Didymin Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis by Regulating Gut Microbiota and Amino Acid Metabolism in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal and Experimental Design
2.3. Disease Activity Index (DAI) Evaluation
2.4. Colon Histopathology Observation
2.5. Protein Extraction and Western Blot Analysis
2.6. RNA Extraction and Quantitative Real-Time PCR Analysis
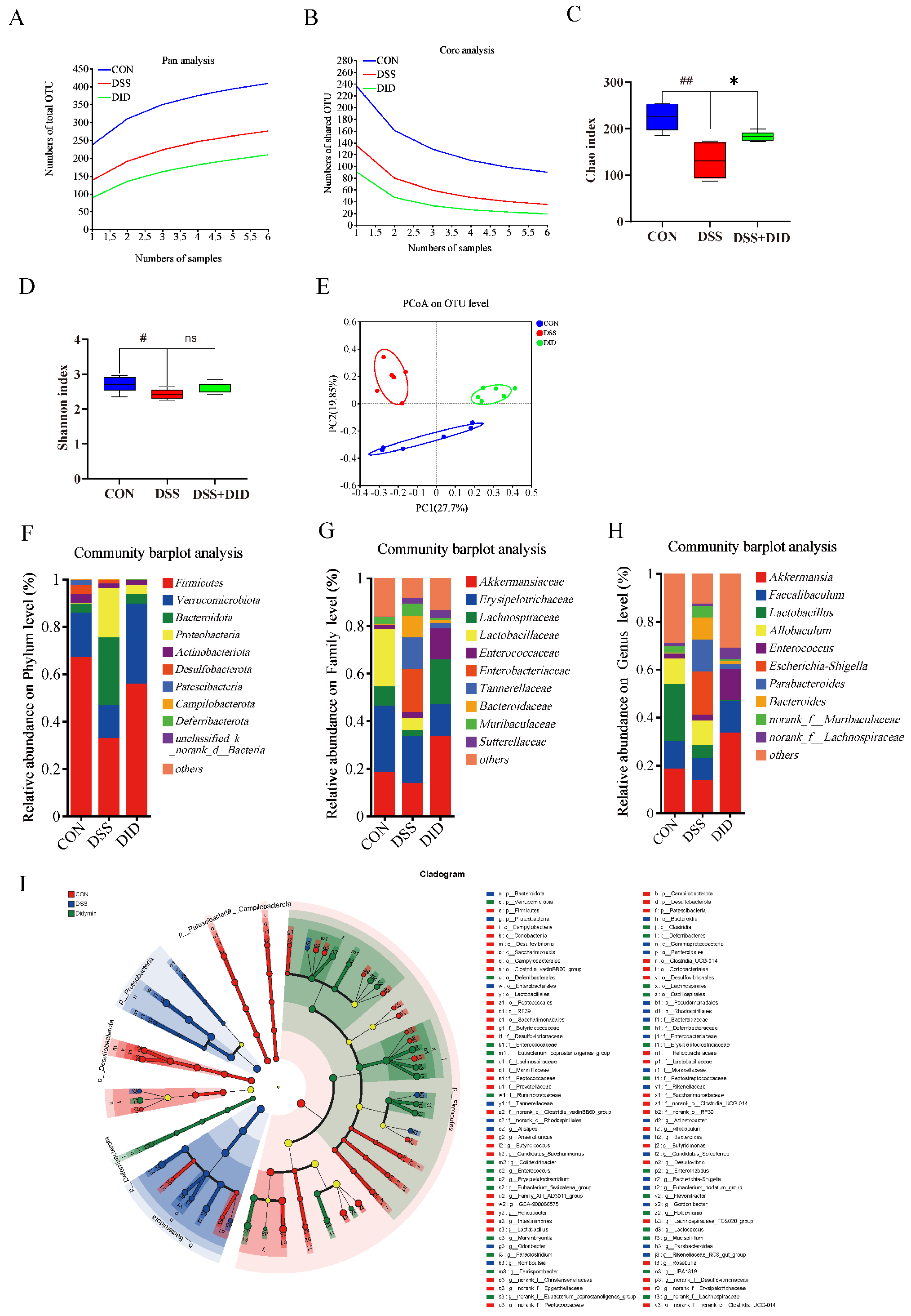
2.7. DNA Sequencing and Gut Microbiota Analysis
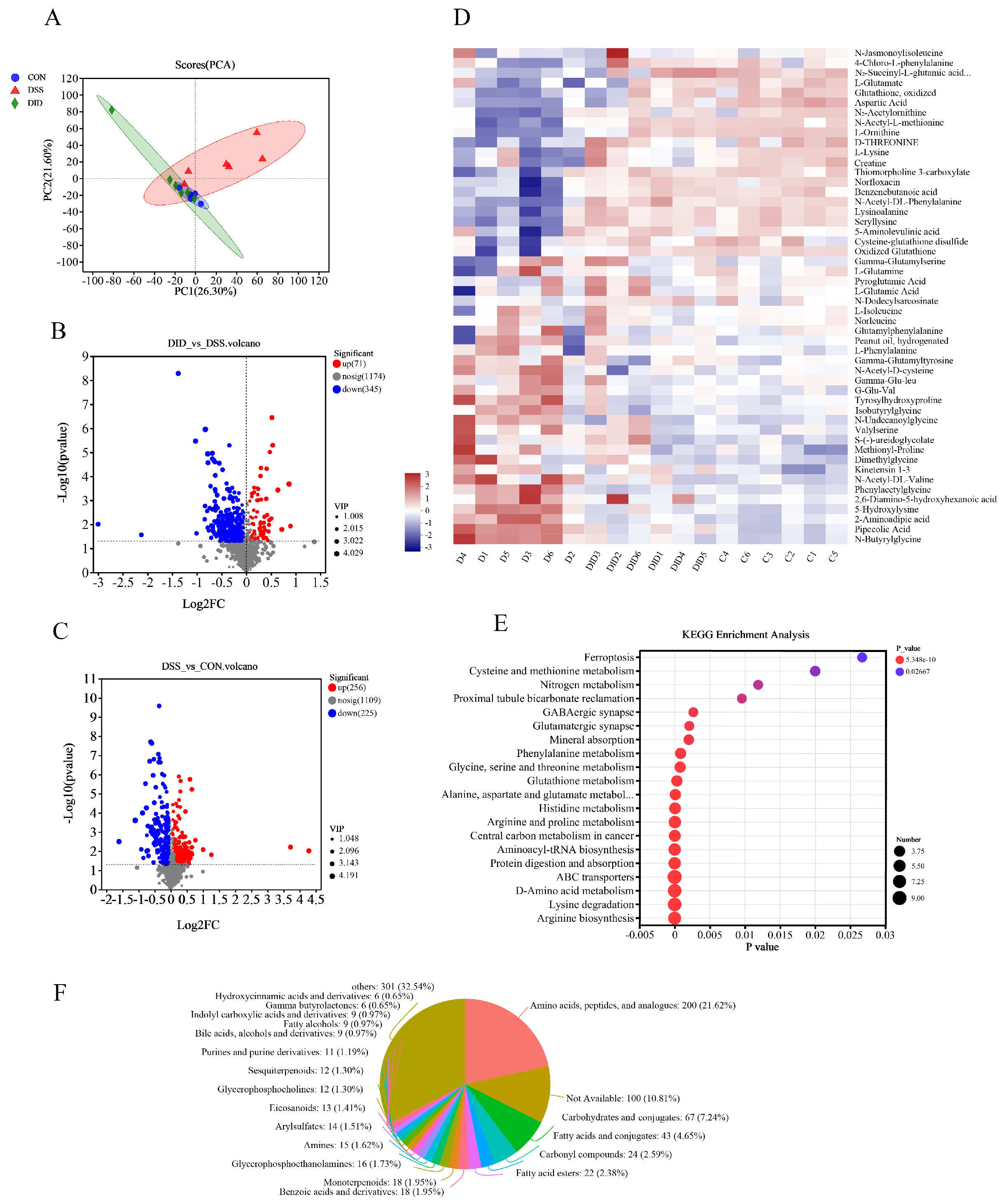
2.8. Non-Target Metabolomics Assay of Serum Metabolites
2.9. Selection of Representative Serum Metabolites
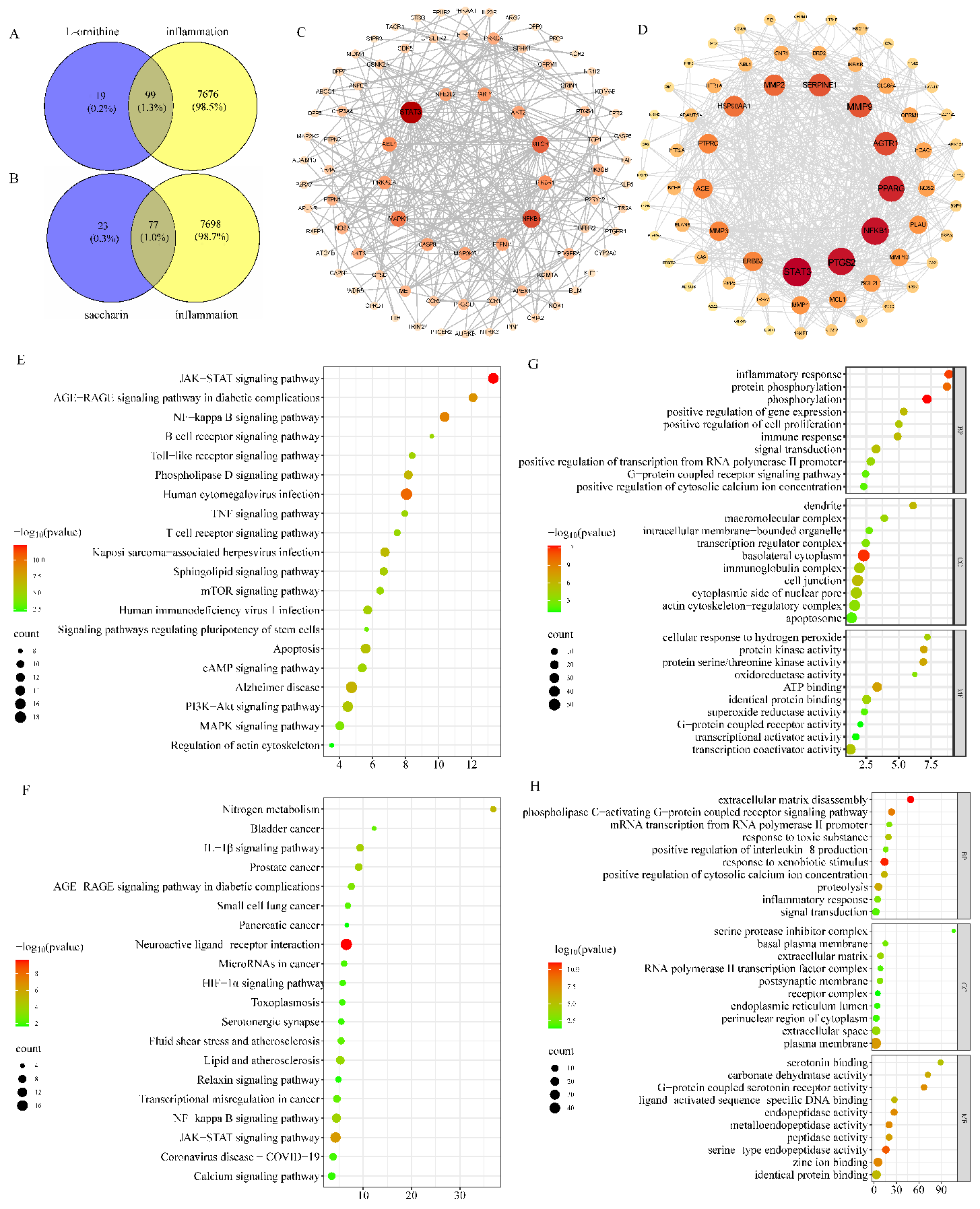
2.10. Network Pharmacological Analysis
2.10.1. Collection and Screening of Active Components of Drugs and Prediction of Targets
2.10.2. Collection of Disease-Related Genes
2.10.3. Intersection of Drug and Disease Target
2.10.4. Data Visualization and Construction of PPI Network
2.10.5. GO Enrichment Analysis and KEGG Pathway Enrichment Analysis
2.11. Molecular Docking
2.12. In Vitro Study of Metabolites in Serum
2.13. Statistical Analysis
3. Results
3.1. Determination of the Dose of DID by Gavage
3.2. Effects of DID Supplementation on the Colitis Symptoms
3.3. Effects of DID Supplementation on the Gut Microbiota in Colitis Mice
3.4. Effect of DID Supplementation on the Serum Metabolites in Colitis Mice
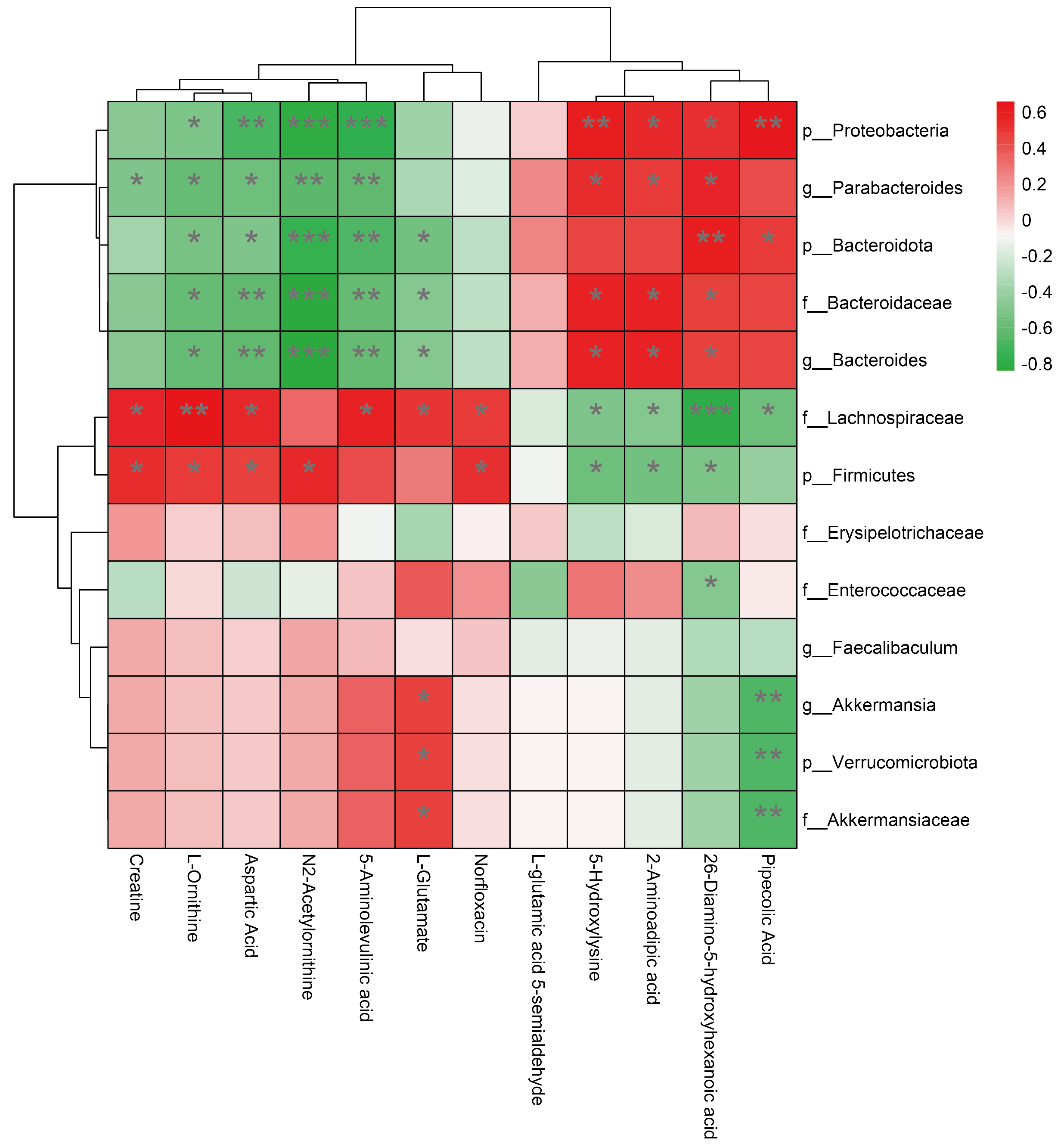
3.5. Association between Changes in Gut Microbiota and Changes in Amino Acid Metabolism
3.6. Results of Network Pharmacology Data Analysis
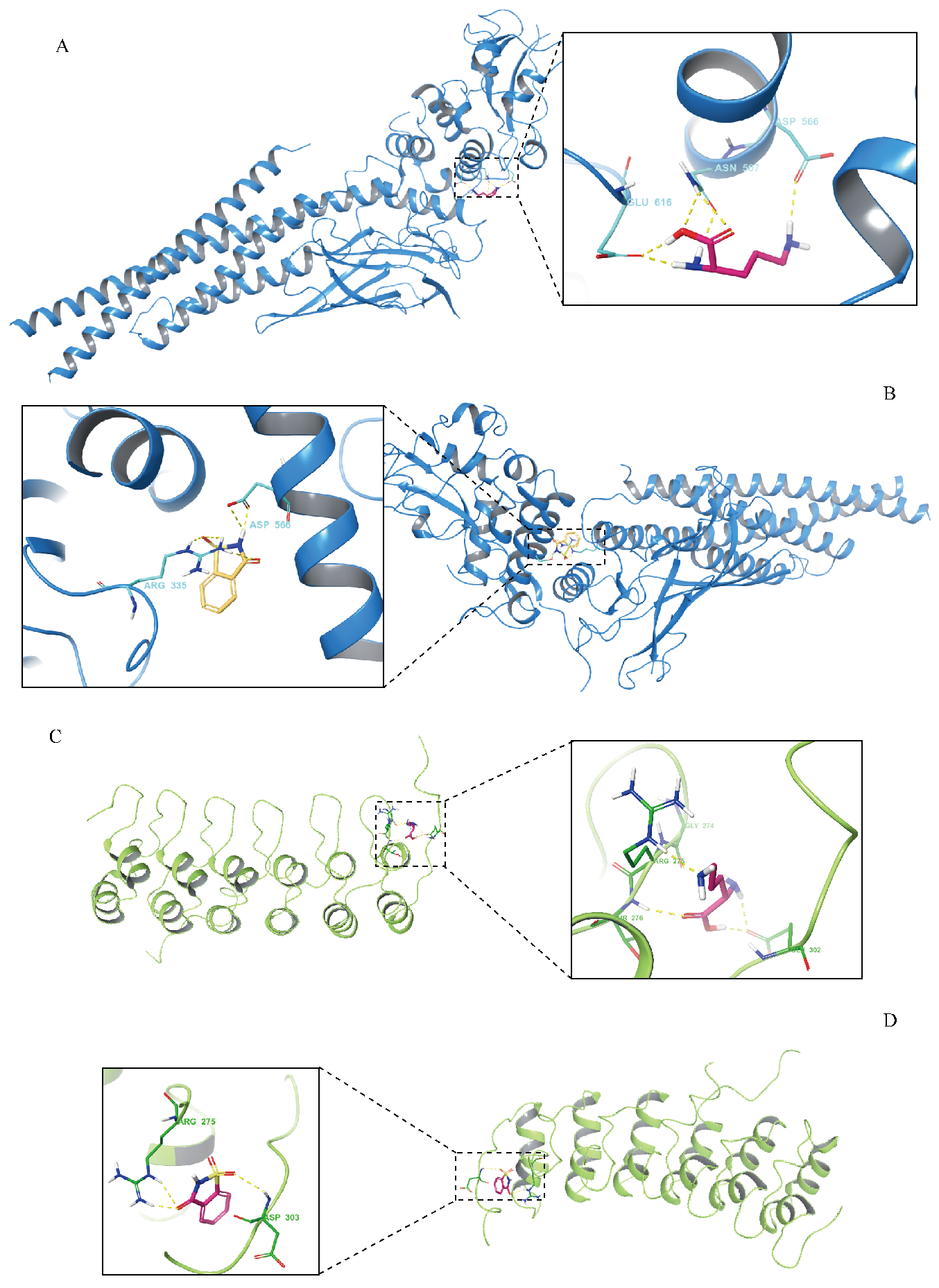
3.7. Results of Molecular-Docking Analysis
3.8. Evaluated Safety and Dosages of Serum Metabolites
3.9. Serum Metabolites Affected the Expressions of Inflammatory Factors
3.10. Metabolites Affected mRNA Expressions of Target Genes and Inflammatory Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| CD | Crohn’s disease |
| DAI | disease activity index |
| DID | didymin |
| DMEM | Dulbecco’s modified essential medium |
| DSS | dextran sulfate sodium |
| FBS | fetal bovine serum |
| H&E | hematoxylin–eosin |
| HPLC | high-performance liquid chromatography |
| IBD | inflammatory bowel disease |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinases |
| NF-κB | nuclear factor-kappa B |
| TJ | tight junction |
| TLR4 | Toll-like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| PCoA | principal coordinate analysis |
| PCR | polymerase chain reaction |
| PI3K | phosphatidyl inositol 3-kinase |
| PMSF | phenylmethanesulfonyl fluoride |
| RIPA | radioimmunoprecipitation assay buffer |
| RT | retention time |
| STAT3 | signal transducer and activator of transcription 3 |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| ZO-1 | zonula occludens protein 1 |
References
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Ferry, G.D. Inflammatory bowel diseases in pediatric and adolescent patients: Clinical, therapeutic, and psychosocial considerations. Gastroenterology 2004, 126, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Berends, S.E.; Strik, A.S.; Löwenberg, M.; D’Haens, G.R.; Mathôt, R.A.A. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin. Pharmacokinet. 2019, 58, 15–37. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Graef, F.A.; Healey, G.R.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018, 155, 36–52. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singhal, S.; Singhal, P.; Singhal, J.; Horne, D.; Awasthi, S. Didymin: An orally active citrus flavonoid for targeting neuroblastoma. Oncotarget 2017, 8, 29428–29441. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem. Interactions 2019, 305, 180–194. [Google Scholar] [CrossRef]
- Yang, F.; Chu, Z.; Wu, Q.; Qu, G.; He, Z.; An, J.; Tang, Y.; Sun, S.; Ci, D.; Luo, F. A peptide from yak ameliorates hypoxia-induced kidney injury by inhibiting inflammation and apoptosis via Nrf2 pathway. Food Biosci. 2024, 60, 104407. [Google Scholar] [CrossRef]
- Guo, T.; Lin, Q.; Li, X.; Nie, Y.; Wang, L.; Shi, L.; Xu, W.; Hu, T.; Guo, T.; Luo, F. Octacosanol attenuates inflammation in both RAW264.7 macrophages and a mouse model of colitis. J. Agric. Food Chem. 2017, 65, 3647–3658. [Google Scholar] [CrossRef]
- Hu, Z.; Chu, Z.; Ling, X.; Wu, Y.; Qin, D.; Yang, F.; Yu, X.; Zhou, Y.; Tang, Y.; Luo, F. Sinensetin from citrus peel alleviates DSS-induced inflammation by regulating gut microbiota and serum metabolism in mice. Food Biosci. 2024, 62, 105066. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Z.; Ye, F.; Guo, T.; Luo, Y.; Zhou, W.; Qin, D.; Tang, Y.; Cao, F.; Luo, F.; et al. Mogroside V exerts anti-inflammatory effect via MAPK-NF-κB/AP-1 and AMPK-PI3K/Akt/mTOR pathways in ulcerative colitis. J. Funct. Foods 2021, 87, 104807. [Google Scholar] [CrossRef]
- Liu, N.; Yang, Z.; Liu, Y.; Dang, X.; Zhang, Q.; Wang, J.; Liu, X.; Zhang, J.; Pan, X. Identification of a putative SARS-CoV-2 main protease inhibitor through in silico screening of self-designed molecular library. Int. J. Mol. Sci. 2023, 24, 11390. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zeng, L.; Yang, F.; Hu, Z.; Luo, Y.; Zhou, Y.; Tang, Y.; Luo, F. Network pharmacology combined with molecular docking and molecular dynamic simulation to reveal the potential mechanism of lentinan ameliorating hyperlipidemia. Food Biosci. 2024, 60, 104306. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Liu, X. Didymin alleviates cerebral ischemia-reperfusion injury by activating the PPAR signaling pathway. Yonsei Med. J. 2022, 63, 956–965. [Google Scholar] [CrossRef]
- Feng, Z.; Pang, L.; Chen, S.; Pang, X.; Huang, Y.; Qiao, Q.; Wang, Y.; Vonglorkham, S.; Huang, Q.; Lin, X.; et al. Didymin ameliorates dexamethasone-induced non-alcoholic fatty liver disease by inhibiting TLR4/NF-κB and PI3K/Akt pathways in C57BL/6J mice. Int. Immunopharmacol. 2020, 88, 107003. [Google Scholar] [CrossRef]
- Gu, L.; Sun, M.; Li, R.; Zhang, X.; Tao, Y.; Yuan, Y.; Luo, X.; Xie, Z. Didymin suppresses microglia pyroptosis and neuroinflammation through the Asc/Caspase-1/GSDMD pathway following experimental intracerebral hemorrhage. Front. Immunol. 2022, 13, 810582. [Google Scholar] [CrossRef]
- Lv, Q.; Xing, Y.; Liu, Y.; Chen, Q.; Xu, J.; Hu, L.; Zhang, Y. Didymin switches M1-like toward M2-like macrophage to ameliorate ulcerative colitis via fatty acid oxidation. Pharmacol. Res. 2021, 169, 105613. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Xie, T.; Wu, Q.; Lu, H.; Hu, Z.; Luo, Y.; Chu, Z.; Luo, F. Functional perspective of leeks: Active components, health benefits and action mechanisms. Foods 2023, 12, 3225. [Google Scholar] [CrossRef]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic functions of TNF-α in the regulation of the intestinal epithelial response to inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Ye, D.; Boivin, M.; Guo, S.; Hashimi, M.; Ereifej, L.; Ma, T.Y. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE 2014, 9, e85345. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.W.; Al-Sadi, R.; Ma, T.Y. IL-1β and the intestinal epithelial tight junction barrier. Front. Immunol. 2021, 12, 767456. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Liu, Y.; Xia, Z. Protective effects of L-arginine on the intestinal epithelial barrier under heat stress conditions in rats and IEC-6 cell line. J. Anim. Physiol. Anim. Nutr. 2020, 104, 385–396. [Google Scholar] [CrossRef]
- Thompson-Chagoyán, O.C.; Maldonado, J.; Gil, A. Aetiology of inflammatory bowel disease (IBD): Role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin. Nutr. 2005, 24, 339–352. [Google Scholar] [CrossRef]
- Li, L.; Qiu, N.; Meng, Y.; Wang, C.; Mine, Y.; Keast, R.S.; Guyonnet, V. Preserved egg white alleviates DSS-induced colitis in mice through the reduction of oxidative stress, modulation of inflammatory cytokines, NF-κB, MAPK and gut microbiota composition. Food Sci. Hum. Wellness 2023, 12, 312–323. [Google Scholar] [CrossRef]
- Fábrega, M.J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal anti-inflammatory effects of outer membrane vesicles from escherichia coli nissle 1917 in DSS-experimental colitis in mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Wang, B.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Suo, H.; Chen, W.; Zhai, Q. Stachyose modulates gut microbiota and alleviates DSS-induced ulcerative colitis in mice. Food Sci. Hum. Wellness 2023, 12, 2211–2220. [Google Scholar] [CrossRef]
- Schwalm, N.D.; Groisman, E.A. Navigating the gut buffet: Control of polysaccharide utilization in bacteroides spp. Trends Microbiol. 2017, 25, 1005–1015. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, Y.; Lu, H.; Xie, T.; Hu, Z.; Chu, Z.; Luo, F. The potential role of vitamin E and the mechanism in the prevention and treatment of inflammatory bowel disease. Foods 2024, 13, 898. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The role of Enterobacteriaceae in gut microbiota dysbiosis in inflammatory bowel diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Di’Narzo, A.F.; Houten, S.M.; Kosoy, R.; Huang, R.; Vaz, F.M.; Hou, R.; Wei, G.; Wang, W.; Comella, P.H.; Dodatko, T.; et al. Integrative analysis of the inflammatory bowel disease serum metabolome improves our understanding of genetic etiology and points to novel putative therapeutic targets. Gastroenterology 2022, 162, 828–843.e11. [Google Scholar] [CrossRef]
- Crovesy, L.; El-Bacha, T.; Rosado, E.L. Modulation of the gut microbiota by probiotics and symbiotics is associated with changes in serum metabolite profile related to a decrease in inflammation and overall benefits to metabolic health: A double-blind randomized controlled clinical trial in women with obesity. Food Funct. 2021, 12, 2161–2170. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Zhou, N.; Peng, W.; Peng, F.; Ma, M.; Li, L.; Fu, F.; Xiang, S.; Zhang, H.; He, X.; et al. Multi-Omics analysis of the microbiome and metabolome reveals the relationship between the gut microbiota and wooden breast myopathy in broilers. Front. Vet. Sci. 2022, 9, 922516. [Google Scholar] [CrossRef] [PubMed]
- Driuchina, A.; Hintikka, J.; Lehtonen, M.; Keski-Rahkonen, P.; O’connell, T.; Juvonen, R.; Kuula, J.; Hakkarainen, A.; Laukkanen, J.A.; Mäkinen, E.; et al. Identification of gut microbial lysine and histidine degradation and CYP-Dependent metabolites as biomarkers of fatty liver disease. mBio 2023, 14, e0266322. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chrysopoulou, M.; Rinschen, M.M. Integrative physiology of lysine metabolites. Physiol. Genom. 2023, 55, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Wang, M.; Chang, Y.; Zhang, F.; Ban, Z.; Tang, R.; Gan, Q.; Wu, S.; Guo, Y.; et al. The lysine catabolite saccharopine impairs development by disrupting mitochondrial homeostasis. J. Cell Biol. 2019, 218, 580–597. [Google Scholar] [CrossRef]
- Conz, A.; Salmona, M.; Diomede, L. Effect of non-nutritive sweeteners on the gut microbiota. Nutrients 2023, 15, 1869. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The nuclear factor kappa B (NF-κB) signaling in cancer development and immune diseases. Genes Dis. 2020, 8, 287–297. [Google Scholar] [CrossRef]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef]
- Stritesky, G.L.; Muthukrishnan, R.; Sehra, S.; Goswami, R.; Pham, D.; Travers, J.; Nguyen, E.T.; Levy, D.E.; Kaplan, M.H. The transcription factor STAT3 is required for T helper 2 cell development. Immunity 2011, 34, 39–49. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. CMLS 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Hu, Z.; Yang, F.; Zhou, Y.; Tang, Y.; Luo, F. Didymin Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis by Regulating Gut Microbiota and Amino Acid Metabolism in Mice. Metabolites 2024, 14, 547. https://doi.org/10.3390/metabo14100547
Chu Z, Hu Z, Yang F, Zhou Y, Tang Y, Luo F. Didymin Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis by Regulating Gut Microbiota and Amino Acid Metabolism in Mice. Metabolites. 2024; 14(10):547. https://doi.org/10.3390/metabo14100547
Chicago/Turabian StyleChu, Zhongxing, Zuomin Hu, Feiyan Yang, Yaping Zhou, Yiping Tang, and Feijun Luo. 2024. "Didymin Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis by Regulating Gut Microbiota and Amino Acid Metabolism in Mice" Metabolites 14, no. 10: 547. https://doi.org/10.3390/metabo14100547
APA StyleChu, Z., Hu, Z., Yang, F., Zhou, Y., Tang, Y., & Luo, F. (2024). Didymin Ameliorates Dextran Sulfate Sodium (DSS)-Induced Ulcerative Colitis by Regulating Gut Microbiota and Amino Acid Metabolism in Mice. Metabolites, 14(10), 547. https://doi.org/10.3390/metabo14100547






