Abstract
With the sustainable increase in agricultural productivity, the need for safer, environmentally friendly pesticide alternatives is also growing. Metabolites of microorganisms (bacteria, fungi, actinomycetes) are emerging as potential bioactive compounds for integrated pest and disease management. These compounds comprise amino acids, carbohydrates, lipids, organic acids, phenolics, peptides, alkaloids, polyketides, and volatile organic compounds. The majority of them have insecticidal, fungicidal, and nematicidal activities. In this review, the classifications, biosynthetic pathways, and ecological functions of primary and secondary metabolites produced by microorganisms are discussed, including their mechanisms of action, ranging from competition to systemic acquired resistance in host plants. The article highlights the importance of microbial genera (viz., Bacillus sp., Pseudomonas sp., Trichoderma sp., Streptomyces sp., etc.) in making chemicals and biopesticides for crop defense. We present the possible applications of microbial biosynthesis strategies and synthetic biology tools in bioprocess development, covering recent innovations in formulation, delivery, and pathway engineering to enhance metabolite production. This review emphasizes the significance of microbial metabolites in improving the plant immunity, yield performance, reduction in pesticide application, and the sustainability of an ecological, sustainable, and resilient agricultural system.
1. Introduction
Contemporary agriculture has a pressing dual need to increase crop yields and to minimize the environmental impact. Pest and disease pressures rank as one of the leading factors of food loss, causing an annual loss of global food production of between 20 and 40% for major staple crops. First, synthetic chemical pesticides were put up as a defense system against insects, but their prolonged use and overuse are responsible for problems including pesticide resistance, ecosystem pollution, loss of biodiversity, and human and other living system health problems [1]. These developments have opened the door for biologically based strategies—such as biological control, microbial metabolites, pheromones, and juvenile hormone-based methods—to emerge as strong alternatives, combining effectiveness with environmental safety. Among these, microbial metabolites stand out as bioactive compounds produced by microorganisms like bacteria, actinomycetes, and fungi. These compounds are often synthesized as part of the microorganisms’ natural survival strategies, helping them compete for resources, inhibit rival species, or establish symbiotic relationships with hosts. There are many types of plant growth promoters whose function depends on other factors (e.g., genotype, age, development, and stage of growth), creating an impact on yield. The mechanisms of the PGPR can be positive or negative. Some of these compounds, categorized as primary (essential to basic cellular functions) or secondary (produced as a response to a given stimulus), show a wide spectrum of action against a series of agricultural challenges, including pests, plant pathogens, and nematodes [2].
From the functional point of view, microbial metabolites represent the effect of microorganisms within the ecosystem of nature, that is, their important contribution to defense, competition, communication, or symbiosis. They act to control pests through modes of action such as antibiosis, the release of degradative enzymes, the induction of systemic resistance in host plants, disruption of pest development, and direct toxicity [3,4,5]. Several of these compounds have been formulated into biopesticides and are in use as components of more environmentally benign, integrated pest management (IPM) practices [6].
It is anticipated that the microbial biopesticides market will witness substantial growth, primarily due to increasing consumer preference for environmentally sustainable products and stricter regulations on synthetic pesticide use. According to a recent study, the global biopesticides market was valued at USD 6.7 billion in 2023 and is projected to reach USD 13.9 billion by 2028, growing at a compound annual growth rate (CAGR) of 15.9% during the forecast period. Notably, microbial biopesticides constitute over 55% of the global biopesticide market, underscoring their significant role in sustainable agriculture [7]. Modern technological advances in microbial fermentation, metabolomics, genomics, and synthetic biology are also expediting the discovery and utilization of these natural compounds in agriculture [8,9,10].
This review provides a comprehensive understanding of the role and potential of microbial secondary metabolites in plant protection and sustainable agriculture. It discusses their modes of action, ecological functions, and real-world applications, along with recent advances in their production and delivery systems. Additionally, it highlights the potential to enhance their efficacy through cutting-edge biotechnological and synthetic biology approaches.
2. Microbial Metabolites
Eukaryotic and prokaryotic microbes inherently produce a diverse array of chemical substances, collectively known as metabolites, which are essential for their survival and role in ecosystems [11]. These compounds are usually small, with molecular weights under 1000 Daltons, and are at the heart of the chemical processes that support microbial metabolism [12]. The full set of these substances produced by a single organism is called its metabolome—a term introduced in 1997 [13]. The metabolome encompasses substances present inside cells, in the surrounding environment (volatile metabolites). Metabolite patterns are intricately linked to an organism’s characteristics; hence their research yields significant insights into microbial behavior and environmental relationships [14,15,16].
The structure and roles of microbial metabolites vary widely, and their presence shifts based on changing conditions. Microorganisms may synthesize, modify, absorb, or release these compounds depending on their environment and physiological state [17]. Generally, metabolites are classified into three types: polar (dissolving in water), nonpolar (not dissolving in water), and volatile. Even with the vast diversity, many structural differences happen at the atomic level rather than creating entirely new forms [18].
Metabolites may be categorized according to their origin and function. They may be generated internally (endogenous) or obtained outside (exogenous) [19]. They are categorized into primary and secondary metabolites based on their function. Primary metabolites are associated with fundamental requirements such as growth, development, and energy metabolism. Examples include amino acids, nucleotides, and organic acids—typically generated during periods of microbial proliferation [20]. A microorganism unable to make a required primary metabolite is called auxotrophic, a condition that can be fatal unless the missing compound is provided externally [21]. Primary metabolites are often consistent across different species [19,22], though they typically appear in low amounts because they are quickly used up. Occasionally, intermediate compounds such as organic acids (e.g., citric acid, malic acid) or amino acid derivatives (e.g., glutamine, ornithine) accumulate and are released into the environment, often as byproducts of microbial metabolism. These metabolites play key roles in the biological control of crop diseases and pests by inhibiting pathogen growth and inducing plant defenses [23,24].
In contrast, secondary metabolites (terpenes, phenolics, peptides, polyketides, etc.) are not essential for immediate survival but provide adaptive advantages for a wide range of microorganisms. They assist in defense, stress reaction, and communication [25]. These chemicals often emerge when cells reach the stationary phase, frequently induced by stress or nutritional deficiencies [26]. Secondary metabolites are often more biologically active in ecological interactions than primary metabolites, as they tend to exhibit targeted effects against pests, pathogens, or competing microbes, making them particularly valuable for biocontrol applications. Although it is helpful to categorize metabolites as primary or secondary, the boundary between them can sometimes shift depending on the situation [19] (Figure 1).
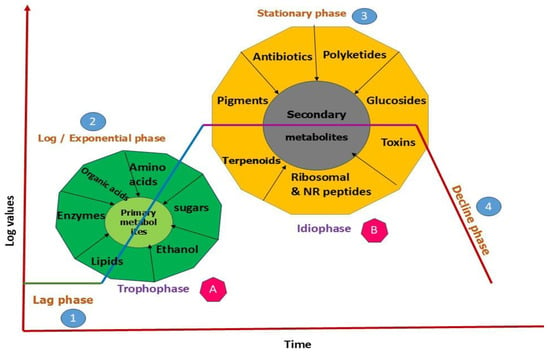
Figure 1.
Different phases of microbial metabolites production.
Secondary metabolites have gained significant attention in agriculture due to their potent bioactive properties, many of which have been harnessed as commercial biopesticides. For example, spinosad, a mixture of macrocyclic lactones produced by Saccharopolyspora spinosa, is employed as an effective insecticide in organic farming [27]. Another notable example is abamectin, a secondary metabolite from Streptomyces avermitilis, which acts as a miticide and insecticide by interfering with neurotransmission in target pests [28]. These examples demonstrate the commercial success and ecological potential of microbial and plant-derived secondary metabolites in sustainable pest management.
3. Primary Metabolites from Microorganisms
Primary metabolites are small molecules, typically weighing less than 900 Daltons; they are found within cells and play essential roles in fundamental biological processes. They support vital cellular functions such as growth, division, maturation, and reproduction—essentially providing everything a cell needs to function efficiently and maintain its health. Basically, by providing everything a cell needs to be healthy and run effectively, they enable cells to grow, divide, mature, and reproduce. Some well-known examples include amino acids, nucleotides, and fermentation products like ethanol and organic acids. These compounds are absolutely crucial for the growth and survival of microorganisms. Out of all the ways to produce them, microbial production of amino acids stands out because it is highly efficient, cost-effective, and environmentally friendly, offering the added bonus of producing pure enantiomers [29,30] (Table 1).
3.1. Amino Acids in Plant Health and Defense
Amino acids are essential for organisms, playing key roles in cellular growth, biofilm formation, and environmental adaptation. They serve not only as building blocks for proteins but also as signaling molecules that support microbial survival and colonization in diverse habitats [31]. These amino acids are grouped by their side chains into three categories: acidic (like aspartic and glutamic acids), basic (such as lysine, arginine, and histidine), and neutral (including serine, threonine, and tyrosine) [32]. Amino acids play a crucial role in the health of both microbes and plants. For plants, they serve as small messengers that direct growth, increase their strength to deal with stress, and help them defend against pests and diseases. A specific amino acid, γ-aminobutyric acid (GABA), allows plants to send signals when they need to activate their defenses. This is especially important during tough conditions or attacks from harmful organisms [33]. Proline, another amino acid, helps maintain cell stability and function in tough conditions [34]. Furthermore, some derivatives of amino acids, like threonine, have natural power to kill harmful microbes. Certain amino acids or their altered forms can also make plants less appealing to herbivores, offering extra protection to the plants [35,36].


Table 1.
Primary metabolites from microorganisms against plant pests and diseases.
Table 1.
Primary metabolites from microorganisms against plant pests and diseases.
| Metabolites | Source Microorganisms | Mode of Action | Target Pests/Pathogens | References |
|---|---|---|---|---|
| Lipids | ||||
| Rhamnolipids (RLs) Fengycins (FGs) | Bacillus subtilis | Induce mycelial de-structuring and hyphal fusions | Botrytis cinerea and Sclerotinia sclerotiorum | [37] |
| Hormones | ||||
| Abscisic acid | Achromobacter xiloxidans, Bacillus pumilus | Enhances plant resistance | Broad-spectrum fungicide | [38] |
| Jasmonic acid (JA) | Pseudomonas, Bacillus, Azoospirillum | Induces systemic resistance (ISR) | Spodoptera exigua | [39] |
| Jasmonic acid (JA | Pochonia chlamydosporia | PR protein | Meloidogyne javanica | [40] |
| Salicylic acid (SA) | Pseudomonas, Bacillus, Azoospirillum | Induces systemic resistance (ISR) | Sclerotinia sclerotiorum | [41] |
| Ethylene (ET) | Paenibacillus lentimorbus | Induces systemic resistance (ISR) | Sclerotium rolfsii. | [42] |
| Indole acetic acid (IAA) | Dysoxylum gotadhora | Induces systemic resistance (ISR) | Verticillium dahliae and Fusarium oxysporum | [43] |
| Gibberelin | Rhizobium, Bacillus, and Penicillium | Induces systemic resistance (ISR) | All pathogens | [44] |
| Turanose sugar and hormones IAA, Gibberelic acid (GA) SA | B. amyloliquefaciens | Modulation of phytohormone signal | Rhizoctonia solani | [45] |
| Organic acids | ||||
| Lactic acid | Lactobacillus plantarum | Antibacterial and antifungal | Pseudomonas campestris, Ralstonia solanacearum, Xanthomonas campestris pv. vesicatoria, Pectobacterium carotovorum | [46] |
| Lactic acid | L. paracasei | Antibacterial and antifungal | R. solanacearum | [47] |
| Organic acid | Most of the microbes | Solubilizes cuticular proteins | Lepidopteran and dipteran pest | [8] |
| Organic acid | L. plantarum | Nematicidal effect | Meloidogyne incognita | [48] |
| Acetic, propionic, formic, benzoic acid | Lactobacillus sp. | Interferes with the membrane functions of the pathogen | Broad-spectrum | [49] |
| Others | ||||
| Meso-2,3-Butanediol | Klebsiella pneumoniae | Induction of systemic resistance | R. solanacearum | [50] |
| Proteinaceous and non-proteinaceous antifungal compounds | Lactobacillus plantarum | Antifungal compounds | Botrytis cinerea, Alternaria solani, Phytophthora drechsleri, Fusarium oxysporum and Glomerella cingulate | [51] |
3.2. Sugars and Their Role in Pest and Disease Control
Microbes also generate sugar molecules such as oligosaccharides and polysaccharides, which heavily contribute to the enhancement of plant resistance. These sugars act as signaling molecules to induce the plant’s endogenous defense mechanisms against pathogen and insect pests [52]. Some microbial oligosaccharides can increase the synthesis of plant defense enzymes and secondary metabolites, e.g., Šphytoalexins (which retard pathogen growth). Chitosan oligosaccharides [53] and oligogalacturonides [54] obstruct the attachment and invasion by pathogens and deter the feeding of herbivorous insects. In conclusion, microbial sugars are natural agents for eco-friendly pest and disease control.
3.3. Organic Acids as Biocontrol Agents
Organic acids, namely those secreted by lactic acid bacteria (LAB), are also fundamental for inhibiting detrimental microorganisms [55]. The most abundant metabolite is lactic acid, although other compounds, including acetic, propionic, formic, benzoic, and polylactic acids, also play a role in the antimicrobial activity of LAB. It has been demonstrated that lactic acid could be used to control the growth of spoilage and pathogenic microorganisms by disorganizing the microorganism’s membrane, reducing intracellular pH levels, and interfering with the metabolic activities, which subsequently leads to cell death [56]. The antimicrobial activity of lactic acid varies depending on LAB species or strain, growth conditions, and microbial consortia [57]. Numerous microorganisms such as bacteria and fungi, are especially sensitive to lactic acid in acidic pH [58].
Some LAB, including L. sakei, and L. curvatus, synthesize metabolites that act as strong nematicides [59]. Furthermore, compounds produced by Oenococcus oeni have demonstrated that they attract spotted wing drosophila and, therefore, could have utility for pest monitoring or behavioral control [60].
3.4. Lipids in Microbial Communication and Plant Interaction
A wide range of microbial lipids was produced by an enormous diversity of microorganisms that may be found as intra- or a cell-bound layers, or may be secreted into the surrounding medium [61]. These microbial lipids have important functions in plant–microbe interactions, which include pathogen recognition, signaling, and immunity [62]. Serving as chemical messengers, they control not just interactions between microbes and plants but also those between different groups of microorganisms. During plant defense, microbial lipids can either prime plants for resistance against deleterious invaders or modulate plant response to beneficial microbes [63].
3.5. Nucleotides and Their Regulatory Functions
Nucleotides are one of the principal classes of signaling molecules used by microorganisms to signal each other, specifically during quorum signaling, in which the activity group level of a microbial community is regulated. Gene expression is regulated by genes, which are responsible for microbial regulation and secondary metabolite production.
In addition they also contribute to plant defenses. This complex interplay between microbial signaling and plant immune responses highlights the importance of these molecules in maintaining ecosystem health. By facilitating communication within microbial communities and enhancing plant defenses, they play a crucial role in promoting resilience against environmental stressors and pathogens.
4. Secondary Metabolites from Microorganisms
Microorganisms are also able to synthesize a wide variety of compounds known as secondary metabolites, generally in the stationary or idiophase stages of growth [26,64]. Primary metabolites are involved in growth and development and are directly associated with essential cellular processes, while secondary metabolites are not critical to the life of the organism. Rather, they contribute to important ecological functions for microbial survival–in competition, defense, signaling, and symbiosis with other organisms [65].
Secondary metabolites, which are often produced during environmental stress or lack of certain nutrients, enable the microbes to cope with the changing environment [66]. Although many of these compounds are well-characterized antimicrobial and bioactive compounds, others remain poorly studied; this is likely due to both the high diversity of microbial species that produce these compounds and the environmental variability experienced [67,68]. They belong to several categories based on their chemical structure, such as antibiotics, toxins, phytohormones, and plant growth regulators [69,70]. Secondary metabolites production is dynamic and dependent upon microbial interactions, environmental signals, and the availability of nutrients [71], and they are commonly grouped into five major structural and functional classes:
- Terpenes (e.g., volatiles, glycosides, sterols);
- Phenolics (e.g., flavonoids, coumarins);
- Nitrogen-containing compounds (e.g., alkaloids);
- Peptides (e.g., surfactin, iturin);
- Polyketides (e.g., 2,4-diacetylphloroglucinol) [72,73,74,75].
Several of these compounds are effective not only as antimicrobials in crops, but also through the induction of host plant disease tolerance, e.g., trichodermin, gliotoxin, and Bacillus thuringiensis (Bt) toxin [76,77,78]. These are valuable tools for sustainable agriculture, as shown in Table 2 [79,80].

Table 2.
Secondary metabolites from microorganisms against plant pests.
4.1. Terpenoids: Diverse Roles in Plant Protection
Terpenoids, or isoprenoids, constitute the most structurally diverse class of natural products, with over 80,000 compounds identified across all domains of life [99]. These molecules play essential roles in both primary and secondary metabolism and are involved in signaling, defense, and growth regulation. Microbial producers of terpenoids include Streptomyces and Penicillium sp., which synthesize bioactive metabolites such as erythromycin and antimicrobial agents. A subset of these compounds—microbial volatile organic compounds (MVOCs)—are released into the environment and have been shown to suppress pathogens, promote plant–microbe communication, and stimulate plant defenses [100].
Compounds such as salicylic acid (SA), jasmonic acid (JA), benzothiazole phenol, and pyrazine (as MVOCs) are more powerful in inducing phytoalexin biosynthesis and plant immunity [101]; MVOCs may be a new avenue for inducing plant immunity and an interesting research subject. Such volatiles are greener substitutes for synthetic pesticides and growth promoters [102]. Another group of terpenoids that are essential for fungal growth are the sterols. Although ergosterol is often employed as a fungal biomass marker, it is not present in every fungus [103], thereby highlighting an area that is rarely studied in the breadth of fungal diversity and ecology within the field.
4.2. Polyketides
Polyketides are a chemically varied category of secondary metabolites recognized for their potent biological activities, including antibacterial, antifungal, and antiviral properties [104]. Polyketides, synthesized by microorganisms like fungus and actinomycetes, have emerged as crucial agents in agriculture. Examples include avermectins and spinosyns—widely used insecticides that target various insect orders—and the commercial polynactin, effective against spider mites [105,106,107]. Plant-associated Pseudomonas species produce 2,4-Diacetylphloroglucinol (DAPG), another well-studied polyketide, which exhibits a broad spectrum of biocontrol activity [108,109]. This compound not only inhibits the growth of various plant pathogens but also promotes plant health by enhancing root development and nutrient uptake. As research continues, the potential applications of polyketides in sustainable agriculture and pest management are becoming increasingly significant. In addition, the B. amyloliquefaciens subsp. plantarum strain produces polyketides like macrolactin, bacillaene, and difficidin, which contribute to its strong antagonistic action against pathogens. These compounds are essential tools for managing plant diseases through natural means.
4.3. Phenolic Compounds
Phenolics, particularly flavonoids, are secondary metabolites found in both plants and microbes. These compounds are involved in defense responses, signal transduction, and plant development. Flavonoids are synthesized through the shikimate, phenylpropanoid, and flavonoid-specific pathways [110]. They play key roles in shaping root growth, regulating auxin transport, and ensuring reproductive success in plants like maize and petunia. Their antimicrobial and antioxidant properties make them important contributors to plant resilience under biotic and abiotic stress conditions.
4.4. Alkaloids
Alkaloids are nitrogen-containing natural products known for their structural diversity and broad-spectrum bioactivities and are produced by bacteria and fungi. Microbial alkaloids such as indole, isoindole, and ergot derivatives possess antifungal, antibacterial, and nematicidal properties [111]. These properties make microbial alkaloids valuable in agricultural applications, particularly in the development of sustainable pest management strategies. Additionally, their unique structures provide a foundation for the synthesis of novel pharmaceuticals, highlighting the potential of these compounds in both ecological and medicinal contexts. Epichloë sp., for instance, produce ergot alkaloids that suppress Pratylenchus sp. in the field [112]. Metarhizium spp. produce lysergic acid derivatives during insect infection, linking alkaloid production to ecological function rather than plant colonization [113]. Recently discovered alkaloids such as spirobrefeldins from Penicillium brefeldianum have demonstrated antimicrobial activity against several pathogens [114].
4.5. Peptides
Antimicrobial peptides (AMPs) are small, cationic, and broad-spectrum antimicrobial molecules. These peptides are generally composed of fewer than 50 amino acids and work by disrupting the microbial membranes, and they are effective against many pathogens [115]. AMPs exist in multiple life forms, and serve important functions to combat microbes, defend plants, and regulate immunity [116]. The synergistic action of AMPs, when used in combination, can enhance the plant immune response to a much higher level than that achieved by a single AMP [117].
Peptide antibiotics of a non-ribosomal nature (NRPs), which constitute one subclass of AMPs, are synthesized through large enzyme complexes, termed non-ribosomal peptide synthetases (NRPSs). Others, like B. subtilis and P. fluorescens, are widely known to produce NRPs, including iturin, surfactin, and fengycin, which are important to plant disease suppression [118] Several NRPs target peptidoglycan synthesis and act as bacteriostatic agents, similar to clinical antibiotics [119,120]. These two peptides as a composite offer a sustainable disease management option in agriculture.
5. Mechanisms of Action of Microbial Metabolites Against Insects Pest and Diseases
Microbial metabolites have emerged as potential and eco-friendly alternatives to synthetic pesticides for controlling insect pests and plant pathogens. In contrast to the broad-spectrum chemical insecticides, these bioactive compounds have high specificity in targeting organisms, with limited noxiousness to beneficial species, causing a low ecological risk [121].
Microbial metabolites are particularly useful because they show activity against the host, allowing specific pest control while leaving the related flora and fauna undisturbed. The targeted approach of such a mode of action not only enhances the efficiency of pest management but is also in line with integrated pest management (IPM) and sustainable agriculture.
Although there is a substantial increase in interest in microbial solutions, only a small percentile of microbial diversity has been explored, which is estimated to be just 5% of fungal species and 0.1% of bacterial species globally. This stands to underscore a vast and largely untapped pool of new bioactive agents that could serve as sustainable pesticides and biotechnological and pharmaceutical resources.
Microbial metabolites, which are produced by various microorganisms, including bacteria, fungi, and viruses, are capable of inhibiting or even killing insect pests, as are nematodes that target insects in their natural environment. These metabolites work in different ways, such as by disrupting nerve functions, blocking digestion and reproduction, or boosting the immune responses of the host [25]. In addition, various other microbial strains isolated from marine and terrestrial sediments have been shown to yield secondary metabolites with a great deal of biotechnological significance [122].
Microbial metabolites directly act as pesticides, but they also exert other protective roles such as rhizosphere microbiome modulation, the promotion of systemic resistance in host plants, and exclusion of competing phytopathogens [123]. The broad spectrum of the functions of these roles emphasizes the capability of microbial metabolites as sustainable pest and disease biocontrol agents.
5.1. Mechanisms of Action Against Insect Pests
5.1.1. Production of Toxins That Disrupt Insect Physiology
The microbial metabolites are frequently toxins that disrupt normal insect physiology, leading to insect death or developmental dysfunction. Some target the nervous or digestive systems, processes that are essential for maintaining life [124]. One of the common ways of action is through degradation of chitin, which is the main component of the exoskeleton and intestines of insects, leading to impaired structures and gut [125,126].
One of the most characterized microorganisms is B. thuringiensis (Bt), which synthesizes Cry and Cyt toxins. These proteins attach to receptors in the gut of the insect, which leads to the lysis and paralysis of the gut, leading to starvation [127]. The specificity of Bt is especially appealing because it reduces collateral damage to less beneficial insect populations and deters environmental risk [128].
5.1.2. Interference with Insect Development and Reproduction
Several microbial metabolites also interfere with hormonal regulation in insects, thereby disrupting development, metamorphosis, or reproduction. For example, decoyinine has been shown to significantly reduce fecundity in small brown planthoppers (SBPH) and to simultaneously alter rice plant physiology, making them less favorable hosts [129,130].
Several other metabolites affect the molting process in insects by interfering with the endocrine system, and most notably by mimicking or blocking juvenile hormone (JH) pathways. Nerolidol, as a sesquiterpenoid compound, alters the expression pattern of the JH-degrading enzyme, resulting in modified hormonal levels and disrupted development [131]. Additionally, sakuranetin and other plant-based compounds can diminish populations of yeast-like symbionts that contribute to insect nutrition and stress resistance, thereby compromising pest success (Figure 2) [130].
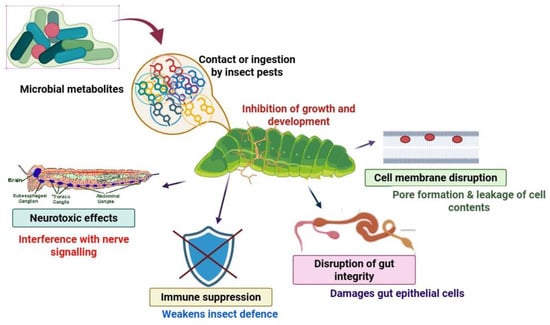
Figure 2.
Mechanism of action of microbial metabolites against plant pests (change diagram in Biorender).
5.1.3. Repellent and Antifeedant Effects
Microbial VOCs are essential in repelling pests. These compounds can function as repellents or antifeedants, disrupting the perception of insect senses and hindering their ability to detect or exploit host plants [132,133].
Chemosensory and mechanosensory systems are essential for insects to find food and oviposition sites. These ways can be targeted by metabolites that impair pest activity or inhibit feeding [134]. Microbial metabolites provide a sustainable tactic for insect control, either by changing the chemical environment or directly impacting insect receptors.
5.1.4. Disruption of Insect Hormonal Balance
Hormonal disruption is another major part of this mechanism. These microbial metabolites can partially imitate or interfere with insect hormones like the juvenile hormone (JH) and ecdysone, causing developmental stunting, deformities, or even sterility [94,135]. For example, nerolidol simulates JH and alters the expression of JH degradation enzymes, producing hormonal disturbances that break the insect life cycle [131]. This disruption leads to improper molting or metamorphosis in the insect, significantly compromising its reproductive fitness and overall survival.
5.1.5. Induction of Plant Defense Mechanisms Against Diseases
Microbial metabolites also have indirect effects on pest suppression through activation of plant defense systems. Through pattern recognition receptors (PRRs), plants recognize specific pathogen- or microbe-associated molecular patterns (PAMPs/MAMPs), triggering immune responses that lead to systemic acquired resistance (SAR) and/or induced systemic resistance (ISR) [136,137]. Such responses include synthesis of antimicrobial compounds, strengthening of cell walls, and induction of defense genes. Several microbes among the plant’s surrounding rhizosphere also provoke ISR, making the whole plant less susceptible to attacks by insect or microbial problems.
The combined benefits of ISR (defense) and better nutrient absorption suggest that microbes that trigger ISR could be useful in managing pests and nutrients together.
5.2. Mechanisms of Action Against Plant Disease
5.2.1. Production of Toxins Against Fungi
Antibiosis is one of the important biological control strategies, through which beneficial microbes secrete antimicrobial compounds to inhibit or damage plant pathogens. These bioactive molecules inhibit crucial metabolic processes, including the integrity of the cell wall, membrane potential, and nucleic acid synthesis within pathogens, ultimately leading to their growth inhibition. For instance, Trichoderma viride is known to produce viridiol, an antifungal phytotoxin. Additionally, Chromobacterium sp. produces chromobactomycin, a cyclic lipopeptide with antifungal activity [59].
Many actinomycetes, especially Streptomyces species, are known to produce such antimicrobial agents in abundance [138]. In a similar manner, endophytic fungi are among the best sources of antifungal metabolites and have potential against various phytopathogens, such as Aspergillus niger and T. harzianum isolated from several wild medicinal plants [139]. Such metabolites can be utilized to produce biopesticide product formulations that can control a broad range of plant diseases. Additionally, even some of the antimicrobial metabolites can induce the host resistance responses such as the plant defensive arsenal, from both yield and health perspectives (Figure 3 and Table 3) [140].
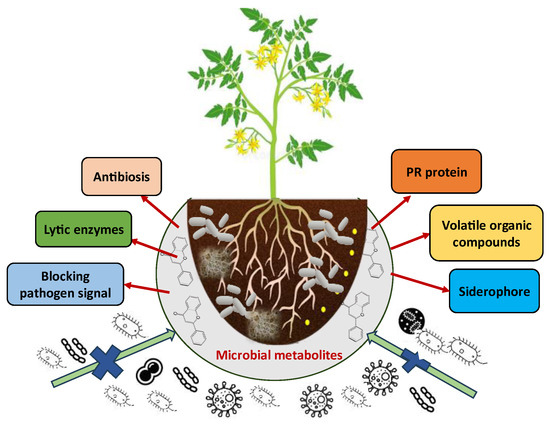
Figure 3.
Mechanism of action of microbial metabolites against plant disease.

Table 3.
Microbial secondary metabolites for disease management.
5.2.2. Induction of Systemic Resistance in Plants
Microbial metabolites induce plant immune responses by priming through ISR or SAR. These resistances are not mediated by direct contact with pathogens but rather by detecting microbe-associated molecules. After recognition, plants initiate a signaling cascade of events, which involves hormones such as salicylic acid (SA), jasmonic acid (JA), or ethylene (ET), known to trigger the synthesis of defense proteins as pathogenesis-related (PR) proteins [163].
For example, B. cereus induces plant resistance pathways via NPR1-dependent signaling, and P. simplicissimum and Paraburkholderia phytofirmans induce systemic resistance in Arabidopsis thaliana against bacterial and fungal pathogens [164].
5.2.3. Enzyme Production to Degrade Pathogen Cell Walls
Lytic enzymes like chitinases, glucanases, proteases, and cellulases break down the structural components of fungal cell walls. These enzymes degrade chitin and β-glucans, major constituents of pathogenic fungal cell walls, thereby weakening and killing the pathogen [165]. Some endophytes from Fagopyrum esculentum, such as those in the Bacillus genus, display multiple enzymatic activities and are effective against pathogens like Fusarium culmorum and Rhizoctonia solani [166]. Their mode of action combines enzymatic degradation with colonization, providing a synergistic approach to disease suppression.
5.2.4. Production of Volatile Organic Compounds (VOCs)
Microbial metabolites are volatile and active metabolites with antimicrobial effects. These alcohols, ketones, terpenes, and sulfur compounds disrupt the pathogen metabolism and membrane structure [167]. In addition, VOCs can induce defense pathways in plants or attract helpful microbes, thus enhancing the aboveground and belowground plant–microbe society network. Pseudomonas and Bacillus spp. are abundant VOC producers; their emission can be used to suppress soil-borne diseases and promote plant growth [168].
5.2.5. Siderophore Production
Siderophores are iron-chelating molecules that restrict iron availability to pathogens while simultaneously supplying iron to the host plant. This dual function not only suppresses pathogen development but also promotes plant vigor [169,170].
Trichoderma and Pseudomonas spp. are effective siderophore producers, and their iron-scavenging activity forms a key element of their biocontrol capacity.
5.2.6. Plant Growth Promotion
PGPRs enhance plant growth through a variety of mechanisms: nitrogen fixation, phosphorus solubilization, phytohormone production (e.g., auxins, cytokinins), and indirect pathogen suppression [171]. Species like Azospirillum sp. and Enterobacter spp. have been shown to increase biomass and yield in rice, sugarcane, and oil palm while also improving plant resistance to diseases such as rice blast [172].
5.2.7. Cell Wall Reinforcement
In response to the microbial elicitors, plants strengthen their cell walls by depositing lignin, callose, and other polysaccharides to prevent pathogen penetration. This signaling network includes the perception of damage-associated molecular patterns (DAMPs) as well as crosstalk between cell wall integrity maintenance and immune signaling [173]. These responses can be intensified, and the structural integrity of host plants can be further strengthened by biocontrol agents such as Trichoderma atroviride and beneficial rhizobacteria.
6. Mechanisms of Action Against Nematodes
Plant-parasitic nematodes, particularly root-knot nematodes, threaten global food production by reducing root function and the ability to take up water and nutrients. Microbial metabolites from bacteria, fungi, and other beneficial microorganisms offer an ecologically sound and sustainable alternative to nematicidal chemicals. These organisms reduce nematode populations by different actions, like the production of toxins and enzymes, plant immunity modulation, and alterations in nematode behavior. This section highlights the integrated approaches adopted by microbial metabolites for the effective management of nematode infestations (Figure 4 and Table 4).
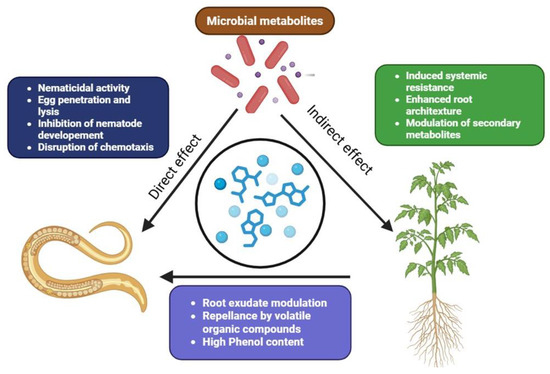
Figure 4.
Mechanism of action of microbial metabolites against plant parasitic nematodes.
6.1. Production of Nematicidal Toxins
The primary mechanism by which microbial metabolites control nematodes is through the production of larvicidal or immobilizing toxic compounds. These metabolites, which include both volatile organic compounds (VOCs) and nonvolatile substances, disrupt essential physiological functions within nematodes, affecting their nervous, muscular, and cellular systems. For instance, B. velezensis GJ-7 emits volatiles that lead to up to 97% mortality of M. hapla juveniles within 48 h [174]. Additionally, B. firmus secretes proteolytic enzymes capable of degrading the nematode cuticle, thereby damaging its protective membrane and reducing pathogenicity [175]. These phytotoxins not only directly eliminate nematodes but also hinder their ability to feed, develop, and reproduce, thus offering both immediate and long-term protection to their host plants.
6.2. Volatile Organic Compounds (VOCs) as Repellents
VOCs, apart from their toxic potential, can also act as strong repellents which prevent nematodes from coming into the proximity of the plant roots. These compounds disrupt nematode chemosensory activity, leading to an inability to find hosts. For example, B. amyloliquefaciens BaNCT02 deterred more than 67% of the juveniles of M. incognita from the reproductive structures of the bean roots [173]. VOCs, 2-heptanone and 3-methyl-1-butanol repellents, also inhibit egg hatching, thus providing dual action in the same direction [176]. These repellent effects may contribute to a reduction in nematode colonization and infection.
6.3. Induction of Plant Resistance to Nematode Infection
Some microbial metabolites have the capacity to induce the plant’s endogenous defense mechanisms, resulting in systemic resistances. This ISR is mediated by jasmonic acid, salicylic acid, and ethylene signaling pathways. For example, the B. velezensis YS-AT-DS1 strain induces JA/SA pathways in tomato plants, thus reinforcing their capability to resist nematodes [177]. Additionally, fungi such as Trichoderma spp. can trigger defense reactions, including the production of nematode-repellent compounds and the reinforcement of the cell wall [178,179]. The induced resistance in plants offers long-term protection and decreases reliance on external treatments.
6.4. Production of Enzymes That Degrade Nematode Structures
Specifically, enzymes, i.e., proteases and chitinases, are involved in the decomposition of the protective cuticle or eggshell of nematodes. B. firmus excrete protease to degrade nematode structural proteins, and B. wiedmamii AzBw1 also produce protease and chitinase to attack egg and juveniles, AzBw1 [180].


Table 4.
Secondary metabolites from microorganisms against plant-parasitic nematodes.
Table 4.
Secondary metabolites from microorganisms against plant-parasitic nematodes.
| Metabolites | Source Microorganisms | Mode of Action | Target Pathogens | References |
|---|---|---|---|---|
| Terpenoids | ||||
| Abamectin | S. rochei | Nematicidal effect | M. incognita | [181] |
| Lactones | T. harnatum | Nematicidal effect | M. incognita | [182] |
| Lactones | Nigrospora sp. P. chalmydospora | Nematicidal effect | M. incognita | [183] |
| Milbemectin | S.bingchenggensis | Inhibits the reproduction of nematodes | M. javanica | [184] |
| Dimethyl disulfide (DMDS), methyl isovalerate (MIV) | Bacillus atrophaeus | Oxidative stress in nematodes lead to death | M. incognita | [185] |
| Dimethyl disulfide, S-methyl ester butanethioic acid | B. cereus | Fumigation and repellent activity | M. incognita | [186] |
| Benzeneacetaldehyde, 2-nonanone, decanal, 2-undecanone | B. megaterium | Nematicidal effect | M. incognita | [187] |
| 3-methoxy-2,5-dimethyl pyrazine,1-undecene, dimethyl disulfide | P. koreensis | Nematicidal effect | M. javanica | [188] |
| 1-octen-3-ol, 3-octanone | M. brunneum | Attraction and kill | M. hapla | [189] |
| 1,8-cineole | Annulohypoxylon sp. | Nematicidal effect | Bursaphelenchus xylophilus | [190] |
| 6-pentyl-2H-pyran-2-one | Trichoderma sp. | Nematicidal effect | B. xylophilus | [191] |
| Polyketides | ||||
| Bikaverin | F. oxysporum | Nematicidal effect | M. incognita Rotylenchulus reiniformis | [192] |
| Butyrolactone | Clonostachys rosea | Nematicidal effect | M. incognita | [193] |
| Alloaureothin and aureothin | Streptomyces sp. | Prevent egg hatching and juvenile mortality | B. xylophilus | [194] |
| 2,4-diacetylpholoroglucinol (DAPG) | P. fluroscens | Prevent egg hatching and juvenile mortality | M. incognita | [28] |
| 2,4-diacetylpholoroglucinol (DAPG) | P. fluroscens | Prevent egg hatching and juvenile mortality | M. javanica | [195] |
| Abamectin | S. avermitilis | Nematicidal effect | M. incognita | [196] |
| 4-heptanone | Daldinia concentrica | Nematicidal effect | M javanica | [197] |
| Phenols | ||||
| Trichostatin and dehydroxytrichostatin | S. nigrescens | Nematicidal effect | M. incognita | [198] |
| Trans cinnamic acid (t-CA) 5-phenylpent-4 enoic acid (PPA) and indole | Photorhabdus luminescens sonorensis | Nematicidal effect | M.incognita T. semipenetrans, | [199] |
| N-acetyltyramine. benzenepropanoic acid | Micromonospora sp. | Prevents egg hatching and juvenile mortality | M. incognita | [200] |
| Napthoquinone Fusarubin | Fusarium oxysporum | Affects the nervous system, leading to paralysis | M. incognita. | [193] |
| Nitrogen-containing compounds/Alkaloids | ||||
| Alkaloids | Penicillium bilaiae | Affects the nervous system, leading to paralysis and death | P. penetrans | [201] |
| Peptides | ||||
| Lucinostatin | Pacilomyces lilacinus | Prevents egg hatching and juvenile mortality | M. javanica | [202] |
| Rhabdopeptide | X. budapestensis | Nematicidal activity | M. incognita | [203] |
| Rhabdopeptides Fabclavines | Xenorhabdus sp. | Prevents egg hatching and juvenile mortality | M. javanica | [204] |
| Omphalotin | Omphalotis olearius | Disrupts nematode biology | M. incognita | [181] |
This enzymatic degradation leads to a weakening of the nematodes and increases their susceptibility to the action of environmental stress and other biocontrol agents.
6.5. Modulation of Plant Metabolism and Immunity
Microbial metabolites modulate plant hormonal and metabolic pathways to improve robustness. PGPR (plant growth-promoting rhizobacteria), such as Bacillus and Pseudomonas spp., enhance the production of auxins, gibberellins, and cytokinins, which help in root promotion and nutrient absorption [8]. These hormones, in association with secondary metabolites, such as alkaloids and phenolics, strengthen the plant defense responses against nematode infestation [179]. Moreover, BCAs can also induce the expression of genes that are associated with systemic acquired resistance (ISR) and further enhance the defense capacity of plants at molecular levels [177].
6.6. Disruption of Nematode Feeding and Reproduction
Microbial metabolites disrupt the feeding and reproductive behaviors of nematodes and impede nematode development. Enzymes, which are chemicals, can interfere with the nematode’s ability to find the feeding site or mate. For instance, B. amyloliquefaciens BaNCT02 and B. wiedmannii AzBw1 were effective in decreasing egg hatching and J2 emergence through the dual action of VOCs and lytic enzymes [180]. The interruption of these vital life processes reduces the rate and severity of nematode infestation.
7. Practical Applications of Secondary Metabolites Against Pests, Diseases, and Nematodes
7.1. Microbial Metabolites for Pest Management
Crop protection is essential in modern agriculture to ensure consistent yields and food security. In response, microbial pest and pathogen management has emerged as a promising alternative, offering high ecological safety and strong target specificity. These microbes often exert their pest-control effects through the production of bioactive metabolites that cause pathogenicity or directly kill host pests. Therefore, selecting an appropriate microbial strain for pest management largely depends on the types of metabolites it produces and their specific bioactivity against the target pest [205].
The bacterium Bacillus thuringiensis produces a β-exotoxin known as thuringiensin, a thermostable secondary metabolite with insecticidal activity against a wide range of insect orders, including Diptera, Coleoptera, Lepidoptera, Hymenoptera, Orthoptera, and Isoptera, as well as several nematode species [206].
Spinosad, a bacterial metabolite derived from Saccharopolyspora spinosa, is another successful example of microbial-based pesticide development for insect control [207]. The insecticidal activity of the metabolites produced by the novel insecticidal bacterium C.subtsugae was later confirmed by Martin [208], who discovered the bacterium through his research. Another well-known example is B.thuringiensis and its various subspecies, which exhibit insecticidal activity against a broad range of pests, including lepidopterans, coleopterans, and dipterans. These have been widely used in commercial formulations for controlling agricultural pests. Similarly, the toxin complex produced by the enterobacterium Xenorhabdus nematophilus has demonstrated insecticidal properties and plays a supportive role in the pathogenicity of its associated entomopathogenic nematodes [209].
Actinomycetes are promising candidates for the development of natural insecticides due to their remarkable ability to synthesize bioactive metabolites. Among these, polyketide compounds represent the primary class of insecticidal agents produced by actinomycetes, including well-known groups such as avermectins, spinosyns, polynactins, tetramycins, and related analogs [107]. Avermectins, derived from the fermentation of S. avermitilis, are widely commercialized for crop protection. Their molecular structure enables strong binding to the neuromuscular junctions of certain insects, acting as agonists of γ-aminobutyric acid (GABA)-gated chloride channels, ultimately leading to paralysis and death [210]. These compounds are effective against a wide range of insect orders, including Blattodea, Coleoptera, Diptera, Hymenoptera, Isoptera, and Lepidoptera. Abamectin and emamectin are commercially important insecticidal derivatives of avermectins. Milbemycins, though structurally distinct, share a similar mode of action and are produced by S. bingchenggensis [211].
7.2. Microbial Metabolites for Disease Management
Bacillus spp. possess various antifungal lipopeptides, such as surfactins, iturins, and fengycins, as well as antibiotics, volatile antifungal compounds, and active biomolecules. Such metabolites can enhance their biocontrol potential against plant pathogenic agents [212].
Metabolites and their derivatives isolated from Streptomyces spp. have also shown efficacious antifungal activity against different phytopathogenic fungi., e.g., salvianolic acid B can be inhibitory to the mycelia cells and spores of Alternaria spp., Fusarium spp., Colletotrichum spp., Cladosporium herbarum, and Botrytis cinerea. Another secondary metabolite, 6-amino-5-nitrosopyrimidine-2,4-diol, isolated from crude extract of Streptomyces amritsarensis V31 also displayed significant inhibitory potential towards the mycelial growth of various test fungi, like Rhizoctonia solani (7.5–65%), Alternaria alternata (5.5–52.7%), Aspergillus flavus (8–30.7%), Fusarium oxysporum f. lycopersici (25–44%), Sarocladium oryzae (11–55.5%), and Sclerotinia sclerotiorum (29.7–40.5%) [213,214].
Bioactive secondary metabolites from actinomycetes were shown to be effective against potato silver scurf disease, induced by the fungus Helminthosporium solani [215]. And above all, the efficiencies of producing antifungal metabolites are remarkably high for Streptomyces species. For instance, ansamycin-type antibiotics, including carbamitocins, are also produced by Amycolatopsis CP2808, from which ansamitocin exhibiting marked anticancer activity can be obtained. An endophyte actinomycete Nocardia sp. was previously reported as a bioresource for ansamitocin [216].
7.3. Microbial Metabolites as Nematode Management
Metabolites produced by actinomycetes—including volatile compounds, fatty acids, hydrogen sulfide, ammonia, alcohols, and phenolic compounds—have been associated with nematicidal activity [217]. Among these, polyketide compounds produced by Streptomyces, such as avermectins and their derivative abamectin, are particularly significant. These metabolites are key components of several widely used commercial biological nematicides. Rashad [218] identified 28 Streptomyces strains exhibiting nematicidal activity against M. incognita, potentially linked to their production of enzymes such as proteases, chitinases, and lipases. A nematicidal strain, Micromonospora sp. WH06, was isolated and identified. Benzenepropanoic acid (compound 4) exhibited the highest effect, causing 99.02% nematode mortality at 200 μg/mL in 72 h, and it also inhibited egg hatching, highlighting WH06 as a strong biocontrol candidate against M. incognita [200].
Volatile organic compounds (VOCs), which are naturally occurring, low-toxicity substances, show promise as biological nematicides. Several fungi are known to produce nematicidal VOCs; for example, F. oxysporum emits 2-methylbutyl acetate, 3-methylbutyl acetate, ethyl acetate, and 2-methylpropyl acetate—compounds effective against plant-parasitic nematodes [219]. Daldinia cf. concentrica produces VOCs that control M. javanica, with 4-heptanone identified as the primary active component. Similarly, Annulohypoxylon sp. FPYF3050 releases 1,8-cineole, which is highly effective against Bursaphelenchus xylophilus [154]. Although many nematicidal metabolites have been reported from nematode-trapping fungi (NTF) VOCs remain less explored. In Duddingtonia flagrans, three antimicrobial metabolites—flagranones A, B, and C—have been identified [181].
8. Formulation and Delivery Methods for Microbial Metabolites
The development of microbial metabolites into biopesticides is an important breakthrough, bringing the use of pesticides in focus for the target-oriented control. A bioformulation is a combination of the biologically active microbial biomass and its metabolites with a carrier. It has eco-friendly uses, including stimulating the growth of plants, improving the uptake of nutrients, and contributing to biocontrol [65]. Bioformulations are of different types, which include solid, liquid, encapsulated, and metabolites and cell-free culture supernatant-based formulations (Figure 5) [220].
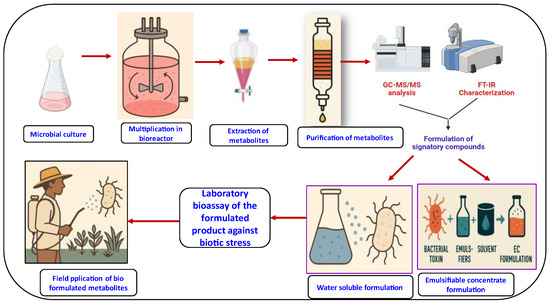
Figure 5.
Formulation of microbial metabolites against pests, diseases, and nematodes.
Formulations need to be developed in such a way that biocontrol agents are prepared with a sufficient high loading, so that effective and stable control of the target disease is obtained. To do so, the organisms need to be able to survive throughout the different processes of production, transport, storage, and application. Generally, the biocontrol agent is manufactured, formulated, packed, and conserved [221].
Limiting the range of acceptable storage conditions within the distribution channel—such as requiring refrigeration—can restrict market reach. However, improved packaging can help maintain viability by protecting the formulation from fluctuations in moisture and oxygen levels. In many cases, inconsistent conditions can be just as damaging as exposure to extreme temperatures. Repeated freeze–thaw cycles or other significant temperature shifts can harm or kill microbial cells and disrupt cell aggregates [222].
Another critical challenge to preserving high levels of viable spores arises during the application of the biocontrol agent. Formulations must be designed to ensure the organisms remain viable when used with standard farming equipment and stay alive long enough to effectively colonize plant roots or leaves. Some important factors to consider include rain fastness, leaf coverage, and soil penetration. One possible solution is to provide a pilot scale process for a formulation which has been designed to perform well with commonly employed adjuvants and to acquire such information with those adjuvants under different conditions [223].
Stickers may improve the adhesion to seeds and the survival of biocontrol agents and thus can be useful in seed treatments. Knowing the characteristics of the sticker is important; it needs to be effective and easy to apply. For instance, GA is a powerful adhesive, but to canvass it should be heated before use, while some adhesives can be coated at room temperature [224].
Foliar spray formulations should include ingredients that reduce water evaporation and often require adjuvants to protect the active agents from UV light [225]. Low-volume sprays applied at high pressure tend to create fine droplets that dry too quickly, which can prevent the moisture needed for the agent to start infecting the target. Using formulation components with specific properties can help address these issues [226]. Adjuvants can be added to slow down evaporation, improve coverage on leaf surfaces, reduce surface tension from waxes and oils, attract and retain moisture, and form films that help keep the surface moist [227].
9. Advances in Biotechnology and Synthetic Biology for Enhanced Metabolite Production
Genetic improvement in microbes is performed by manipulating and improving the strains for enhanced metabolite production and also for improving the efficacy of the metabolites and also for the exclusion of unwanted cometabolites. Microbial strain improvement can be performed by classical genetic methods (including genetic recombination) and by molecular genetic methods (Figure 6) [228]. Classical genetic methods for improvement in microbial metabolites rely mostly on mutation (using both physical and chemical mutagens) followed by rational screening. Rational screening is made for a particular characteristic which is different from that of the final interest but easier to detect. Microorganisms possess regulatory mechanisms that regulate metabolite production and thus prevent overproduction. So the mutants are to be selected for overproduction of desired metabolites. Genetic recombination methods for improvement are by sexual or parasexual cross in fungi and conjugation in actinomycetes and by protoplast fusion in both [229].
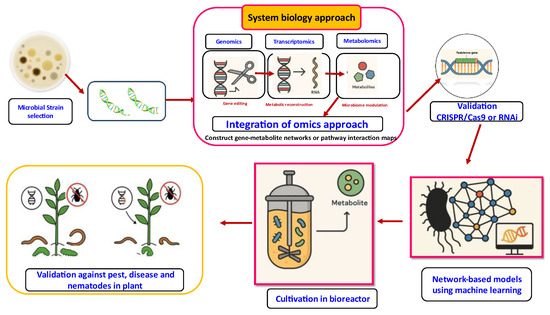
Figure 6.
Application of synthetic biology in microbial metabolites for plant health management.
There are many methods used for molecular genetic improvement for secondary metabolite production. It is reported that the genes responsible for metabolite biosynthesis are found in clusters in the organisms and which are amplified for higher copies. Molecular improvements can also be made by targeted duplication or amplification of secondary metabolite production genes and amplification of the whole pathway. The negative process of inactivating the competing pathways, silencing the regulatory genes, etc., can also used be in the genetic improvement process [8]. Exogenous manipulation of the microbial genome has increased the production of metabolites and also the effectiveness of biocontrol agents, optimizing their production levels. The expressions of certain genes required for metabolite biosynthesis are enhanced, leading to an augmentation in the formation of compounds of interest. Some of the genes encoding the metabolite degradation are down-regulated, thus preventing the degradation of precious metabolites.
Microbial synthetic biology (SynBio) has been defined as a branch of synthetic biology that uses microorganisms for manufacturing fine chemicals. SynBio in essence integrates and reconstitutes the genetic elements (genes, promoters, transcription factors) from different organisms (source) onto an expression chassis (mostly microorganisms but also advanced organisms have been considered) to produce a certain product of interest [230]. SynBio offers an attractive avenue in this regard, as it allows targeted engineering of prominent microbial strains, thereby unlocking the potential of the microbiome to enhance agricultural sustainability. New, strong strains, such as those found for both of these new species, and root colonizing strains from original communities could be genetically manipulated to increase plant growth without the use of chemical fertilizers and pesticides. This may be a relatively consistent benefit that can be offered by the introduction of plant growth-promoting (PGP) traits to these strains, even as crops change or conditions of culture vary [231].
Microbes engineered to produce antibiotics have also proved their ability to protect plants from biotic stresses [232]. L-threonine transaldolase (LTTA) catalyzed the formation of β-hydroxy-α-amino acids and was essential for the biosynthesis of (2S,3R)-2-amino-3-hydroxy-4-(4-nitrophenyl)butanoate in the plant-associated bacterium P. fluorescens. This metabolite has biocontrol properties and is also a precursor for the antibacterial agent obafluorin [232,233].
10. Conclusions
The microbial metabolites have potential to be used as environmentally safe pest control agents, providing the multiple effects of protecting the crops and causing negligible environmental harm.
Microbial secondary metabolites can act as an elicitor of plants to enhance the resistance of plants against biotic stress, such as pests, pathogens, and nematodes. They work in two key ways: directly, by generating substances that either damage or kill these threats, and indirectly, by stimulating the plant’s own defense mechanisms. While lab studies with the second greenhouse gas to be recognized show promise, translating those results to growing fields out in the real world can be more challenging. And as environmental conditions—such as temperature and humidity—shift, so too can the ways in which microbes grow and function, resulting in variable outcomes in the field. These are secondary metabolites produced by a variety of microorganisms that have various insecticidal, nematicidal, and antimicrobial activities and thus are excellent candidates for use in integrated pest management (IPM). Compared to conventional pesticides, microbial-based products may currently face higher production costs, scalability issues, and regulatory hurdles, which can impact their commercial viability. For agrochemical companies considering market entry, the cost of production, formulation stability, shelf life, and farmer adoption are critical factors that need to be addressed. Despite these challenges, the growing demand for sustainable and eco-friendly pest control solutions continues to drive research and investment into microbial metabolites, highlighting their transformative potential in IPM strategies.
Author Contributions
Writing—original draft, conceptualization: S.P.; writing—review and editing, supervision, resources: R.P.; supervision, conceptualization, writing—review and editing, validation: L.D.; visualization, supervision, conceptualization, resources: S.P. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Reveglia, P.; Corso, G.; Evidente, A. Advances on Bioactive Metabolites with Potential for the Biocontrol of Plant Pathogenic Bacteria. Pathogens 2024, 13, 1000. [Google Scholar] [CrossRef] [PubMed]
- Sansinenea, E. Bacillus Spp.: As Plant Growth-Promoting Bacteria. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 225–237. [Google Scholar]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Mechanisms and Impact of Rhizosphere Microbial Metabolites on Crop Health, Traits, Functional Components: A Comprehensive Review. Molecules 2024, 29, 5922. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Mathivanan, N. Management of Plant Diseases by Microbial Metabolites. In Bacteria in Agrobiology: Plant Nutrient Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 237–265. [Google Scholar]
- Ribeiro, V.P.; Bajsa-Hirschel, J.; Tamang, P.; Harries, M.D.; Meepagala, K.M. Bioactive Secondary Metabolites from Curvularia Spp.: Natural Alternatives for Pest Management in Agriculture. J. Nat. Pestic. Res. 2025, 12, 100117. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Genetically Modified Plants Based on Bacillus Genes and Commercial Bacillus-Based Biopesticides for Sustainable Agriculture. Horticulturae 2023, 9, 963. [Google Scholar] [CrossRef]
- Wend, K.; Zorrilla, L.; Freimoser, F.; Gallet, A. Microbial Pesticides–Challenges and Future Perspectives for Testing and Safety Assessment with Respect to Human Health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef]
- Subbanna, A.R.N.S.; Stanley, J.; Rajasekhara, H.; Mishra, K.K.; Pattanayak, A.; Bhowmick, R. Perspectives of Microbial Metabolites as Pesticides in Agricultural Pest Management. In Co-Evolution of Secondary Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–28. [Google Scholar]
- Rutledge, P.J.; Challis, G.L. Discovery of Microbial Natural Products by Activation of Silent Biosynthetic GENE clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef]
- Keasling, J.D. Synthetic Biology and the Development of Tools for Metabolic Engineering. Metab. Eng. 2012, 14, 189–195. [Google Scholar] [CrossRef]
- Salazar, F.; Ortiz, A.; Sansinenea, E. Characterisation of Two Novel Bacteriocin-Like Substances Produced by Bacillus amyloliquefaciens ELI149 with Broad-Spectrum Antimicrobial Activity. J. Glob. Antimicrob. Resist. 2017, 11, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Villas-Boas, S.G. Extracellular Microbial Metabolomics: The State of the Art. Metabolites 2017, 7, 43. [Google Scholar] [CrossRef]
- Beale, D.J.; Kouremenos, K.A.; Palombo, E. Microbial Metabolomics: Applications in Clinical, Environmental, and Industrial Microbiology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R. Metabolomic Tools for Secondary Metabolite Discovery from Marine Microbial Symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef]
- Shahriar, S.A.; Islam, M.N.; Chun, C.N.W.; Kaur, P.; Rahim, M.A.; Islam, M.M.; Uddain, J.; Siddiquee, S. Microbial Metabolomics Interaction and Ecological Challenges of Trichoderma Species as Biocontrol Inoculant in Crop Rhizosphere. Agronomy 2022, 12, 900. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hanooq, S.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of Metabolites of Bacillus Spp. and Their Application in Sustainable Plant Growth Promotion and Biocontrol. Front. Sustain. Food Syst. 2021, 5, 605195. [Google Scholar] [CrossRef]
- Villas-Bôas, S.G.; Roessner, U.; Hansen, M.A.E.; Smedsgaard, J.; Nielsen, J. Metabolome Analysis: An Introduction; Willey-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Aldridge, B.B.; Rhee, K.Y. Microbial Metabolomics: Innovation, Application, Insight. Curr. Opin. Microbiol. 2014, 19, 90–96. [Google Scholar] [CrossRef]
- Vaidyanathan, S. Profiling Microbial Metabolomes: What Do We Stand to Gain? Metabolomics 2005, 1, 17–28. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G.; Aggio, R. Analysis of Intracellular Metabolites from Microorganisms: Quenching and Extraction Protocols. Metabolites 2017, 7, 53. [Google Scholar] [CrossRef]
- Pande, S.; Kost, C. Bacterial Unculturability and the Formation of Intercellular Metabolic Networks. Trends Microbiol. 2017, 25, 349–361. [Google Scholar] [CrossRef]
- Maharana, C.; Kumar, P.; Hubballi, A.; Raj, M.; Paschapur, A.; Bhat, C.; Singh, A.; Arns, S. Secondary Metabolites of Microbials as Potential Pesticides. In Sustainable Management of Potato Pests and Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 111–142. [Google Scholar]
- Harman, G.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic Strains of Trichoderma Increase Plants’ Photosynthetic Capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a Promising Biological Control Agents for Plant Pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef] [PubMed]
- Dara, S.K. Microbial Metabolites as Pesticides. In Microbes for Sustainable lnsect Pest Management: Hydrolytic Enzyme & Secondary Metabolite–Volume 2; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 75–88. [Google Scholar]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of Microbial Secondary Metabolites: Regulation by the Carbon Source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, Z.; Chen, J.; Shen, Z.; Chen, W.; Chen, S.; Li, J. Enhancement of Spinosad Production in Saccharopolyspora Spinosa by Overexpression of the Complete 74-kb Spinosyn Gene Cluster. Microb. Cell Factories 2025, 24, 1–10. [Google Scholar] [CrossRef]
- Radwan, W.H.; Abdelhafez, A.A.; Mahgoub, A.E.; Zayed, M.S. Streptomyces Avermitilis MICNEMA2022: A New Biorational Strain for Producing Abamectin as an Integrated Nematode Management Agent. BMC Microbiol. 2024, 24, 329. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shen, X.; Jain, R.; Lin, Y.; Wang, J.; Sun, J.; Wang, J.; Yan, Y.; Yuan, Q. Synthesis of Chemicals by Metabolic Engineering of Microbes. Chem. Soc. Rev. 2015, 44, 3760–3785. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial Synthesis of Primary Metabolites: Current Trends and Future Prospects. In Fermentation Microbiology and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 57–74. [Google Scholar]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Ye, D.-X.; Liu, Y.; Zhang, X.-Y.; Zhou, Y.-L.; Zhang, L.; Yang, X.-L. Peptides, New Tools for Plant Protection in Eco-Agriculture. Adv. Agrochem. 2023, 2, 58–78. [Google Scholar] [CrossRef]
- Moe, L.A. Amino Acids in the Rhizosphere: From Plants to Microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef]
- Rai, V.K. Role of Amino Acids in Plant Responses to Stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Moormann, J.; Heinemann, B.; Hildebrandt, T.M. News About Amino Acid Metabolism in Plant-Microbe Interactions. Trends Biochem. Sci. 2022, 47, 839–850. [Google Scholar] [CrossRef]
- Botcazon, C.; Bergia, T.; Lecouturier, D.; Dupuis, C.; Rochex, A.; Acket, S.; Nicot, P.; Leclère, V.; Sarazin, C.; Rippa, S. Rhamnolipids and Fengycins, very Promising Amphiphilic Antifungal Compounds from Bacteria Secretomes, Act on Sclerotiniaceae Fungi Through Different Mechanisms. Front. Microbiol. 2022, 13, 977633. [Google Scholar] [CrossRef]
- Kumar, A.; Radhakrishnan, E. Microbial Endophytes: Functional Biology and Applications; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria Activates (+)-Δ-Cadinene Synthase Genes and Induces Systemic Resistance in Cotton Against Beet Armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhao, J.-L.; Bai, M.; Ping, X.-X.; Li, Y.-L.; Yang, Y.-H.; Mao, Z.-C.; Yang, G.-S.; Xie, B.-Y. Pochonia Chlamydosporia Isolate Pc-170-Induced Expression of Marker Genes for Defense Pathways in Tomatoes Challenged by Different Pathogens. Microorganisms 2021, 9, 1882. [Google Scholar] [CrossRef]
- He, S.; Huang, K.; Li, B.; Lu, G.; Wang, A. Functional Analysis of a Salicylate Hydroxylase in Sclerotinia sclerotiorum. J. Fungi 2023, 9, 1169. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Agrawal, L.; Singh, S.P.; Prateeksha; Singh, P.C.; Prasad, V.; Chauhan, P.S. Paenibacillus lentimorbus Induces Autophagy for Protecting Tomato from Sclerotium rolfsii Infection. Microbiol. Res. 2018, 215, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Ntemafack, A.; Chouhan, R.; Gandhi, S.G. Anti-Phytopathogenic and Plant Growth Promoting Potential of Endophytic Fungi Isolated from Dysoxylum gotadhora. Arch. Phytopathol. Plant Prot. 2022, 55, 454–473. [Google Scholar] [CrossRef]
- Wang, H.N.; Ke, X.; Jia, R.; Huang, L.G.; Liu, Z.Q.; Zheng, Y.G. Multivariate Modular Metabolic Engineering for Enhanced Gibberellic Acid Biosynthesis in Fusarium Fujikuroi. Bioresour. Technol. 2022, 364, 128033. [Google Scholar] [CrossRef]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia Solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Visser, R.; Holzapfel, W.H.; Bezuidenhout, J.J.; Kotzé, J.M. Antagonism of Lactic Acid Bacteria against Phytopathogenic Bacteria. Appl. Environ. Microbiol. 1986, 52, 552–555. [Google Scholar] [CrossRef]
- Murthy, K.N.M.M.; Savitha, J.; Srinivas, C. Lactic Acid Bacteria (LAB) as Plant Growth Promoting Bacteria (Pgpb) for the Control of Wilt of Tomato Caused by Ralstonia solanacearum. Pest Manag. Hortic. Ecosyst. 2012, 18, 60–65. [Google Scholar]
- Kim, S.; Kim, H.M.; Seo, H.J.; Yeon, J.; Park, A.R.; Yu, N.H.; Jeong, S.G.; Chang, J.Y.; Kim, J.C.; Park, H.W. Root-Knot Nematode (Meloidogyne incognita) Control Using a Combination of Lactiplantibacillus plantarum WiKim0090 and Copper Sulfate. J. Microbiol. Biotechnol. 2022, 32, 960–966. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus Plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef]
- Kim, B.; Park, A.R.; Song, C.W.; Song, H.; Kim, J.-C. Biological Control Efficacy and Action Mechanism of Klebsiella pneumoniae Jck-2201 Producing Meso-2,3-Butanediol Against Tomato Bacterial Wilt. Front. Microbiol. 2022, 13, 914589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Pan, H.X.; Tang, G.L. Production of Doramectin by Rational Engineering of the Avermectin Biosynthetic Pathway. Bioorg. Med. Chem. Lett. 2011, 21, 3320–3323. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial Volatiles as Plant Growth Inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Meza, L.C.; Quintana-Obregón, E.A.; Vargas-Arispuro, I.; Falcón-Rodríguez, A.B.; Aispuro-Hernández, E.; Virgen-Ortiz, J.J.; Martínez-Téllez, M.Á. Oligosaccharins as Elicitors of Defense Responses in Wheat. Polymers 2021, 13, 3105. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense Against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Battistelli, N.; Perpetuini, G.; Valbonetti, L.; Rossetti, A.P.; Perla, C.; Zulli, C.; Arfelli, G. Oenococcus oeni Lifestyle Modulates Wine Volatilome and Malolactic Fermentation Outcome. Front. Microbiol. 2021, 12, 736789. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Lactic Acid Bacteria: Their Antimicrobial Compounds and Their Uses in Food Production. Ann. Biol. Res. 2010, 1, 218–228. [Google Scholar]
- Olaoye, O.A.; Onilude, A.A. Quantitative Estimation of Antimicrobials Produced by Lactic Acid Bacteria Isolated from Nigerian Beef. Int. Food Res. J. 2011, 18, 1104–1110. [Google Scholar]
- Jaffar, N.S.; Jawan, R.; Chong, K.P. The Potential of Lactic Acid Bacteria in Mediating the Control of Plant Diseases and Plant Growth Stimulation in Crop Production-A Mini Review. Front. Plant Sci. 2023, 13, 1047945. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Choi, I.S.; Wi, S.G.; Ha, S.; Chun, H.H.; Hwang, I.M.; Chang, J.Y.; Choi, H.J.; Kim, J.C.; et al. Effective Approach to Organic Acid Production from Agricultural Kimchi Cabbage Waste and Its Potential Application. PLoS ONE 2018, 13, e0207801. [Google Scholar] [CrossRef]
- Alawamleh, A.; Ðurović, G.; Maddalena, G.; Guzzon, R.; Ganassi, S.; Hashmi, M.M.; Wäckers, F.; Anfora, G.; Cristofaro, A.D. Selection of Lactic Acid Bacteria Species and Strains for Efficient Trapping of Drosophila Suzukii. Insects 2021, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or Facilitators? Roles of Lipids in Plant Root–Microbe Interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, E.F.; Hashem, A.; Ezzat, S.M. Lipid Metabolism in Tomato and Bean as a Sensitive Monitor for Biocontrol of Wilt Diseases. Phytoparasitica 2006, 34, 516–522. [Google Scholar] [CrossRef]
- Siebers, M.; Brands, M.; Wewer, V.; Duan, Y.; Hölzl, G.; Dörmann, P. Lipids in Plant-Microbe Interactions. Biochim. Biophys. Acta 2016, 1861, 1379–1395. [Google Scholar] [CrossRef]
- Sansinenea, E.; Ortiz, A. Secondary Metabolites of Soil Bacillus Spp. Biotechnol. Lett. 2011, 33, 1523–1538. [Google Scholar] [CrossRef]
- Aamir, M.; Rai, K.; Zehra, A.; Dubey, M.; Kumar, S.; Shukla, V.; Upadhyay, R. Microbial Bioformulation-Based Plant Biostimulants: A Plausible Approach Toward Next Generation of Sustainable Agriculture. In Microbial Endophytes; Woodhead Publishing: Cambridge, UK, 2020; pp. 195–225. [Google Scholar]
- Abdel-Aziz, S.; Abo Elsoud, M.; Anise, A. Microbial Biosynthesis: A Repertory of Vital Natural Products. Food Biosynth. 2017, 1, 25–54. [Google Scholar]
- Firn, R.D.; Jones, C.G. The Evolution of Secondary Metabolism-a Unifying Model. Mol. Microbiol. 2000, 37, 989–994. [Google Scholar] [CrossRef]
- Junkins, E.N.; McWhirter, J.B.; McCall, L.-I.; Stevenson, B.S. Environmental Structure Impacts Microbial Composition and Secondary Metabolism. ISME Commun. 2022, 2, 15. [Google Scholar] [CrossRef]
- Chaabouni, I.; Guesmi, A.; Cherif, A. Secondary Metabolites of Bacillus: Potentials in Biotechnology. In Bacillus Thuringiensis Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 347–366. [Google Scholar]
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 1087–1119. [Google Scholar] [CrossRef]
- Song, H.C.; Shen, W.Y.; Dong, J.Y. Nematicidal Metabolites from Gliocladium roseum Ymf1.00133. Appl. Biochem. Microbiol. 2016, 52, 324–330. [Google Scholar] [CrossRef]
- Dhanarasu, S. Chromatography and Its Applications. Chem. Biol. 2012, 1, 212. [Google Scholar] [CrossRef]
- Balthazar, C.; St-Onge, R.; Léger, G.; Lamarre, S.G.; Joly, D.L.; Filion, M. Pyoluteorin and 2,4-Diacetylphloroglucinol are Major Contributors to Pseudomonas protegens Pf-5 Biocontrol Against Botrytis cinerea in Cannabis. Front. Microbiol. 2022, 13, 945498. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, P.; Zhao, W.; Su, Z.; Zhang, X.; Lu, X.; Li, S.; Ma, P.; Qinggang, G. Surfactin and Fengycin Contribute Differentially to the Biological Activity of Bacillus subtilis NCD-2 Against Cotton Verticillium Wilt. Biol. Control 2022, 174, 104999. [Google Scholar] [CrossRef]
- Tang, R.; Tan, H.; Dai, Y.; Li, L.a.; Huang, Y.; Yao, H.; Cai, Y.; Yu, G. Application of Antimicrobial Peptides in Plant Protection: Making Use of the Overlooked Merits. Front. Plant Sci. 2023, 14, 1139539. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Oviya, R.; Premalatha, K.; Mehetre, S.T.; Paramasivam, M.; Kannan, R.; Theradimani, M.; Pallavi, M.S.; Mukherjee, P.K.; Ramamoorthy, V. Production, Stability and Degradation of Trichoderma Gliotoxin in Growth Medium, Irrigation Water and Agricultural Soil. Sci. Rep. 2021, 11, 16536. [Google Scholar] [CrossRef]
- Tijerino, A.; Cardoza, R.E.; Moraga, J.; Malmierca, M.G.; Vicente, F.; Aleu, J.; Collado, I.G.; Gutiérrez, S.; Monte, E.; Hermosa, R. Overexpression of the Trichodiene Synthase Gene Tri5 Increases Trichodermin Production and Antimicrobial Activity in Trichoderma brevicompactum. Fungal. Genet. Biol. 2011, 48, 285–296. [Google Scholar] [CrossRef]
- Whalon, M.E.; Wingerd, B.A. Bt: Mode of Action and Use. Arch. Insect Biochem. Physiol. 2003, 54, 200–211. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef]
- Kumar, V.; Nautiyal, C.S. Plant Abiotic and Biotic Stress Alleviation: From an Endophytic Microbial Perspective. Curr. Microbiol. 2022, 79, 311. [Google Scholar] [CrossRef]
- Toopaang, W.; Bunnak, W.; Srisuksam, C.; Wattananukit, W.; Tanticharoen, M.; Yang, Y.-L.; Amnuaykanjanasin, A. Microbial Polyketides and Their Roles in Insect Virulence: From Genomics to Biological Functions. Nat. Prod. Rep. 2022, 39, 2008–2029. [Google Scholar] [CrossRef]
- Waldron, C.; Madduri, K.; Crawford, K.; Merlo, D.J.; Treadway, P.; Broughton, M.C.; Baltz, R.H. A Cluster of Genes for the Biosynthesis of Spinosyns, Novel Macrolide Insect Control Agents Produced by Saccharopolyspora Spinosa. Antonie Van Leeuwenhoek 2000, 78, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.A.; Dybas, R.A. Avermectins, a Novel Class of Compounds: Implications for Use in Arthropod Pest Control. Annu. Rev. Entomol. 1991, 36, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.E.; Andres, M.F.; Lacret, R.; Cabrera, R.; Gimenez, C.; Kaushik, N.; Gonzalez-Coloma, A. Antifeedant, Antifungal and Nematicidal Compounds from the Endophyte Stemphylium Solani Isolated from Wormwood. Sci. Rep. 2024, 14, 13500. [Google Scholar] [CrossRef]
- Díaz-González, S.; Andrés, M.F.; González-Sanz, C.; Sacristán, S.; González-Coloma, A. Nematicidal and Antifeedant Activity of Ethyl Acetate Extracts from Culture Filtrates of Arabidopsis Thaliana Fungal Endophytes. Sci. Rep. 2025, 15, 11332. [Google Scholar] [CrossRef]
- Horikoshi, R.; Goto, K.; Mitomi, M.; Oyama, K.; Hirose, T.; Sunazuka, T.; Ōmura, S. Afidopyropen, a Novel Insecticide Originating from Microbial Secondary Extracts. Sci. Rep. 2022, 12, 2827. [Google Scholar] [CrossRef]
- Liu, F.; Wang, N.; Wang, Y.; Yu, Z. The Insecticidal Activity of Secondary Metabolites Produced by Streptomyces Sp. SA61 Against Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Microorganisms 2024, 12, 2031. [Google Scholar] [CrossRef]
- Horikoshi, R.; Goto, K.; Mitomi, M.; Oyama, K.; Sunazuka, T.; Ōmura, S. Insecticidal Properties of Pyripyropene A, a Microbial Secondary Metabolite, Against Agricultural Pests. J. Pestic. Sci. 2018, 43, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ruan, L.; Peng, D.; Li, L.; Sun, M.; Yu, Z. Thuringiensin: A Thermostable Secondary Metabolite from Bacillus thuringiensis with Insecticidal Activity Against a Wide Range of Insects. Toxins 2014, 6, 2229–2238. [Google Scholar] [CrossRef]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae Enzymes and Toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Gupta, S. Identification and Directed Biosynthesis of Efrapeptins in the Fungus Tolypocladium geodes Gams (Deuteromycotina: Hyphomycetes). J. Chem. Ecol. 1991, 17, 1953–1962. [Google Scholar] [CrossRef]
- Olombrada, M.; Martínez-del-Pozo, A.; Medina, P.; Budia, F.; Gavilanes, J.G.; García-Ortega, L. Fungal Ribotoxins: Natural Protein-Based Weapons Against Insects. Toxicon 2014, 83, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Binnington, K.C.; Baule, V.J. Naturally Occurring Insecticidal Molecules as Candidates for Genetic Engineering. In Molecular Approaches to Fundamental and Applied Entomology; Oakeshott, J., Whitten, M.J., Eds.; Springer: New York, NY, USA, 1993; pp. 38–89. [Google Scholar]
- Islam, S.M.N.; Chowdhury, M.Z.H.; Mim, M.F.; Momtaz, M.B.; Islam, T. Biocontrol Potential of Native Isolates of Beauveria bassiana Against Cotton Leafworm Spodoptera Litura (Fabricius). Sci. Rep. 2023, 13, 8331. [Google Scholar] [CrossRef] [PubMed]
- Stilwell, M.D.; Cao, M.; Goodrich-Blair, H.; Weibel, D.B. Studying the Symbiotic Bacterium Xenorhabdus Nematophila in Individual, Living Steinernema Carpocapsae Nematodes Using Microfluidic Systems. mSphere 2018, 3, e00530-17. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, D.; Prabhu, S.; Rani, W.B.; Anandham, R. Isolation and Characterization of Toxins from Xenorhabdus nematophilus Against Ferrisia Virgata (Ckll.) on Tuberose, Polianthes Tuberosa. Toxicon 2018, 146, 42–49. [Google Scholar] [CrossRef]
- Wright, D.; Zydenbos, S.; Wessman, P.; O’Callaghan, M.; Townsend, R.; Jackson, T.; van Koten, C.; Mansfield, S. Surface Coating aids Survival of Serratia entomophila (Enterobacteriaceae) in Granules for Surface Application. Biocontrol. Sci. Technol. 2017, 27, 1–17. [Google Scholar] [CrossRef]
- Koivunen, M.; Chanbusarakum, L.; Fernández, L.; Asolkar, R.; Tan, E.; Wallner, D.; Marrone, P. Development of a New Microbial Insecticide Based on Chromobacterium Subtsugae; Crickmore, N., Enkerli, J., Glazer, I., Lopez-Ferber, M., Tkaczukeditors, C., Ehlers, R.U., Eds.; International Organization for Biological and Integrated Control of Noxious Animals and Plants (OIBC/OILB), West Palaearctic Regional Section (WPRS/SROP): Dijon, France, 2009; pp. 183–186. [Google Scholar]
- Tu, Y. The Discovery and Development of Artemisinins and Antimalarial agentsindex. In From Artemisia Annua L. to Artemisinins; Tu, Y., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 413–426. [Google Scholar]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms 2023, 11, 42. [Google Scholar] [CrossRef]
- Thomas, G.; Withall, D.; Birkett, M. Harnessing Microbial Volatiles to Replace Pesticides and Fertilizers. Microb. Biotechnol. 2020, 13, 1366–1376. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Bioprospecting Bacterial and Fungal Volatiles for Sustainable Agriculture. Trends Plant Sci. 2015, 20, 206–211. [Google Scholar] [CrossRef]
- Der, C.; Courty, P.E.; Recorbet, G.; Wipf, D.; Simon-Plas, F.; Gerbeau-Pissot, P. Sterols, Pleiotropic Players in Plant-Microbe Interactions. Trends Plant Sci. 2024, 29, 524–534. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of Polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, E.S.; Hwang, Y.S.; Choi, C.Y. Avermectin: Biochemical and Molecular Basis of its Biosynthesis and Regulation. Appl. Microbiol. Biotechnol. 2004, 63, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical Mode of Action, Biological Activity and Agricultural Importance. In Insecticides with Novel Modes of Action: Mechanisms and Application; Ishaaya, I., Degheele, D., Eds.; Springer: Berlin, Heidelberg, 1998; pp. 152–170. [Google Scholar]
- Li, S.; Yang, B.; Tan, G.-Y.; Ouyang, L.-M.; Qiu, S.; Wang, W.; Xiang, W.; Zhang, L. Polyketide Pesticides from Actinomycetes. Curr. Opin. Biotechnol. 2021, 69, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kankariya, R.A.; Chaudhari, A.B.; Gavit, P.M.; Dandi, N.D. 2,4-Diacetylphloroglucinol: A Novel Biotech Bioactive Compound for Agriculture. In Microbial Interventions in Agriculture and Environment: Volume 1: Research Trends, Priorities and Prospects; Singh, D.P., Gupta, V.K., Prabha, R., Eds.; Springer: Singapore, 2019; pp. 419–452. [Google Scholar]
- Almario, J.; Bruto, M.; Vacheron, J.; Prigent-Combaret, C.; Moënne-Loccoz, Y.; Muller, D. Distribution of 2,4-Diacetylphloroglucinol Biosynthetic Genes among the Pseudomonas spp. Reveals Unexpected Polyphyletism. Front. Microbiol. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-Borne Flavonoids Released into the Rhizosphere: Impact on Soil Bio-Activities Related to Plant Nutrition. A Review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Gören Sağlam, N.; Albayrak, F.Ö.; Ortaş, İ. Endophytic Fungal Alkaloids: Production and Applications. In Fungal Endophytes Volume I: Biodiversity and Bioactive Materials; Springer: Berlin/Heidelberg, Germany, 2025; pp. 341–363. [Google Scholar]
- Bacetty, A.A.; Snook, M.E.; Glenn, A.E.; Noe, J.P.; Hill, N.; Culbreath, A.; Timper, P.; Nagabhyru, P.; Bacon, C.W. Toxicity of Endophyte-Infected Tall Fescue Alkaloids and Grass Metabolites on Pratylenchus scribneri. Phytopathology 2009, 99, 1336–1345. [Google Scholar] [CrossRef]
- Leadmon, C.E.; Sampson, J.K.; Maust, M.D.; Macias, A.M.; Rehner, S.A.; Kasson, M.T.; Panaccione, D.G. Several Metarhizium Species Produce Ergot Alkaloids in a Condition-Specific Manner. Appl. Environ. Microbiol. 2020, 86, e00373-20. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, J.; Zhang, H.; Jiang, H.; Su, Z.; Liu, D.; Jie, L.; He, F. Antibacterial Spirooxindole Alkaloids from Penicillium brefeldianum Inhibit Dimorphism of Pathogenic Smut Fungi. Front. Microbiol. 2022, 13, 1046099. [Google Scholar] [CrossRef]
- Tang, Z.; Cao, X.; Zhang, H. Production of iturin A by Bacillus velezensis ND and Its Biological Control Characteristics. J. Basic Microbiol. 2023, 63, 179–189. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption Vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Sarkar, T.; Chetia, M.; Chatterjee, S. Antimicrobial Peptides and Proteins: From Nature’s Reservoir to the Laboratory and Beyond. Front. Chem. 2021, 9, 691532. [Google Scholar] [CrossRef]
- Niu, X.; Thaochan, N.; Hu, Q. Diversity of Linear Non-Ribosomal Peptide in Biocontrol Fungi. J. Fungi 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, D.; Finking, R.; Marahiel, M.A. Nonribosomal Peptides: From Genes to Products. Nat. Prod. Rep. 2003, 20, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Prazdnova, E.V.; Gurnani, M.; Bhardwaj, P.; Sharma, S.; Sushkova, S.; Mandzhieva, S.S.; Minkina, T.; Sudan, J.; et al. Nature’s Antimicrobial Arsenal: Non-Ribosomal Peptides from Pgpb for Plant Pathogen Biocontrol. Fermentation 2023, 9, 597. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Sansinenea, E. Antimicrobial Secondary Metabolites from Agriculturally Important Bacteria as Next-Generation Pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.A.; Ahmad, I.Z.; Saeed, M. Current Approaches Toward Production of Secondary Plant Metabolites. J. Pharm. Bioallied. Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Geat, N.; Jadon, K.S.; Verma, A.; Sharma, R.; Rajput, L.S.; Mahla, H.R.; Kakani, R.K. Isolation and Characterization of Biocontrol Microbes for Development of Effective Microbial Consortia for Managing Rhizoctonia Bataticola Root Rot of Cluster Bean Under Hot Arid Climatic Conditions. Microorganisms 2024, 12, 2331. [Google Scholar] [CrossRef]
- Thangaraj, P.; Balamurali, A.S.; Muthusamy, N. Biological Control of Trichoderma spp.: Mechanisms of Action Against Phytopathogens, Insect Pests, and Its Multifaceted Roles in Agro-Ecosystems. Environ. Conserv. J. 2025, 26, 302–314. [Google Scholar] [CrossRef]
- Suganthi, M.; Arvinth, S.; Senthilkumar, P. Comparative Bioefficacy of Bacillus and Pseudomonas Chitinase Against Helopeltis Theivora in Tea (Camellia sinensis (L.) O.Kuntze. Physiol. Mol. Biol. Plants 2020, 26, 2053–2060. [Google Scholar] [CrossRef]
- Attia, Z.; Dalal, A.; Moshelion, M. Vascular Bundle Sheath and Mesophyll Cells Modulate Leaf Water Balance in Response to Chitin. Plant J. Cell Mol. Biol. 2020, 101, 1368–1377. [Google Scholar] [CrossRef]
- Shaana, O.M.; Nisha, M.S. Harnessing Bacteria for Sustainable Pest Management: A Biological Approach. Arch. Curr. Res. Int. 2025, 25, 262–274. [Google Scholar] [CrossRef]
- Bangaru, N. Bacillus thuringiensis Cry and Cyt Toxins: Mechanisms of Action, Resistance Management and Impact on Host Immune Responses. Res. J. Chem. Environ. 2025, 29, 101–110. [Google Scholar] [CrossRef]
- Shah, A.Z.; Ma, C.; Zhang, Y.; Zhang, Q.; Xu, G.; Yang, G. Decoyinine Induced Resistance in Rice against Small Brown Planthopper Laodelphax Striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- McMillan, H.M. Plants Target Gut Microbes to Reduce Insect Herbivore Damage. Proc. Natl. Acad. Sci. USA 2023, 120, e2308568120. [Google Scholar] [CrossRef]
- Dai, H.; Liu, B.; Yang, L.; Yao, Y.; Liu, M.; Xiao, W.; Li, S.; Ji, R.; Sun, Y. Investigating the Regulatory Mechanism of the Sesquiterpenol Nerolidol from a Plant on Juvenile Hormone-Related Genes in the Insect Spodoptera exigua. Int. J. Mol. Sci. 2023, 24, 13330. [Google Scholar] [CrossRef]
- Gao, Q.; Song, L.; Sun, J.; Cao, H.Q.; Wang, L.; Lin, H.; Tang, F. Repellent Action and Contact Toxicity Mechanisms of the Essential oil Extracted from Chinese Chive Against Plutella Xylostella Larvae. Arch. Insect Biochem. Physiol. 2019, 100, e21509. [Google Scholar] [CrossRef]
- Kandasamy, D.; Gershenzon, J.; Andersson, M.N.; Hammerbacher, A. Volatile Organic Compounds Influence the Interaction of the Eurasian Spruce Bark Beetle (Ips typographus) with Its Fungal Symbionts. ISME J. 2019, 13, 1788–1800. [Google Scholar] [CrossRef]
- Mi, T.; Sheng, C.; Lee, C.K.; Nguyen, P.; Zhang, Y.V. Harnessing Insect Chemosensory and Mechanosensory Receptors Involved in Feeding for Precision Pest Management. Life 2025, 15, 110. [Google Scholar] [CrossRef]
- Durak, R.; Jedryczka, M.; Czajka, B.; Dampc, J.; Wielgusz, K.; Borowiak-Sobkowiak, B. Mild Abiotic Stress Affects Development and Stimulates Hormesis of Hemp Aphid Phorodon cannabis. Insects 2021, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.M. The Fog of War: How Network Buffering Protects Plants’ Defense Secrets from Pathogens. PLOS Genet. 2017, 13, e1006713. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (Isr) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Neha, T.; Kumar, A.; Sayrav, K.; Prasad, B. Isolation and Screening of Potent Antibiotic-Producing Actinomycete. J. Adv. Sci. Res. 2024, 15, 22–26. [Google Scholar] [CrossRef]
- Sukar, N.A.; Elazab, N.T. Molecular Characterization and Fungicidal Activity of Some Isolated Endophytic Fungi from Some Wild Plants in Egypt. J. Plant Prot. Pathol. 2020, 11, 627–634. [Google Scholar] [CrossRef]
- Sulaiman, M.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; Rajagopal, M.; Rusdi, N.A.; Seelan, J.S.S.; Suleiman, M.; Zakaria, Z.A.; Wiart, C. Antimicrobial Secondary Metabolites from the Mangrove Plants of Asia and the Pacific. Mar. Drugs 2022, 20, 643. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, G.; Mathur, V. Plant Endophytes: A Treasure House of Antimicrobial Compounds. In Medicinal Plants and Antimicrobial Therapies; Kumar, V., Shriram, V., Dey, A., Eds.; Springer Nature: Singapore, 2024; pp. 107–123. [Google Scholar]
- Intra, B.; Mungsuntisuk, I.; Nihira, T.; Igarashi, Y.; Panbangred, W. Identification of Actinomycetes from Plant Rhizospheric Soils with Inhibitory Activity Against Colletotrichum spp., the Causative Agent of Anthracnose Disease. BMC Res. Notes 2011, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural Products in Crop Protection. Bioorganic Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef]
- Saxena, S.; Pandey, A.K. Microbial Metabolites as Eco-Friendly Agrochemicals for the Next Millennium. Appl. Microbiol. Biotechnol. 2001, 55, 395–403. [Google Scholar] [CrossRef]
- Ruiu, L. Insect Pathogenic Bacteria in Integrated Pest Management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef]
- Valan Arasu, M.; Esmail, G.A.; Al-Dhabi, N.A. Hypersaline Actinomycetes and Their Biological Applications. In Actinobacteria-Basics and Biotechnological Applications; Dhanasekaran, D., Jiang, Y., Eds.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Zaid, R.; Koren, R.; Kligun, E.; Gupta, R.; Leibman-Markus, M.; Mukherjee, P.K.; Kenerley, C.M.; Bar, M.; Horwitz, B.A. Gliotoxin, an Immunosuppressive Fungal Metabolite, Primes Plant Immunity: Evidence from Trichoderma virens-Tomato Interaction. mBio 2022, 13, e0038922. [Google Scholar] [CrossRef] [PubMed]
- Chengzeng, Z.; Yichao, G.; Bin, W. Harzianolides H-J: Three New Butenolides Isolated from the Fungus Trichoderma harzianum. Nat. Prod. J. 2025, 15, 1–5. [Google Scholar] [CrossRef]
- Montesinos, L.; Bundó, M.; Izquierdo, E.; Campo, S.; Badosa, E.; Rossignol, M.; Montesinos, E.; San Segundo, B.; Coca, M. Production of Biologically Active Cecropin a Peptide in Rice Seed Oil Bodies. PLoS ONE 2016, 11, e0146919. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wu, L.; Gao, W.; Jiang, J. Iturin a Extracted from Bacillus subtilis Wl-2 Affects Phytophthora Infestans via Cell Structure Disruption, Oxidative Stress, and Energy Supply Dysfunction. Front. Microbiol. 2020, 11, 536083. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, D.; Phelan, A.; Murphy, C.D.; Cobb, S.L. Fengycin a Analogues with Enhanced Chemical Stability and Antifungal Properties. Org. Lett. 2021, 23, 4672–4676. [Google Scholar] [CrossRef]
- Liu, L.; Jin, X.; Lu, X.; Guo, L.; Lu, P.; Yu, H.; Lv, B. Mechanisms of Surfactin from Bacillus subtilis Sf1 against Fusarium foetens: A Novel Pathogen Inducing Potato Wilt. J. Fungi 2023, 9, 367. [Google Scholar] [CrossRef]
- Clough, S.E.; Jousset, A.; Elphinstone, J.G.; Friman, V.P. Combining in Vitro and in Vivo Screening to Identify Efficient Pseudomonas Biocontrol Strains Against the Phytopathogenic Bacterium Ralstonia solanacearum. Microbiologyopen 2022, 11, e1283. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dou, G.; Gao, M.; Ren, F.; Li, R.; Zhang, X.; Yan, D.-H. Annulohypoxylon sp. Fpyf3050 Produces Volatile Organic Compounds against the Pine Wood Nematode, Bursaphelenchus xylophilus. Nematology 2020, 22, 245–255. [Google Scholar] [CrossRef]
- Chen, D.M.; Yang, H.J.; Huang, J.G.; Yuan, L. Lysobacter enzymogenes Le16 Autolysates Have Potential as Biocontrol Agents-Lysobacter sp. Autolysates as Biofungicide. J. Appl. Microbiol. 2020, 129, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cui, R.; Wang, D.; Yi, Y.; Feng, D. Antagonistic Effects and Action Mechanism of Bacillus velezensis HX-6 Against Ralstonia solanacearum. Chin. J. Pestic. Sci. 2025, 27, 322–331. [Google Scholar]
- Huang, K.; Zhang, B.; Shen, Z.-Y.; Cai, X.; Liu, Z.-Q.; Zheng, Y.-G. Enhanced Amphotericin B Production by Genetically Engineered Streptomyces nodosus. Microbiol. Res. 2021, 242, 126623. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D Produced by Bacillus amyloliquefaciens Is Involved in the Antagonistic Interaction with the Plant-Pathogenic Fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef]
- Lin, X.; Kück, U. Cephalosporins as Key Lead Generation Beta-Lactam Antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 8007–8020. [Google Scholar] [CrossRef]
- Léger, G.; Novinscak, A.; Biessy, A.; Lamarre, S.; Filion, M. In Tuber Biocontrol of Potato Late Blight by a Collection of Phenazine-1-Carboxylic Acid-Producing Pseudomonas spp. Microorganisms 2021, 9, 2525. Microorganisms 2021, 9, 2525. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial Peptaibols from Trichoderma pseudokoningii Induce Programmed Cell Death in Plant Fungal Pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Durán, N.; Castro, G.R.; Portela, R.W.; Fávaro, W.J.; Durán, M.; Tasic, L.; Nakazato, G. Violacein and its Antifungal Activity: Comments and Potentialities. Lett. Appl. Microbiol. 2022, 75, 796–803. [Google Scholar] [CrossRef]
- Timmermann, T.; Poupin, M.J.; Vega, A.; Urrutia, C.; Ruz, G.A.; González, B. Gene Networks Underlying the Early Regulation of Paraburkholderia phytofirmans PsJN Induced Systemic Resistance in Arabidopsis. PLoS ONE 2019, 14, e0221358. [Google Scholar] [CrossRef]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus Ar156 through a Ja/Et- and Npr1-Dependent Signaling Pathway and Activates Pamp-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Panicker, S.; Sayyed, R. Hydrolytic Enzymes from Pgpr Against Plant Fungal Pathogens. In Antifungal Metabolites of Rhizobacteria for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2022; pp. 211–238. [Google Scholar]
- Agarwal, M.; Dheeman, S.; Dubey, R.C.; Kumar, P.; Maheshwari, D.K.; Bajpai, V.K. Differential Antagonistic Responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum Causing Fungal Diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017, 205, 40–47. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.-K.; Han, J.A.; Lee, H.-S. Exploiting Bacterial Genera as Biocontrol Agents: Mechanisms, Interactions and Applications in Sustainable Agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Ge, W.; Zhang, L.; Meng, F.; Tian, C. Study on Biocontrol Potential of Volatile Organic Compounds Produced by Pseudomonas atacamensis GZ-3 on Poplar Anthracnose. Ind. Crops Prod. 2025, 224, 120402. [Google Scholar] [CrossRef]
- Ghosh, P.; De, T.; Maiti, T. Role of Acc Deaminase as a Stress Ameliorating Enzyme of Plant Growth-Promoting Rhizobacteria Useful in Stress Agriculture: A Review. In Role of Rhizospheric Microbes in Soil: Volume 1: Stress Management and Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2018; pp. 57–106. [Google Scholar]
- Ehinmitan, E.; Losenge, T.; Mamati, E.; Ngumi, V.; Juma, P.; Siamalube, B. Biosolutions for Green Agriculture: Unveiling the Diverse Roles of Plant Growth-Promoting Rhizobacteria. Int. J. Microbiol. 2024, 2024, 6181491. [Google Scholar] [CrossRef]
- Toyota, K.; Watanabe, T. Recent Trends in Microbial Inoculants in Agriculture. Microbes Environ. JSME 2013, 28, 403–404. [Google Scholar] [CrossRef]
- Lorrai, R.; Ferrari, S. Host Cell Wall Damage During Pathogen Infection: Mechanisms of Perception and Role in Plant-Pathogen Interactions. Plants 2021, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zeng, Y.; Yan, X.; Wang, Z.; Guo, L.; Zhu, Y.; Wang, Y.; He, X. Volatile Organic Compounds of Bacillus velezensis Gj-7 against Meloidogyne hapla through Multiple Prevention and Control Modes. Molecules 2023, 28, 3182. [Google Scholar] [CrossRef] [PubMed]
- Settu, V.; Annaiyan, S.; Manu, J. Revealing the Genetic Arsenal of Bacillus firmus TNAU1: Unleashing Nematicidal and Plant Growth Promotion Traits. Physiol. Mol. Plant Pathol. 2023, 129, 102177. [Google Scholar] [CrossRef]
- Moreira, A.C.S.; Lopes, E.A.; Visôtto, L.E.; Soares, M.S.; Londe, M.L.A.; Ribeiro, L.B.; Terra, W.C.; Moreira, S.I.; Pedroso, M.P.; Pereira, L.F.; et al. Bacillus amyloliquefaciens Strain Banct02: An Antagonist with Multiple Mechanisms of Action Against Meloidogyne incognita. Plant Pathol. 2025, 74, 320–329. [Google Scholar] [CrossRef]
- Hu, L. Integration of Multiple Volatile Cues into Plant Defense Responses. New Phytol. 2022, 233, 618–623. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Kalia, N.; Bhardwaj, R.; Sidhu, G.P.S.; Sharma, A. Mechanisms of Plant Defense Under Pathogen Stress: A Review. Curr. Protein. Pept. Sci. 2021, 22, 376–395. [Google Scholar] [CrossRef]
- Fallahzadeh-Mamaghani, V.; Shahbazi-Ezmareh, R.; Shirzad, A.; Moslehi, S. Possible Mechanisms of Action of Bacillus wiedmannii AzBw1, a Biocontrol Agent of the Root-Knot Nematode, Meloidogyne arenaria. Egypt. J. Biol. Pest Control 2023, 33, 28. [Google Scholar] [CrossRef]
- Mani, J.; Kandasamy, D.; Vendan, R.T.; Sankarasubramanian, H.; Mannu, J.; Nagachandrabose, S. Unlocking the Potential of Streptomyces species as Promising Biological Control Agents Against Phytonematodes. Physiol. Mol. Plant Pathol. 2024, 134, 102465. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In Vitro Antibacterial, Antifungal, Nematocidal and Growth Promoting Activities of Trichoderma hamatum Fb10 and Its Secondary Metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from Nematophagous Fungi and Nematicidal Natural Products from Fungi as an Alternative for Biological Control. Part I: Metabolites from Nematophagous Ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef]
- Li, G.-H.; Zhang, K.-Q. Natural Nematicidal Metabolites and Advances in Their Biocontrol Capacity on Plant Parasitic Nematodes. Nat. Prod. Rep. 2023, 40, 646–675. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus Gbsc56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Yin, N.; Liu, R.; Zhao, J.L.; Khan, R.A.A.; Li, Y.; Ling, J.; Liu, W.; Yang, Y.H.; Xie, B.Y.; Mao, Z.C. Volatile Organic Compounds of Bacillus cereus Strain Bc-Cm103 Exhibit Fumigation Activity against Meloidogyne incognita. Plant Dis. 2021, 105, 904–911. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Ma, L.; Zhang, K.; Duan, C.; Mo, M. Characterisation of Volatiles Produced from Bacillus megaterium YFM3.25 and Their Nematicidal Activity Against Meloidogyne incognita. Eur. J. Plant Pathol. 2009, 126, 417–422. [Google Scholar] [CrossRef]
- Wolfgang, A.; Taffner, J.; Guimarães, R.A.; Coyne, D.; Berg, G. Novel Strategies for Soil-Borne Diseases: Exploiting the Microbiome and Volatile-Based Mechanisms Toward Controlling Meloidogyne-Based Disease Complexes. Front. Microbiol. 2019, 10, 1296. [Google Scholar] [CrossRef]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the Entomopathogenic Fungus, Metarhizium brunneum, Attract and Kill Plant Parasitic Nematodes. Biol. Control. 2021, 152, 104472. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; de Vries Reinder, H.; Chakraborty, P.; Song, C.; Zhao, X.; Scheffers, D.-J.; Roelfes, G.; Kuipers Oscar, P. Novel Modifications of Nonribosomal Peptides from Brevibacillus laterosporus Mg64 and Investigation of Their Mode of Action. Appl. Environ. Microbiol. 2020, 86, e01981-20. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Z.; Lei, L.; Xia, Z.; Shao, L.; Zhang, K.; Li, G. Nematicidal Effect of Volatiles Produced by Trichoderma sp. J. Asia-Pac. Entomol. 2012, 15, 647–650. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Dutta, T.K. Anti-Nemic Secondary Metabolites Produced by Fusarium oxysporum f. sp. ciceris. J. Asia-Pac. Entomol. 2016, 19, 631–636. [Google Scholar] [CrossRef]
- Nagaraj, G.; Rengasamy, K.; Thiruvengadam, R.; Swarnakumari, N.; Saravanakumar, D. Nematicidal Action of Clonostachys rosea Against Meloidogyne incognita: In-Vitro and In-Silico Analyses. J. Taibah Univ. Sci. 2024, 18, 2288723. [Google Scholar] [CrossRef]
- Kang, M.-K.; Kim, J.-H.; Liu, M.-J.; Jin, C.-Z.; Park, D.-J.; Kim, J.; Sung, B.-H.; Kim, C.-J.; Son, K.-H. New Discovery on the Nematode Activity of Aureothin and Alloaureothin Isolated from Endophytic Bacteria Streptomyces sp. Ae170020. Sci. Rep. 2022, 12, 3947. [Google Scholar] [CrossRef]
- Meyer, S.L.; Halbrendt, J.M.; Carta, L.K.; Skantar, A.M.; Liu, T.; Abdelnabby, H.M.; Vinyard, B.T. Toxicity of 2,4-Diacetylphloroglucinol (Dapg) to Plant-Parasitic and Bacterial-Feeding Nematodes. J. Nematol. 2009, 41, 274–280. [Google Scholar] [PubMed]
- Siddiqui, I.; Shaukat, S. Suppression of Root-Knot Disease by Pseudomonas fluorescens Cha0 in Tomato: Importance of Bacterial Secondary Metabolite, 2,4-Diacetylpholoroglucinol. Soil Biol. Biochem. 2003, 35, 1615–1623. [Google Scholar] [CrossRef]
- Liarzi, O.; Bucki, P.; Braun Miyara, S.; Ezra, D. Bioactive Volatiles from an Endophytic Daldinia Cf. Concentrica Isolate Affect the Viability of the Plant Parasitic Nematode Meloidogyne javanica. PLoS ONE 2016, 11, e0168437. [Google Scholar] [CrossRef]
- Park, E.J.; Jang, H.J.; Park, J.Y.; Yang, Y.K.; Kim, M.J.; Park, C.S.; Lee, S.; Yun, B.S.; Lee, S.J.; Lee, S.W.; et al. Efficacy Evaluation of Streptomyces nigrescens Ka-1 Against the Root-Knot Nematode Meloidogyne incognita. Biol. Control 2023, 179, 105150. [Google Scholar] [CrossRef]
- Kusakabe, A.; Wang, C.; Xu, Y.M.; Molnár, I.; Stock, S.P. Selective Toxicity of Secondary Metabolites from the Entomopathogenic Bacterium Photorhabdus luminescens Sonorensis Against Selected Plant Parasitic Nematodes of the Tylenchina Suborder. Microbiol. Spectr. 2022, 10, e0257721. [Google Scholar] [CrossRef]
- Ran, Y.; Zhang, Y.; Wang, X.; Li, G. Nematicidal Metabolites from the Actinomycete Micromonospora sp. Wh06. Microorganisms 2022, 10, 2274. [Google Scholar] [CrossRef]
- Nakahara, S.; Kusano, M.; Fujioka, S.; Shimada, A.; Kimura, Y. Penipratynolene, a Novel Nematicide from Penicillium bilaiae Chalabuda. Biosci. Biotechnol. Biochem. 2004, 68, 257–259. [Google Scholar] [CrossRef]
- Park, J.O.; Hargreaves, J.R.; McConville, E.J.; Stirling, G.R.; Ghisalberti, E.L.; Sivasithamparam, K. Production of Leucinostatins and Nematicidal Activity of Australian Isolates of Paecilomyces lilacinus (Thom) Samson. Lett. Appl. Microbiol. 2004, 38, 271–276. [Google Scholar] [CrossRef]
- Bi, Y.; Gao, C.; Yu, Z. Rhabdopeptides from Xenorhabdus budapestensis Sn84 and Their Nematicidal Activities against Meloidogyne incognita. J. Agric. Food Chem. 2018, 66, 3833–3839. [Google Scholar] [CrossRef] [PubMed]
- Abebew, D.; Sayedain, F.S.; Bode, E.; Bode, H.B. Uncovering Nematicidal Natural Products from Xenorhabdus Bacteria. J. Agric. Food Chem. 2022, 70, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, S.; Rajadurai, G. Biological Control of Plant Parasitic-Nematodes by Plant Growth Promoting-Rhizobacteria. Plant Health Arch. 2023, 1, 5–7. [Google Scholar] [CrossRef]
- Aswathi, N.; Balakrishnan, N.; Srinivasan, T.; Kokiladevi, E.; Raghu, R. Diversity of Bt toxins and Their Utility in Pest Management. Egypt. J. Biol. Pest Control 2024, 34, 40. [Google Scholar] [CrossRef]
- Sparks, T.C.; Dripps, J.E.; Watson, G.B.; Paroonagian, D. Resistance and Cross-Resistance to the Spinosyns–A Review and Analysis. Pestic. Biochem. Physiol. 2012, 102, 1–10. [Google Scholar] [CrossRef]
- Martin, P.A.W.; Gundersen-Rindal, D.; Blackburn, M.; Buyer, J. Chromobacterium subtsugae sp. nov., a Betaproteobacterium Toxic to Colorado potato Beetle and Other Insect Pests. Int. J. Syst. Evol. Microbiol. 2007, 57, 993–999. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Xenorhabdus spp.: An Overview of the Useful Facets of Mutualistic Bacteria of Entomopathogenic Nematodes. Life 2022, 12, 1360. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, K.V. Recent Advancement in the Development of Biopesticides by Actinomycetes for the Control of Insect Pests. In Plant Growth Promoting Actinobacteria: A New Avenue for Enhancing the Productivity and Soil Fertility of Grain Legumes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 47–62. [Google Scholar]
- He, H.; Ye, L.; Li, C.; Wang, H.; Guo, X.; Wang, X.; Zhang, Y.; Xiang, W. Sbbr/Sbba, an Important Arpa/Afsa-Like System, Regulates Milbemycin Production in Streptomyces bingchenggensis. Front. Microbiol. 2018, 9, 1064. [Google Scholar] [CrossRef]
- González-Jaramillo, L.M.; Aranda, F.J.; Teruel, J.A.; Villegas-Escobar, V.; Ortiz, A. Antimycotic Activity of Fengycin C Biosurfactant and its Interaction with Phosphatidylcholine Model Membranes. Colloids Surf. B Biointerfaces 2017, 156, 114–122. [Google Scholar] [CrossRef]
- Sharma, M.; Manhas, R.K. Purification and Characterization of Salvianolic acid B from Streptomyces sp. M4 Possessing Antifungal Activity Against Fungal Phytopathogens. Microbiol. Res. 2020, 237, 126478. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, B.N.; Verma, S.; Choudhary, P.; Das, S.; Chakdar, H.; Murugan, K.; Goswami, S.K.; Saxena, A.K. Bioactive Antifungal Metabolites Produced by Streptomyces amritsarensis V31 Help to Control Diverse Phytopathogenic Fungi. Braz J. Microbiol. 2021, 52, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ning, Q.; Yang, Y.; Liu, Y.; Niu, S.; Hu, X.; Pan, H.; Bu, Z.; Chen, N.; Guo, J.; et al. Endophytic Streptomyces hygroscopicus Osish-2-Mediated Balancing between Growth and Disease Resistance in Host Rice. mBio 2021, 12, e0156621. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary Metabolites and Biodiversity of Actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Silva, G.d.C.; Kitano, I.T.; Ribeiro, I.A.d.F.; Lacava, P.T. The Potential Use of Actinomycetes as Microbial Inoculants and Biopesticides in Agriculture. Front. Soil Sci. 2022, 2, 833181. [Google Scholar] [CrossRef]
- Rashad, F.M.; Fathy, H.M.; El-Zayat, A.S.; Elghonaimy, A.M. Isolation and Characterization of Multifunctional Streptomyces Species with Antimicrobial, Nematicidal and Phytohormone Activities from Marine Environments in Egypt. Microbiol. Res. 2015, 175, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.C.; Campos, V.P.; Martins, S.J.; Costa, L.S.A.S.; da Silva, J.C.P.; Barros, A.F.; Lopez, L.E.; Santos, T.C.N.; Smant, G.; Oliveira, D.F. Volatile Organic Molecules from Fusarium oxysporum Strain 21 with Nematicidal Activity Against Meloidogyne incognita. Crop Prot. 2018, 106, 125–131. [Google Scholar] [CrossRef]
- Mishra, J.; Arora, N. Bioformulations for Plant Growth Promotion and Combating Phytopathogens: A Sustainable Approach. Bioformulations Sustain. Agric. 2016, 3–33. [Google Scholar]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. In How Research Can Stimulate the Development of Commercial Biological Control Against Plant Diseases; De Cal, A., Melgarejo, P., Magan, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 275–293. [Google Scholar]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial Bioformulation: A Microbial Assisted Biostimulating Fertilization Technique for Sustainable Agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef]
- Leggett, M.; Leland, J.; Kellar, K.; Epp, B. Formulation of Microbial Biocontrol agents–an Industrial Perspective. Can. J. Plant Pathol. 2011, 33, 101–107. [Google Scholar] [CrossRef]
- Temprano, F.J.; Albareda, M.; Camacho, M.; Daza, A.; Santamaría, C.; Rodríguez-Navarro, D.N. Survival of Several Rhizobium/Bradyrhizobium Strains on Different Inoculant Formulations and Inoculated Seeds. Int. Microbiol. 2002, 5, 81–86. [Google Scholar] [CrossRef]
- Fravel, D. ASDS Keynote Address Session 7. Hurdles and Bottlenecks on the Road to Biocontrol of Plant Pathogens. Australas. Plant Pathol. 1999, 28, 53–56. [Google Scholar] [CrossRef]
- Green, S.; Stewart-Wade, S.M.; Boland, G.J.; Teshler, M.P.; Liu, S.H. Formulating Microorganisms for Biological Control of Weeds. Plant-Microbe Interact. Biol. Control 1998, 249–280. [Google Scholar]
- Burges, H.D. Formulation of Mycoinsecticides. In Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments; Burges, H.D., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 131–185. [Google Scholar]
- Barrios-Gonzalez, J.; Fernández, F.; Tomasini, A. Microbial Secondary Metabolites Production and Strain Improvement. Indian J. Biotechnol. 2003, 2, 322–333. [Google Scholar]
- Elander, R.; Lowe, D. Fungal Biotechnology: An Overview. Handb. Appl. Mycol. 1994, 4, 1–34. [Google Scholar]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome Engineering: Synthetic Biology of Plant-Associated Microbiomes in Sustainable Agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar] [CrossRef]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering Rhizobacteria for Sustainable Agriculture. Isme J. 2021, 15, 949–964. [Google Scholar] [CrossRef]
- Patel, J.K.; Archana, G. Engineered Production of 2,4-Diacetylphloroglucinol in the Diazotrophic Endophytic Bacterium Pseudomonas sp. Ws5 and Its Beneficial Effect in Multiple Plant-Pathogen Systems. Appl. Soil Ecol. 2018, 124, 34–44. [Google Scholar] [CrossRef]
- Xu, L.; Wang, L.C.; Su, B.M.; Xu, X.Q.; Lin, J. Multi-Enzyme Cascade for Improving β-Hydroxy-α-Amino Acids Production by Engineering L-Threonine Transaldolase and Combining Acetaldehyde Elimination System. Bioresour. Technol. 2020, 310, 123439. [Google Scholar] [CrossRef]
- Han, S.-W.; Yoshikuni, Y. Microbiome Engineering for Sustainable Agriculture: Using Synthetic Biology to Enhance Nitrogen Metabolism in Plant-Associated Microbes. Curr. Opin. Microbiol. 2022, 68, 102172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).