extraction Conditions on the Microalgal Lipids Yield, Fatty Acids Profile, and Biodiesel Properties. Bioresour. Technol. 2022, 344, 126303. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Heo, S.; Lee, D.-Y.; Choi, H.-K.; Lee, J.; Kim, J.-H.; Cho, S.-M.; Lee, H.J.; Auh, J.-H. Metabolite Fingerprinting of Bokbunja (Rubus coreanus Miquel) by UPLC-qTOF-MS. Food Sci. Biotechnol. 2011, 20, 567–570. [Google Scholar] [CrossRef]
- Dumez, J.-N. NMR Methods for the Analysis of Mixtures. Chem. Commun. 2022, 58, 13855–13872. [Google Scholar] [CrossRef]
- Qiu, F.; McAlpine, J.B.; Lankin, D.C.; Burton, I.; Karakach, T.; Chen, S.-N.; Pauli, G.F. 2D NMR Barcoding and Differential Analysis of Complex Mixtures for Chemical Identification: The Actaea Triterpenes. Anal. Chem. 2014, 86, 3964–3972. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. GC-MS Analysis of Bioactive Compounds from the Whole Plant Ethanolic Extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, L.; Linghu, K.-G.; Zhao, G.; Chen, Q.; Shen, L.; Dar, P.; Chen, M.; Hu, Y.; Zhang, J.; et al. Comprehensive Comparison on the Anti-Inflammation and GC-MS-Based Metabolomics Discrimination between Bupleuri Chinense DC. and B. Scorzonerifolium Willd. Front. Pharmacol. 2022, 13, 1005011. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, G.; Seymour, A.B. High-Throughput and Multiplexed LC/MS/MRM Method for Targeted Metabolomics. Anal. Chem. 2010, 82, 5527–5533. [Google Scholar] [CrossRef]
- Zhang, F.; Bruschweiler-Li, L.; Brüschweiler, R. High-Resolution Homonuclear 2D NMR of Carbon-13 Enriched Metabolites and Their Mixtures. J. Magn. Reson. 2012, 225, 10–13. [Google Scholar] [CrossRef] [PubMed]



| Methods | Advantages | Disadvantages |
|---|---|---|
| Maceration | It is a simple extraction method with minimal set-up needed. It can be used for the extraction of thermolabile metabolites. It can be considered economically beneficial due to the lack of heat. | Long extraction time and low extraction efficiency. Large volume of extraction solvent is required. |
| Percolation | Percolation is efficient as it is a continuous process in which the saturated solvent is replaced with fresh solvent constantly. | Long extraction time and low extraction efficiency. Large volume of extraction solvent is required. |
| Soxhlet extraction | Simple method to set up. Temperature in the extraction system can be maintained. Temperature could help to rupture the plant tissues and the metabolites can be extracted with greater efficiency. | Requires excessive extraction time. Uses large amounts of solvents. No agitation is applied to accelerate the process. Heat-sensitive compounds can be thermally decomposed. |
| Sequential extraction method | Methodological simplicity as it involves subsequential addition and removal of solvents. Simple apparatus required. | Labor-intensive and consumption of large volume of solvents. It can be environmentally hazardous as many solvents are used. Low selectivity and handling of large sample volumes. |
| Microwave-assisted extraction | Moderate or no volume of organic solvent consumed. Applicable for both industrial and laboratory scales. Less time-consuming than conventional methods. High efficiency as it changes the cell structure due to electromagnetic waves. | Efficiency of MWE is very poor for non-polar compounds or solvents. Less efficiency for extremely viscous solvents. Not appropriate for heat-sensitive organic compounds. Expensive instrumental set-up and difficult to operate. |
| Ultrasound-based extraction | Moderate volume of organic solvent consumed.Less extraction time. Less damage to bioactive compounds. Uniform distribution of energy enhances extraction efficiency. | Expensive set-up and requires optimization. It can cause some unwanted changes to bioactive compounds. |
| Technique | Advantages | Disadvantages |
|---|---|---|
| NMR | Chemical shift advantage provides information about various groups in compounds. Qualitative and quantitative analysis of compounds can be performed in mixtures. Extensive 1D and 2D NMR experiments help to elucidate the structures of compounds. Minimal sample preparation and no method development required once optimized. | Low sensitivity for some of the NMR active nuclei (i.e., 13C) due to low gyromagnetic ratio and very low natural abundance. Low resolution in proton NMR spectroscopy prohibits identification of overlapped signals. High magnetic field instruments are costly, but resolution could be enhanced. |
| LC-MS | Positively and negatively charged adduct ions formed with atoms or molecules can help to determine the exact molecular masses of compounds. Linear dynamic range with low detection limit. Capability to quantify multiple analytes simultaneously. MS/MS fragmentation pattern is very helpful in characterizing the compounds. | Extensive method development needed based on the class of compounds to be analyzed. Testing of several columns for different classes of compounds. High purchase, maintenance, and operational costs. Different ionization sources in mass spectrometer are required for different polarities of compounds. |
| GC-MS | High resolution power and higher sensitivity compared to other methods. GC-MS has high accuracy and precision and can resolve closely related compounds. Small sample volume can be separated using gas chromatography. Fragmentation pattern provides unique fingerprint for each chemical structure. | GC-MS is limited to volatile compounds. Compounds can decompose at high temperatures. Thermal stability is necessary for separation through gas chromatography. It is not suitable for high-boiling-point and polar analytes. Chemical derivatization is needed to make compounds volatile. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthwal, R.; Mahar, R. Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures. Metabolites 2024, 14, 119. https://doi.org/10.3390/metabo14020119
Barthwal R, Mahar R. Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures. Metabolites. 2024; 14(2):119. https://doi.org/10.3390/metabo14020119
Chicago/Turabian StyleBarthwal, Ruchi, and Rohit Mahar. 2024. "Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures" Metabolites 14, no. 2: 119. https://doi.org/10.3390/metabo14020119
APA StyleBarthwal, R., & Mahar, R. (2024). Exploring the Significance, Extraction, and Characterization of Plant-Derived Secondary Metabolites in Complex Mixtures. Metabolites, 14(2), 119. https://doi.org/10.3390/metabo14020119







