Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Botanical Substances and Cultivation Circumstances
2.2. Sample Collection
2.3. Measurement of Physiological and Biochemical Indices
Sample Preparation
2.4. Extraction, Identification, and Analysis of Metabolites
2.5. Quantitative Detection of Flavonoids and Phenolic Substances
2.6. Data Analysis of Flavonoids and Phenolic Substances
2.7. Physiological and Biochemical Data Analysis
3. Results
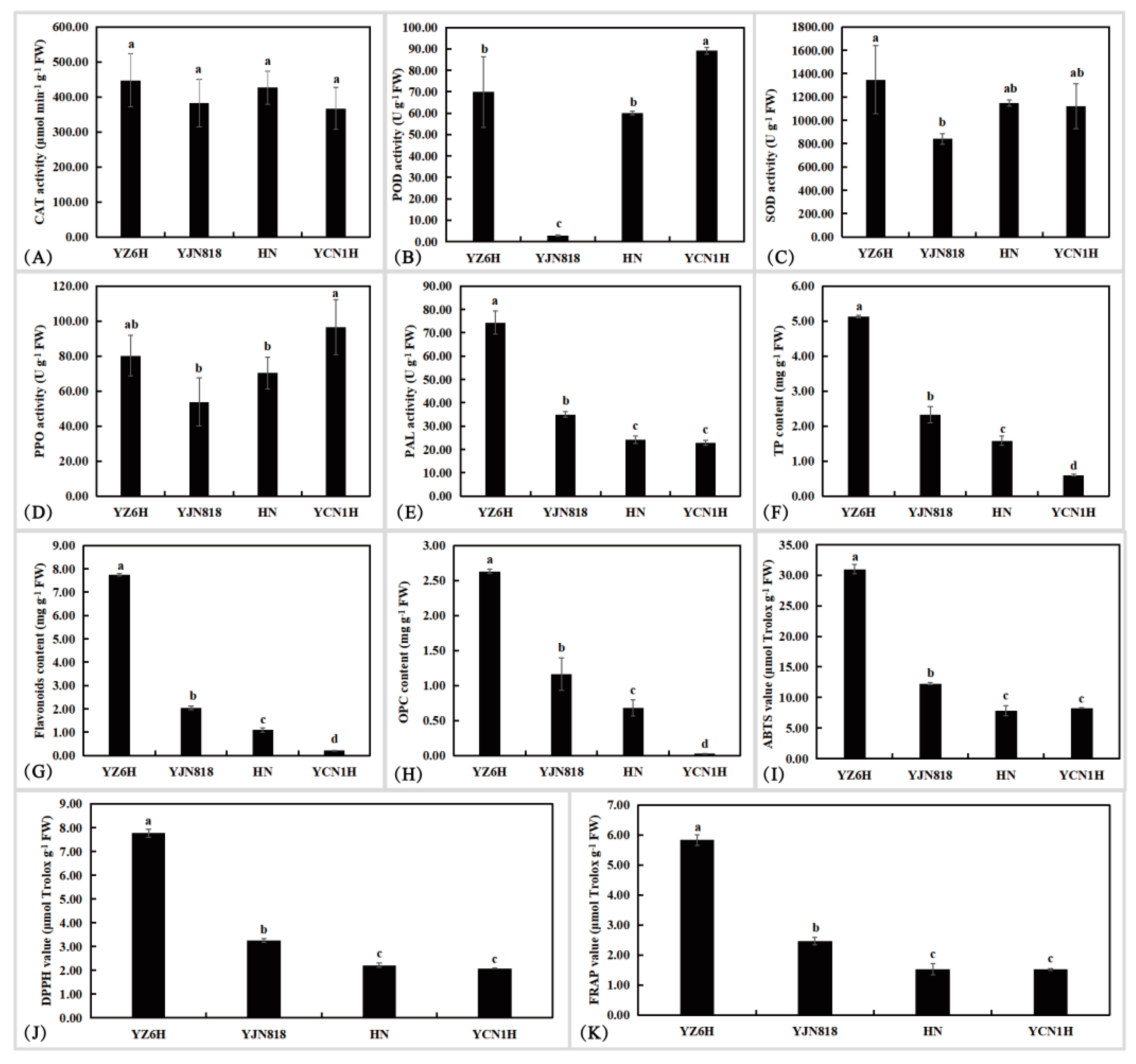
3.1. Analysis of Physiological and Biochemical Indices
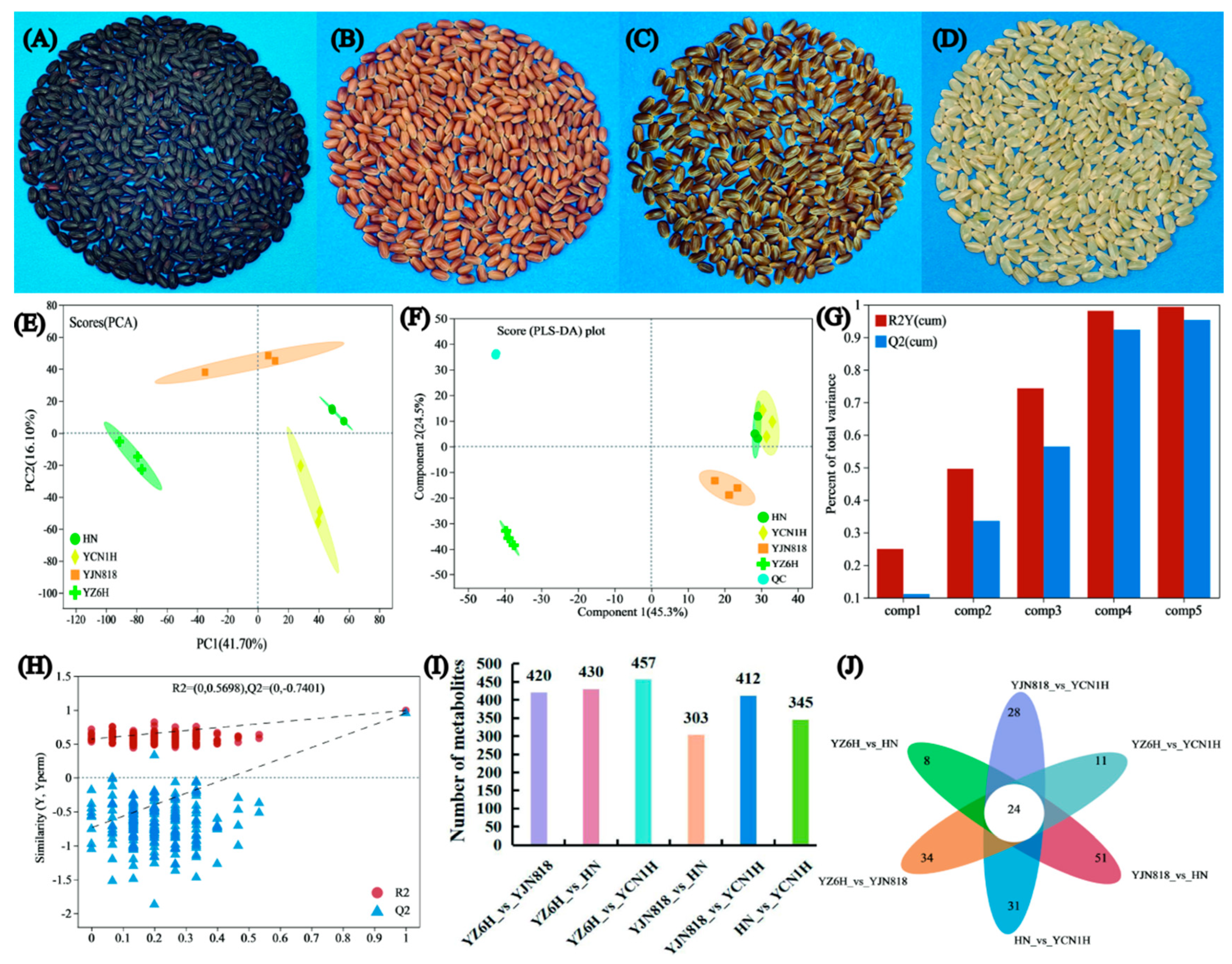
3.2. Multiple Statistical Analysis
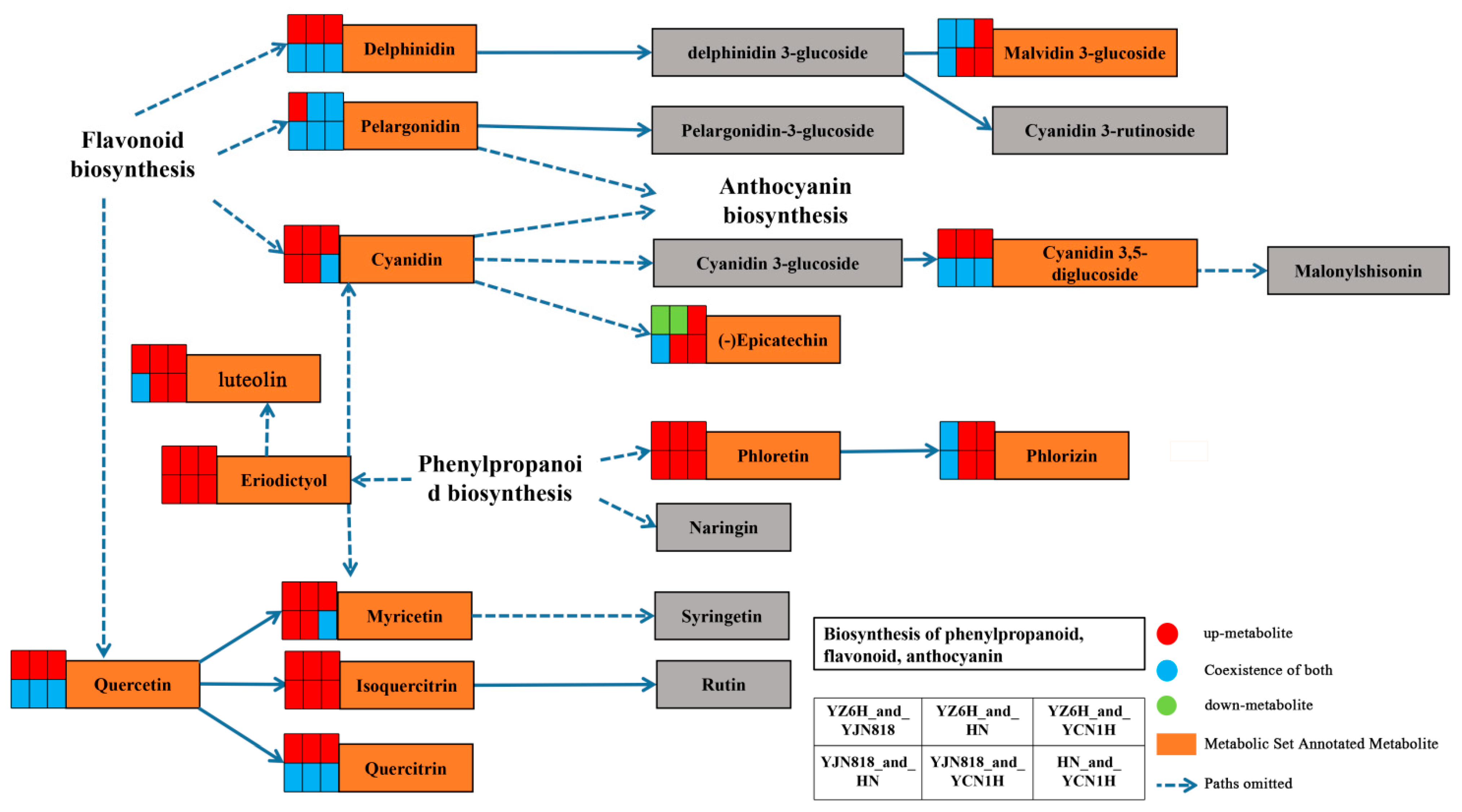
3.3. KEGG Analysis
3.4. Quantitative Analysis of Flavonoids and Phenolic Compounds
3.5. Evaluation of the Relationships between Physiological and Biochemical Indices and Flavonoid Metabolites through Correlation Analysis
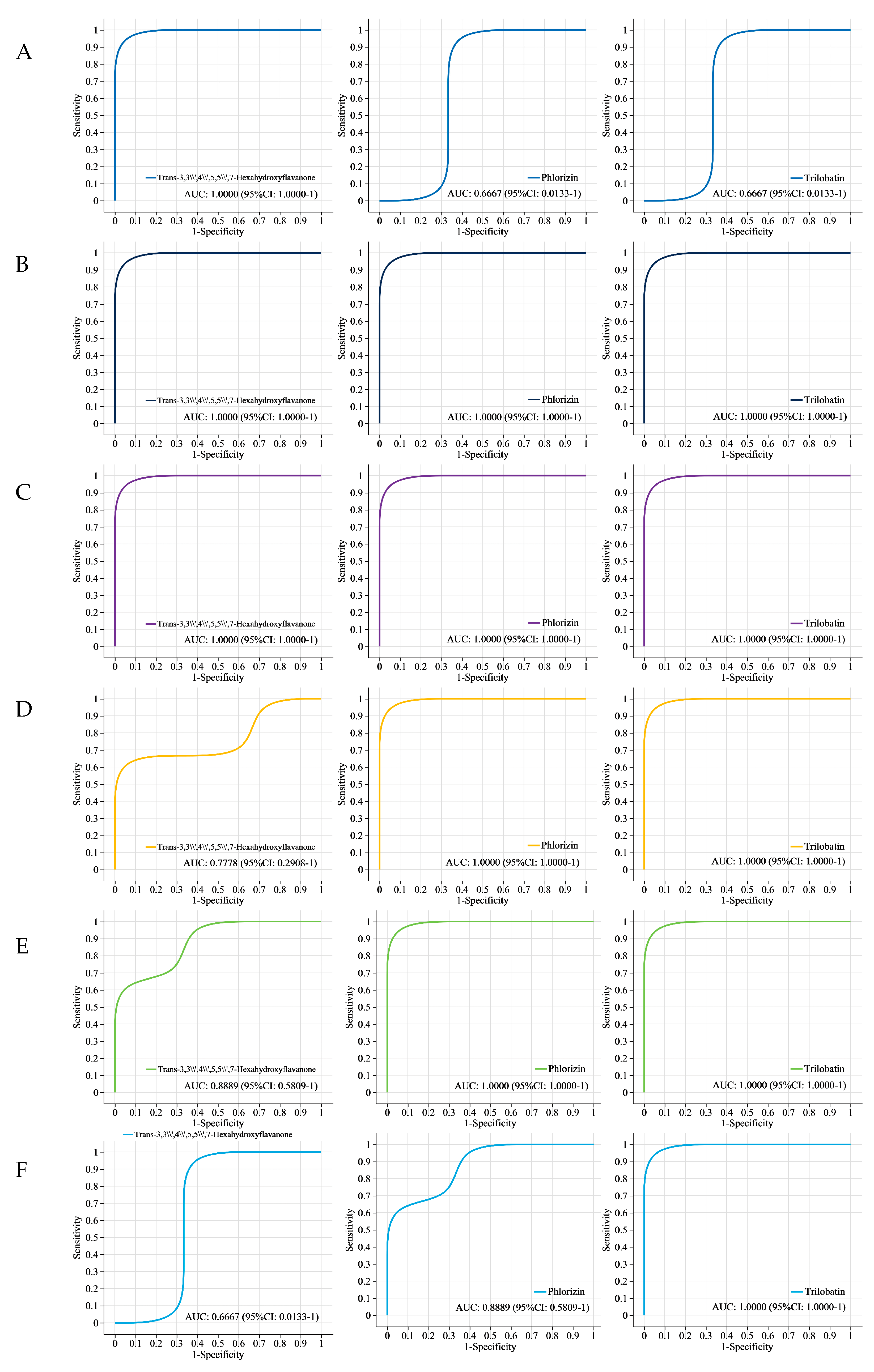
3.6. Receiver Operating Characteristic (ROC) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice: Review on paradigm and its potential benefit in human health. Sci. Technol. 2020, 97, 355–365. [Google Scholar]
- Chen, X.; Tao, Y.; Ali, A.; Zhuang, Z.; Guo, D.; Guo, Q.; Riaz, A.; Zhang, H.; Xu, P.; Liao, Y.; et al. Transcriptome and proteome profiling of different colored rice reveals physiological dynamics involved in the flavonoid pathway. Int. J. Mol. Sci. 2019, 20, 2463. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhu, H.; Huang, C.; Liu, C.; Chang, Y.; Kong, Z.; Zhou, Z.; Wang, G.; Lin, Y.; et al. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol. 2019, 223, 705–721. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, X.; Li, A.; Wang, R.; Ni, X.; Hu, J.; Wei, H.; Zhang, H.; Xiong, Q. The main nutritional components in colored rice grains. LWT-Food Sci. Technol. 2024, 191, 115663. [Google Scholar] [CrossRef]
- Aalim, H.; Wang, D.; Luo, Z. Black rice (Oryza sativa L.) processing: Evaluation of physicochemical properties, in vitro starch digestibility, and phenolic functions linked to type 2 diabetes. Food Res. Int. 2021, 141, 109898. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Sun, Y.; Wang, X.; Zheng, L.; Zhang, H.; Jiang, L.; Zhu, X.; Xiong, H. Drum drying-and extrusion-black rice anthocyanins exert anti-inflammatory effects via suppression of the NF-κB/MAPKs signaling pathways in LPS-induced RAW 264.7 cells. Food Biosci. 2021, 41, 100841. [Google Scholar] [CrossRef]
- Liu, D.; Ji, Y.L.; Zhao, J. Black rice (Oryza sativa L.) reduces obesity and improves lipid metabolism in C57BL/6J mice fed a high-fat diet. J. Funct. Foods. 2020, 64, 103605. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Feng, Y.; Liu, J.; Pan, R.; Lee, S.M.; Lin, L. Myricanol mitigates lipid accumulation in 3T3-L1 adipocytes and high fat diet-fed zebrafish via activating AMP-activated protein kinase. Food Chem. 2019, 270, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Qi, S.S.; He, J. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, S.Y.; Park, Y.J.; Lim, S.-H.; Ha, S.-H.; Park, S.U.; Lee, B.; Kim, J.K. Metabolite profiling reveals distinct modulation of complex metabolic networks in non-pigmented, black, and red rice (Oryza sativa L.) cultivars. Metabolites 2021, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Samyor, D.; Deka, S.C.; Das, A.B. Effect of extrusion conditions on the physicochemical and phytochemical properties of red rice and passion fruit powder based extrudates. J. Food Sci. Tech. 2018, 55, 5003–5013. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The genetic basis and nutritional benefits of pigmented rice grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Rathna Priya, T.S.; Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. Nutritional and functional properties of coloured rice varieties of South India: A review. J. Ethn. Foods. 2019, 6, 11. [Google Scholar] [CrossRef]
- Upanan, S.; Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Wongpoomchai, R.; Limtrakul, P. The proanthocyanidin-rich fraction obtained from red rice germ and bran extract induces HepG2 hepatocellular carcinoma cell apoptosis. Molecules 2019, 24, 813. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhang, R.; Yang, X.; Sun, Y.; Shi, L.; Xue, P. Optimization of ultrasound-assisted extraction by response surface methodology, antioxidant capacity, and tyrosinase inhibitory activity of anthocyanins from red rice bran. Food Sci. Nutr. 2020, 8, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Lim, M.J.; Kim, N.H.; Barathikannan, K.; Vijayalakshmi, S.; Elahi, F.; Ham, H.J.; Oh, D.H. Quantification of amino acids, phenolic compounds profiling from nine rice varieties and their antioxidant potential. Antioxidants 2022, 11, 839. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Park, W.J.; Shelton, D.R.; Peterson, C.J.; Martin, T.J.; Wehling, R.L.; Kachman, S.D.; Wehling, R.L. Variation in polyphenol oxidase activity and quality characteristics among hard white wheat and hard red winter wheat samples. Cereal Chem. 1997, 74, 7–11. [Google Scholar] [CrossRef]
- Aydaş, S.B.; Ozturk, S.; Aslım, B. Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem. 2013, 136, 164–169. [Google Scholar] [CrossRef]
- Reuveni, R. Peroxidase Activity as a Biochemical Marker for Resistance of Muskmelon (Cucumis melo) to Pseudoperonospora cubensis. Phytopathology 1992, 82, 749–753. [Google Scholar] [CrossRef]
- Chu, M.J.; Du, Y.M.; Liu, X.M.; Yan, N.; Wang, F.Z.; Zhang, Z.F. Extraction of proanthocyanidins from Chinese wild rice (Zizania latifolia) and analyses of structural composition and potential bioactivities of different fractions. Molecules 2019, 24, 1681. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive compounds and antioxidant capacity of small berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, J.; Shi, Q.; Zhang, Y.; Sun, C.; Li, A.; Lu, W.; Hu, J.; Zhou, N.; Wei, H.; et al. The key metabolites associated with nutritional components in purple glutinous rice. Food Res. Int. 2022, 160, 111686. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Sun, C.; Li, A.; Zhang, J.; Shi, Q.; Zhang, Y.; Hu, J.; Zhou, N.; Wei, H.; Liu, B.; et al. Metabolomics and biochemical analyses revealed metabolites important for the antioxidant properties of purple glutinous rice. Food Chem. 2022, 389, 133080. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, J.; Sun, C.; Wang, R.; Wei, H.; He, H.; Zhou, D.; Zhang, H.; Zhu, J. Metabolomics revealed metabolite biomarkers of antioxidant properties and flavonoid metabolite accumulation in purple rice after grain filling. Food Chem. X 2023, 18, 100720. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice-not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Amrinola, W.; Sitanggang, A.B.; Kusnandar, F.; Budijanto, S.E. Characterization of three cultivars of Indonesian glutinous rice: A basis for developing rice-based functional food. Ann. Univ. Dunarea Galati Fascicle VI-Food Technol. 2021, 45, 141–156. [Google Scholar] [CrossRef]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Li, W.; Yu, X.; Zhang, B.; Shang, L.; Xie, Y.; Li, Y.; Ding, A.; Shi, J.; Dou, Y.; et al. Genome-wide analysis, metabolomics, and transcriptomics reveal the molecular basis of ZlRc overexpression in promoting phenolic compound accumulation in rice seeds. Food Front. 2023, 4, 849–866. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Júnior, M.R.M.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar]
- Rodboon, T.; Okada, S.; Suwannalert, P. Germinated riceberry rice enhanced protocatechuic acid and vanillic acid to suppress melanogenesis through cellular oxidant-related tyrosinase activity in B16 cells. Antioxidants 2020, 9, 247. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Luangsuphabool, T.; Wongpoomchai, R. Protocatechuic acid as a potent anticarcinogenic compound in purple rice bran against diethylnitrosamine-initiated rat hepatocarcinogenesis. Sci. Rep. 2022, 12, 10548. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059. [Google Scholar]
- Indrianingsih, A.W.; Prihantini, A.I.; Tachibana, S. α-Glucosidase inhibitor and antioxidant activity of procyanidin, an isolated compound from Quercus gilva Blume leaves. J. Appl. Pharm. Sci. 2022, 12, 213–218. [Google Scholar] [CrossRef]
- Hibi, Y.; Yanase, E. Oxidation of procyanidins with various degrees of condensation: Influence on the color-deepening phenomenon. J. Agric. Food Chem. 2019, 67, 4940–4946. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Yang, X.; Zhu, G.; Lu, X.; Jia, F.; Diao, B.; Yu, S.; Ali, A.; Zhang, H.; et al. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res. Int. 2022, 161, 111726. [Google Scholar] [CrossRef]
- Bennett, C.; Sookwong, P.; Jakmunee, J.; Mahatheeranont, S. Smartphone digital image colorimetric determination of the total monomeric anthocyanin content in black rice via the pH differential method. Anal. Methods 2021, 13, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Carrington, A.M.; Manuel, D.G.; Fieguth, P.W.; Ramsay, T.; Osmani, V.; Wernly, B.; Bennett, C.; Hawken, S.; Magwood, O.; Sheikh, Y.; et al. Deep ROC analysis and AUC as balanced average accuracy, for improved classifier selection, audit and explanation. IEEE Trans. Pattern Anal. 2022, 45, 329–341. [Google Scholar] [CrossRef]
| Pathway Description | Pathway_ID | Metabolites | p Value |
|---|---|---|---|
| KEGG pathways between YZ6H and YJN818 | |||
| Citrate cycle (TCA cycle) | map00020 | C00158; C00311; C00417 | 0.0016 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | map00400 | C00251; C00944; C00493 | 0.0074 |
| Anthocyanin biosynthesis | map00942 | C05905; C05904; C05908; C08639 | 0.0075 |
| Flavone and flavonol biosynthesis | map00944 | C10107; C00389; C01750; C01514; C01265; C05623 | 0 |
| Flavonoid biosynthesis | map00941 | C10107; C09727; C06562; C00389; C05631; C01709; C01514; C05908; C05905; C05904; C00774 | 0 |
| KEGG pathways between YZ6H and HN | |||
| Anthocyanin biosynthesis | map00942 | C05905; C05908; C08639 | 0.0597 |
| Flavone and flavonol biosynthesis | map00944 | C10107; C00389; C01750; C01514; C01265; C05623 | 0.0001 |
| Flavonoid biosynthesis | map00941 | C10107; C09727; C00389; C05631; C01709; C00774; C01514; C05908; C05905; C01604; C12127; C12136 | 0 |
| KEGG pathways between YZ6H and YCN1H | |||
| Phenylalanine, tyrosine and tryptophan biosynthesis | map00400 | C00251; C00944; C00493 | 0.012 |
| Anthocyanin biosynthesis | map00942 | C05905; C12140; C05908; C08639 | 0.0138 |
| Flavone and flavonol biosynthesis | map00944 | C10107; C00389; C01750; C01514; C01265; C05623; C12627 | 0 |
| Flavonoid biosynthesis | map00941 | C10107; C09727; C06562; C00389; C05631; C01709; C00774; C01514; C05908; C05905; C01604; C12127; C12136 | 0 |
| KEGG pathways between YJN818 and HN | |||
| Anthocyanin biosynthesis | map00942 | C05905 | 0.5967 |
| Flavone and flavonol biosynthesis | map00944 | C10107; C05623 | 0.1518 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | map00400 | C00251; C00944; C00493 | 0.0106 |
| Flavonoid biosynthesis | map00941 | C10107; C05631; C05905; C12136; C12127; C00774 | 0.0004 |
| Alanine, aspartate and glutamate metabolism | map00250 | C00025; C00158; C00026; C00064; C00940; C00049 | 0 |
| KEGG pathways between YJN818 and YCN1H | |||
| Anthocyanin biosynthesis | map00942 | C05905; C12140 | 0.2973 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | map00400 | C00078; C00251; C00944; C00493 | 0.0022 |
| Phenylpropanoid biosynthesis | map00940 | C00482; C05158; C02666; C02325; C01197 | 0.005 |
| Flavone and flavonol biosynthesis | map00944 | C10107; C01514; C12627; C05623 | 0.0095 |
| Flavonoid biosynthesis | map00941 | C10107; C09727; C06562; C05631; C01709; C00774; C01514; C05905; C01604; C12127; C12136 | 0 |
| KEGG pathways between HN and YCN1H | |||
| Phenylalanine, tyrosine and tryptophan biosynthesis | map00400 | C00078; C00944 | 0.0575 |
| Anthocyanin biosynthesis | map00942 | C12140 | 0.5359 |
| Flavone and flavonol biosynthesis | map00944 | C01514; C12627; C05623 | 0.0202 |
| Phenylpropanoid biosynthesis | map00940 | C00482; C05158; C02666; C01197; C12205 | 0.001 |
| Flavonoid biosynthesis | map00941 | C09727; C06562; C01514; C01709; C05631; C12136; C12127; C00774 | 0 |
| Metabolites | YZ6H | YJN818 | HN | YCN1H |
|---|---|---|---|---|
| 2,4-Dihydroxybenzoic acid | 153.57 ± 25.45a | 128.03 ± 5.62a | 138.46 ± 15.14a | 152.92 ± 2.3a |
| 2,6-Dihydroxybenzoic acid | 85.49 ± 5.66c | 112.67 ± 5.96ab | 105.39 ± 2.42b | 123.29 ± 4.7a |
| 3,4-Dihydroxybenzaldehyde | 48.12 ± 4.98c | 823.57 ± 54.3a | 143.67 ± 15.19b | 0 ± 0c |
| 4-Hydroxybenzoic acid | 174.26 ± 14.95b | 55.53 ± 8.22d | 92.58 ± 6.27c | 233.96 ± 1.98a |
| 4-Hydroxycinnamic acid | 1180.27 ± 16.56b | 828.89 ± 36.26c | 1131.57 ± 20.34b | 1250.14 ± 22.18a |
| Apiin | 5.9 ± 0.34b | 11.06 ± 1.25a | 11.29 ± 1.18a | 11.81 ± 1.54a |
| Aromadendrin | 16.81 ± 0.36a | 13.24 ± 0.96b | 12.37 ± 0.72b | 1.42 ± 0.02c |
| Caffeic acid | 181.23 ± 22.01b | 253.5 ± 33.52b | 345.82 ± 47.58a | 79.02 ± 1.32c |
| Caftaric acid | 8.55 ± 1.36c | 11.06 ± 0.47bc | 28.61 ± 3.04a | 13.84 ± 0.85b |
| Catechin | 35.51 ± 2.69c | 9363.44 ± 357.32a | 3681.15 ± 195.08b | 0 ± 0c |
| Chlorogenic acid | 8.94 ± 0.56a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Coniferaldehyde | 0 ± 0b | 0 ± 0b | 0 ± 0b | 2.07 ± 0.42a |
| Cosmosiin | 12.92 ± 0.88a | 13.25 ± 0.28a | 4.52 ± 0.38b | 3.06 ± 0.21b |
| Cyanidin 3-O-rutinoside chloride | 17,133.01 ± 669.36a | 24.97 ± 2.85b | 0 ± 0b | 6.16 ± 1.43b |
| Epicatechin | 0 ± 0b | 53.35 ± 5.88a | 0 ± 0b | 0 ± 0b |
| Eriodictyol | 25.38 ± 1.19a | 8.36 ± 0.29b | 1.7 ± 0.34c | 0 ± 0c |
| Ferulic acid | 1655.96 ± 70.62d | 2075.78 ± 50.11c | 3901.8 ± 101.15b | 4441.52 ± 127.71a |
| Gallic acid | 74.28 ± 1.63a | 4.38 ± 0.19b | 3.27 ± 0.42b | 3 ± 0.47b |
| Gentisic acid | 81.9 ± 7.35a | 46.04 ± 8.89b | 47.6 ± 5.8b | 22.64 ± 1.51c |
| Hesperidin | 125.36 ± 12.51a | 6.33 ± 1.46b | 4.75 ± 0.23b | 4.96 ± 1.04b |
| Isoorientin | 860.2 ± 17.39a | 184.69 ± 9.25b | 121.38 ± 7.86c | 19.55 ± 1.82d |
| Isorhamnetin | 2.2 ± 0.13a | 0.45 ± 0.02b | 0 ± 0c | 0 ± 0c |
| Isorhamnetin-3-O-glucoside | 1008.38 ± 20.38a | 69.4 ± 6.45b | 29.06 ± 1.63c | 3.35 ± 0.13c |
| Methyl gallate | 136.84 ± 2.98a | 2.28 ± 0.23b | 0.35 ± 0.04b | 0.48 ± 0.05b |
| Morin | 11.59 ± 0.52a | 0.62 ± 0.03b | 0 ± 0b | 0 ± 0b |
| Myricetin 3-galactoside | 343.38 ± 16.36a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Narcissin | 346.68 ± 16.05a | 4.62 ± 0.56b | 1.44 ± 1.05b | 3.42 ± 0.78b |
| Naringenin | 11.3 ± 0.51b | 48.79 ± 2.09a | 14.16 ± 0.66b | 0 ± 0c |
| Naringin | 19.64 ± 3.14a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Nicotiflorin | 4.96 ± 0.4a | 0 ± 0c | 1.12 ± 0.16b | 0 ± 0c |
| Orientin | 39.5 ± 1.1a | 2.82 ± 0.29b | 1.83 ± 0.06b | 0 ± 0c |
| Pelargonidin-3-glucoside | 1878.84 ± 127.67a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Phloretin | 0.38 ± 0.01a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Phlorizin | 148.86 ± 8.03a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Procyanidin B3 | 41.87 ± 5.16c | 39,397.65 ± 3851.68a | 11,397.62 ± 860.57b | 0 ± 0c |
| Protocatechuic acid | 21,480.88 ± 1200.16a | 1052.57 ± 8.97b | 184.97 ± 14.37b | 43.4 ± 1.56b |
| Prunin | 34.77 ± 4.68a | 0.82 ± 0.14b | 0 ± 0b | 0 ± 0b |
| Quercetin 3-galactoside | 5969.76 ± 294.12a | 39.62 ± 4.34b | 12.78 ± 1.65b | 0 ± 0b |
| Quercetin | 11.66 ± 0.47a | 0.52 ± 0.03b | 0 ± 0b | 0 ± 0b |
| Rutin | 1388.95 ± 155a | 3.96 ± 0.35b | 0 ± 0b | 3.05 ± 0.31b |
| Salicylic acid | 1174.88 ± 16.27a | 672.46 ± 9.05b | 588.47 ± 20.49c | 508.82 ± 16.92d |
| Sinapic acid | 995.84 ± 15.07a | 420.6 ± 14.87c | 768.46 ± 15.07b | 269.58 ± 5.62d |
| Syringaldehyde | 39.65 ± 2.78a | 29.15 ± 1.46b | 37 ± 2.83a | 43.56 ± 2.19a |
| Syringic acid | 152.78 ± 27.37a | 65.3 ± 8.39b | 66.3 ± 5.02b | 50.12 ± 4.59b |
| Taxifolin | 3997.79 ± 120.18a | 445 ± 19.93b | 146.86 ± 9.25c | 0 ± 0c |
| trans-3,3′,4′,5,5′,7-Hexahydroxyflavanone | 228.27 ± 18.71a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| trans-Cinnamic acid | 37.56 ± 2.33c | 62.54 ± 2.44a | 68.33 ± 3.57a | 48.7 ± 1.18b |
| trans-Piceid | 336.9 ± 10.03a | 11.67 ± 0.47b | 4.03 ± 0.55b | 0 ± 0b |
| Trilobatin | 1.04 ± 0.04a | 0 ± 0b | 0 ± 0b | 0 ± 0b |
| Vanillic acid | 8942.13 ± 407.93a | 156.65 ± 10.53b | 272.31 ± 27.35b | 247.69 ± 25.61b |
| Vitexin | 150.11 ± 14.69a | 67.62 ± 6.22b | 39.84 ± 5.21c | 18.53 ± 1.12c |
| Index | CAT | POD | SOD | PPO | PAL | TP | Flavonoids | OPC | ABTS | DPPH | FRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,4-Dihydroxybenzoic acid | 0.732 ** | 0.51 | 0.678 * | 0.018 | 0.314 | 0.261 | 0.3 | 0.235 | 0.254 | 0.26 | 0.232 |
| 2,6-Dihydroxybenzoic acid | −0.157 | −0.271 | −0.36 | −0.352 | −0.797 ** | −0.717 ** | −0.791 ** | −0.686 * | −0.849 ** | −0.835 ** | −0.843 ** |
| 3,4-Dihydroxybenzaldehyde | −0.259 | −0.863 ** | −0.691 * | −0.586 * | −0.143 | −0.09 | −0.188 | −0.048 | −0.18 | −0.172 | −0.144 |
| 4-Hydroxybenzoic acid | 0.428 | 0.393 | 0.471 | 0.099 | 0.149 | 0.226 | 0.237 | 0.213 | 0.161 | 0.178 | 0.15 |
| 4-Hydroxycinnamic acid | 0.265 | 0.812 ** | 0.699 * | 0.536 | 0.09 | 0.059 | 0.141 | 0.026 | 0.118 | 0.114 | 0.083 |
| Apiin | −0.288 | −0.182 | −0.525 | −0.161 | −0.897 ** | −0.872 ** | −0.910 ** | −0.853 ** | −0.914 ** | −0.919 ** | −0.921 ** |
| Aromadendrin | −0.076 | −0.038 | 0.083 | 0.128 | 0.655 * | 0.538 | 0.585 * | 0.518 | 0.667 * | 0.647 * | 0.667 * |
| Caffeic acid | −0.436 | 0.082 | −0.176 | 0.342 | −0.18 | −0.314 | −0.252 | −0.327 | −0.142 | −0.174 | −0.153 |
| Caftaric acid | −0.404 | 0.56 | −0.037 | 0.618 * | −0.613 * | −0.763 ** | −0.664 * | −0.793 ** | −0.585 * | −0.614 * | −0.611 * |
| Catechin | −0.394 | −0.732 ** | −0.718 ** | −0.434 | −0.301 | −0.283 | −0.358 | −0.248 | −0.328 | −0.329 | −0.3 |
| Chlorogenic acid | 0.405 | 0.247 | 0.545 | 0.145 | 0.962 ** | 0.927 ** | 0.973 ** | 0.899 ** | 0.980 ** | 0.978 ** | 0.971 ** |
| Coniferaldehyde | 0.152 | 0.079 | 0.089 | −0.159 | −0.399 | −0.278 | −0.325 | −0.262 | −0.412 | −0.396 | −0.425 |
| Cosmosiin | 0.06 | −0.577 * | −0.151 | −0.386 | 0.706 * | 0.735 ** | 0.683 * | 0.751 ** | 0.694 * | 0.699 * | 0.718 ** |
| Cyanidin 3−O−rutinoside chloride | 0.37 | 0.245 | 0.583 * | 0.164 | 0.965 ** | 0.929 ** | 0.975 ** | 0.901 ** | 0.983 ** | 0.981 ** | 0.975 ** |
| Epicatechin | −0.233 | −0.920 ** | −0.674 * | −0.658 * | −0.113 | −0.029 | −0.144 | 0.019 | −0.155 | −0.142 | −0.117 |
| Eriodictyol | 0.351 | −0.011 | 0.374 | −0.032 | 0.985 ** | 0.961 ** | 0.977 ** | 0.946 ** | 0.987 ** | 0.987 ** | 0.989 ** |
| Ferulic acid | −0.133 | 0.431 | 0.003 | 0.287 | −0.801 ** | −0.803 ** | −0.773 ** | −0.810 ** | −0.788 ** | −0.0792 ** | −0.810 ** |
| Gallic acid | 0.389 | 0.235 | 0.562 | 0.142 | 0.970 ** | 0.933 ** | 0.978 ** | 0.906 ** | 0.985 ** | 0.983 ** | 0.977 ** |
| Gentisic acid | 0.036 | 0.127 | 0.365 | 0.293 | 0.847 ** | 0.774 ** | 0.830 ** | 0.749 ** | 0.887 ** | 0.871 ** | 0.879 ** |
| Hesperidin | 0.425 | 0.266 | 0.606 * | 0.129 | 0.976 ** | 0.926 ** | 0.971 ** | 0.899 ** | 0.973 ** | 0.972 ** | 0.964 ** |
| Isoorientin | 0.341 | 0.163 | 0.496 | 0.11 | 0.984 ** | 0.934 ** | 0.975 ** | 0.909 ** | 0.992 ** | 0.988 ** | 0.987 ** |
| Isorhamnetin | 0.352 | 0.073 | 0.499 | 0.028 | 0.995 ** | 0.968 ** | 0.992 ** | 0.949 ** | 0.996 ** | 0.997 ** | 0.995 ** |
| Isorhamnetin-3-O-glucoside | 0.37 | 0.216 | 0.566 | 0.137 | 0.979 ** | 0.938 ** | 0.981 ** | 0.912 ** | 0.990 ** | 0.988 ** | 0.983 ** |
| Methyl gallate | 0.402 | 0.247 | 0.580 * | 0.141 | 0.974 ** | 0.933 ** | 0.978 ** | 0.905 ** | 0.983 ** | 0.981 ** | 0.975 ** |
| Morin | 0.381 | 0.214 | 0.582 * | 0.125 | 0.981 ** | 0.941 ** | 0.983 ** | 0.916 ** | 0.988 ** | 0.987 ** | 0.982 ** |
| Myricetin 3-galactoside | 0.384 | 0.243 | 0.558 | 0.156 | 0.962 ** | 0.928 ** | 0.974 ** | 0.901 ** | 0.982 ** | 0.980 ** | 0.974 ** |
| Narcissin | 0.389 | 0.237 | 0.555 | 0.15 | 0.964 ** | 0.931 ** | 0.976 ** | 0.904 ** | 0.983 ** | 0.981 ** | 0.975 ** |
| Naringenin | −0.306 | −0.804 ** | −0.624 * | −0.484 | −0.022 | 0.012 | −0.074 | 0.051 | −0.052 | −0.05 | −0.021 |
| Naringin | 0.383 | 0.221 | 0.483 | 0.147 | 0.938 ** | 0.915 ** | 0.959 ** | 0.888 ** | 0.969 ** | 0.966 ** | 0.961 ** |
| Nicotiflorin | 0.344 | 0.393 | 0.574 | 0.306 | 0.903 ** | 0.828 ** | 0.904 ** | 0.790 ** | 0.936 ** | 0.925 ** | 0.920 ** |
| Orientin | 0.371 | 0.217 | 0.54 | 0.143 | 0.973 ** | 0.933 ** | 0.978 ** | 0.907 ** | 0.990 ** | 0.987 ** | 0.982 ** |
| Pelargonidin-3-glucoside | 0.385 | 0.263 | 0.621 * | 0.16 | 0.971 ** | 0.926 ** | 0.973 ** | 0.898 ** | 0.978 ** | 0.976 ** | 0.970 ** |
| Phloretin | 0.367 | 0.248 | 0.593 * | 0.167 | 0.966 ** | 0.928 ** | 0.975 ** | 0.900 ** | 0.982 ** | 0.980 ** | 0.974 ** |
| Phlorizin | 0.371 | 0.255 | 0.610 * | 0.166 | 0.969 ** | 0.927 ** | 0.974 ** | 0.899 ** | 0.981 ** | 0.979 ** | 0.972 ** |
| Procyanidin B3 | −0.362 | −0.795 ** | −0.708 * | −0.478 | −0.257 | −0.211 | −0.303 | −0.166 | −0.287 | −0.284 | −0.258 |
| Protocatechuic acid | 0.414 | 0.22 | 0.536 | 0.114 | 0.975 ** | 0.938 ** | 0.980 ** | 0.911 ** | 0.986 ** | 0.984 ** | 0.978 ** |
| Prunin | 0.441 | 0.23 | 0.497 | 0.107 | 0.959 ** | 0.925 ** | 0.967 ** | 0.898 ** | 0.973 ** | 0.971 ** | 0.965 ** |
| Quercetin 3-galactoside | 0.411 | 0.249 | 0.554 | 0.14 | 0.967 ** | 0.929 ** | 0.974 ** | 0.901 ** | 0.981 ** | 0.979 ** | 0.972 ** |
| Quercetin | 0.365 | 0.211 | 0.572 | 0.139 | 0.975 ** | 0.940 ** | 0.982 ** | 0.914 ** | 0.989 ** | 0.987 ** | 0.982 ** |
| Rutin | 0.452 | 0.271 | 0.579 * | 0.122 | 0.971 ** | 0.922 ** | 0.967 ** | 0.894 ** | 0.970 ** | 0.969 ** | 0.961 ** |
| Salicylic acid | 0.352 | 0.113 | 0.478 | 0.079 | 0.985 ** | 0.940 ** | 0.975 ** | 0.918 ** | 0.992 ** | 0.989 ** | 0.988 ** |
| Sinapic acid | 0.093 | 0.519 | 0.505 | 0.516 | 0.707 * | 0.548 | 0.662 * | 0.499 | 0.743 ** | 0.717 ** | 0.720 ** |
| Syringaldehyde | 0.309 | 0.603 * | 0.433 | 0.381 | 0.039 | 0.061 | 0.119 | 0.032 | 0.079 | 0.089 | 0.066 |
| Syringic acid | 0.168 | 0.202 | 0.631 * | 0.274 | 0.907 ** | 0.859 ** | 0.910 ** | 0.837 ** | 0.932 ** | 0.927 ** | 0.927 ** |
| Taxifolin | 0.384 | 0.179 | 0.514 | 0.103 | 0.982 ** | 0.945 ** | 0.984 ** | 0.920 ** | 0.994 ** | 0.992 ** | 0.988 ** |
| trans-3,3′,4′,5,5′,7-Hexahydroxyflavanone | 0.345 | 0.236 | 0.579 * | 0.176 | 0.958 ** | 0.925 ** | 0.972 ** | 0.898 ** | 0.981 ** | 0.978 ** | 0.973 ** |
| trans-Cinnamic acid | −0.511 | −0.156 | −0.576 | 0.034 | −0.757 ** | −0.812 ** | −0.807 ** | −0.803 ** | −0.742 ** | −0.761 ** | −0.742 ** |
| trans-Piceid | 0.394 | 0.229 | 0.55 | 0.136 | 0.972 ** | 0.935 ** | 0.979 ** | 0.907 ** | 0.986 ** | 0.984 ** | 0.979 ** |
| Trilobatin | 0.399 | 0.262 | 0.602 * | 0.153 | 0.972 ** | 0.928 ** | 0.975 ** | 0.900 ** | 0.980 ** | 0.978 ** | 0.971 ** |
| Vanillic acid | 0.411 | 0.275 | 0.604 * | 0.154 | 0.970 ** | 0.923 ** | 0.972 ** | 0.895 ** | 0.977 ** | 0.975 ** | 0.968 ** |
| Vitexin | 0.261 | −0.025 | 0.349 | 0.019 | 0.950 ** | 0.921 ** | 0.947 ** | 0.902 ** | 0.972 ** | 0.969 ** | 0.975 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wang, R.; Zhang, Y.; Lu, Y.; Cai, S.; Xiong, Q. Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains. Metabolites 2024, 14, 120. https://doi.org/10.3390/metabo14020120
Zhu J, Wang R, Zhang Y, Lu Y, Cai S, Xiong Q. Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains. Metabolites. 2024; 14(2):120. https://doi.org/10.3390/metabo14020120
Chicago/Turabian StyleZhu, Jinyan, Ruizhi Wang, Yu Zhang, Yanyao Lu, Shuo Cai, and Qiangqiang Xiong. 2024. "Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains" Metabolites 14, no. 2: 120. https://doi.org/10.3390/metabo14020120
APA StyleZhu, J., Wang, R., Zhang, Y., Lu, Y., Cai, S., & Xiong, Q. (2024). Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains. Metabolites, 14(2), 120. https://doi.org/10.3390/metabo14020120






