Therapeutic Strategies to Modulate Gut Microbial Health: Approaches for Chronic Metabolic Disorder Management
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Configuration of a working group: Three operators skilled in clinical nutrition were gathered (one acting as a methodological operator and two participating as clinical operators).
- (2)
- Formulation of the revision question on the basis of considerations made in the abstract: “the state of the art regarding ideal therapy with probiotics and prebiotics in order to obtain the reversion of dysbiosis (alteration in microbiota) to eubiosis during metabolic diseases, such as metabolic syndrome, diabetes, prediabetes, obesity, hyperhomocysteinemia, dyslipidemia, sarcopenia, and non-alcoholic fatty liver disease”.
- (3)
- Identification of relevant studies: A research strategy was planned on PubMed (Public MEDLINE run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bethesda (Bethesda, MD, USA)) as follows: (a) definition of the keywords (metabolic syndrome, diabetes, prediabetes, obesity, hyperhomocysteinemia, dyslipidemia, sarcopenia, non-alcoholic fatty liver disease, microbiota, probiotics, prebiotics, and dietary supplements), allowing the definition of the interest field of the documents to be searched, grouped in inverted commas (“. . .”), and used separately or in combination; (b) use of the Boolean (a data type with only two possible values: true or false) AND operator, which allows the establishment of logical relations among concepts; (c) research modalities: advanced search; (d) limits (time limits: papers published in the last 20 years; humans; languages: English); (e) manual search performed by senior researchers experienced in clinical nutrition through the revision of reviews and individual articles on the state of the art regarding ideal therapy with probiotics and prebiotics in order to obtain the reversion of dysbiosis (alteration in microbiota) to eubiosis during metabolic diseases, such as metabolic syndrome, diabetes, prediabetes, obesity, hyperhomocysteinemia, dyslipidemia, sarcopenia, and non-alcoholic fatty liver disease published in journals qualified in the Index Medicus.
- (4)
- Analysis and presentation of the outcomes: The data extrapolated from the “revised studies” were collocated in tables, particularly, for each study specified, the author and year of publication and the study characteristics; for each topic, we built three types of tables depending on the type of study: tables with reviews and meta-analyses, tables with observational human studies, and tables with interventional human studies. In the tables (obviously except for reviews and meta-analyses), only studies on humans are reported, while, in the text, in vitro studies and studies on animal models are also cited, if useful to explain some mechanisms of action. Moreover, in tables, for all studies, the level of evidence has been added [31].
- (5)
- The analysis was carried out in the form of a narrative review of the reports. At the beginning of each section, the keywords considered and the kind of studies chosen have been reported. We evaluated, as suitable for the narrative review, the studies of any design that considered the state of the art of ideal therapy with probiotics and prebiotics in order to obtain the reversion of dysbiosis (alteration in microbiota) to eubiosis during metabolic diseases, such as metabolic syndrome, diabetes, prediabetes, obesity, hyperhomocysteinemia, dyslipidemia, sarcopenia, and non-alcoholic fatty liver disease. Figure 1 shows the eligible studies.
3. Results
3.1. Metabolic Syndrome
3.2. Diabetes
3.3. Prediabetes
3.4. Obesity
3.5. Hyperhomocisteinemia
3.6. Dyslipidemia
3.7. Sarcopenia
3.8. NAFLD
4. Discussion
4.1. Metabolic Syndrome
Probiotics
4.2. Diabetes
Probiotics
4.3. Prediabetes
4.3.1. Probiotics
4.3.2. Symbiotics
4.4. Obesity
4.4.1. Probiotics
4.4.2. Symbiotics
4.5. Hyperhomocysteinemia
Probiotics
4.6. Dyslipidemia
4.6.1. Probiotics
4.6.2. Probiotics
4.6.3. Symbiotic
4.7. Sarcopenia
Probiotics
4.8. Non-Alcoholic Fatty Liver Disease (NAFLD)
Probiotics
5. Conclusions: Management of Chronic Metabolic Disorders with Probiotics
5.1. Metabolic Syndrome
5.2. Diabetes
5.3. Prediabetes
5.4. Obesity
5.5. Hyperhomocysteinemia
5.6. Dyslipidemia
5.7. Sarcopenia
5.8. NAFLD
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The Links between the Gut Microbiome and Non-Alcoholic Fatty Liver Disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Busnelli, M.; Manzini, S.; Chiesa, G. The Gut Microbiota Affects Host Pathophysiology as an Endocrine Organ: A Focus on Cardiovascular Disease. Nutrients 2019, 12, 79. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The Role of Diet on Gut Microbiota Composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Nova, E.; Gómez-Martinez, S.; González-Soltero, R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms 2022, 10, 1368. [Google Scholar] [CrossRef]
- Troci, A.; Rausch, P.; Waschina, S.; Lieb, W.; Franke, A.; Bang, C. Long-Term Dietary Effects on Human Gut Microbiota Composition Employing Shotgun Metagenomics Data Analysis. Mol. Nutr. Food Res. 2023, 67, e2101098. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, J.B.; Kang, J.; Ahn, D.-W.; Kim, J.W.; Kim, B.G.; Lee, K.L.; Oh, S.; Yoon, S.H.; Park, S.J.; et al. Association between Sarcopenia Level and Metabolic Syndrome. PLoS ONE 2021, 16, e0248856. [Google Scholar] [CrossRef]
- Olofsson, L.E.; Bäckhed, F. The Metabolic Role and Therapeutic Potential of the Microbiome. Endocr. Rev. 2022, 43, 907–926. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role of the Gut Microbiome and Its Metabolites in Metabolic Diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, M. Gut Microbiota and Metabolic Diseases. J. Biosci. Med. 2022, 10, 113–141. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and Metabolic Diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Hur, K.Y.; Lee, M.-S. Gut Microbiota and Metabolic Disorders. Diabetes Metab. J. 2015, 39, 198. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Gil, Á.; Gómez-Llorente, C. Effects of Probiotics on Metabolic Syndrome: A Systematic Review of Randomized Clinical Trials. Nutrients 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.-H.; Gao, Z.-X.; Liu, D.-W.; Liu, Z.-S.; Wu, P. Gut Microbiota and Its Metabolites–Molecular Mechanisms and Management Strategies in Diabetic Kidney Disease. Front. Immunol. 2023, 14, 1124704. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Olivares, M.; Moya-Pérez, Á.; Agostoni, C. Understanding the Role of Gut Microbiome in Metabolic Disease Risk. Pediatr. Res. 2015, 77, 236–244. [Google Scholar] [CrossRef]
- Li, Z.; Quan, G.; Jiang, X.; Yang, Y.; Ding, X.; Zhang, D.; Wang, X.; Hardwidge, P.R.; Ren, W.; Zhu, G. Effects of Metabolites Derived From Gut Microbiota and Hosts on Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 314. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Zikou, E.; Dovrolis, N.; Dimosthenopoulos, C.; Gazouli, M.; Makrilakis, K. The Effect of Probiotic Supplements on Metabolic Parameters of People with Type 2 Diabetes in Greece—A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 4663. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Yin, J.; Peng, X.; King, L.; Li, L.; Xu, Z.; Zhou, L.; Peng, Z.; Ze, X.; et al. Effect of Synbiotic Supplementation on Immune Parameters and Gut Microbiota in Healthy Adults: A Double-Blind Randomized Controlled Trial. Gut Microbes 2023, 15, 2247025. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Altman, D. Systematic Reviews in Health Care. Meta-Analysis in Context. Int. J. Epidemiol. 2002, 31, 697. [Google Scholar] [CrossRef]
- How To Read A Paper. Available online: https://www.bmj.com/about-bmj/resources-readers/publications/how-read-paper (accessed on 20 October 2024).
- Owens, D.K.; Lohr, K.N.; Atkins, D.; Treadwell, J.R.; Reston, J.T.; Bass, E.B.; Chang, S.; Helfand, M. AHRQ Series Paper 5: Grading the Strength of a Body of Evidence When Comparing Medical Interventions—Agency for Healthcare Research and Quality and the Effective Health-Care Program. J. Clin. Epidemiol. 2010, 63, 513–523. [Google Scholar] [CrossRef]
- Portela-Cidade, J.P.; Borges-Canha, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Systematic Review of the Relation Between Intestinal Microbiota and Toll-Like Receptors in the Metabolic Syndrome: What Do We Know So Far? GE Port. J. Gastroenterol. 2015, 22, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, J.; Liu, Z.; Gao, F. Effect of Supplementation with Probiotics or Synbiotics on Cardiovascular Risk Factors in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front Endocrinol (Lausanne) 2024, 14, 1282699. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut Microbiota as a Potential Target of Metabolic Syndrome: The Role of Probiotics and Prebiotics. Cell Biosci. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Mallappa, R.; Rokana, N.; Duary, R.; Panwar, H.; Batish, V.; Grover, S. Management of Metabolic Syndrome through Probiotic and Prebiotic Interventions. Indian. J. Endocrinol. Metab. 2012, 16, 20. [Google Scholar] [CrossRef]
- Horvath, A.; Zukauskaite, K.; Hazia, O.; Balazs, I.; Stadlbauer, V. Human Gut Microbiome: Therapeutic Opportunities for Metabolic Syndrome-Hype or Hope? Endocrinol. Diabetes Metab. 2024, 7, e436. [Google Scholar] [CrossRef]
- Hadi, A.; Arab, A.; Khalesi, S.; Rafie, N.; Kafeshani, M.; Kazemi, M. Effects of Probiotic Supplementation on Anthropometric and Metabolic Characteristics in Adults with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Clin. Nutr. 2021, 40, 4662–4673. [Google Scholar] [CrossRef]
- Leber, B.; Tripolt, N.J.; Blattl, D.; Eder, M.; Wascher, T.C.; Pieber, T.R.; Stauber, R.; Sourij, H.; Oettl, K.; Stadlbauer, V. The Influence of Probiotic Supplementation on Gut Permeability in Patients with Metabolic Syndrome: An Open Label, Randomized Pilot Study. Eur. J. Clin. Nutr. 2012, 66, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Tripolt, N.J.; Leber, B.; Blattl, D.; Eder, M.; Wonisch, W.; Scharnagl, H.; Stojakovic, T.; Obermayer-Pietsch, B.; Wascher, T.C.; Pieber, T.R.; et al. Short Communication: Effect of Supplementation with Lactobacillus Casei Shirota on Insulin Sensitivity, β-Cell Function, and Markers of Endothelial Function and Inflammation in Subjects with Metabolic Syndrome—A Pilot Study. J. Dairy. Sci. 2013, 96, 89–95. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Leber, B.; Lemesch, S.; Trajanoski, S.; Bashir, M.; Horvath, A.; Tawdrous, M.; Stojakovic, T.; Fauler, G.; Fickert, P.; et al. Lactobacillus Casei Shirota Supplementation Does Not Restore Gut Microbiota Composition and Gut Barrier in Metabolic Syndrome: A Randomized Pilot Study. PLoS ONE 2015, 10, e0141399. [Google Scholar] [CrossRef]
- Barreto, F.M.; Colado Simão, A.N.; Morimoto, H.K.; Batisti Lozovoy, M.A.; Dichi, I.; Helena da Silva Miglioranza, L. Beneficial Effects of Lactobacillus Plantarum on Glycemia and Homocysteine Levels in Postmenopausal Women with Metabolic Syndrome. Nutrition 2014, 30, 939–942. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; Alfieri, D.F.; Lozovoy, M.A.B.; Mari, N.L.; de Souza, C.H.B.; Dichi, I.; Costa, G.N. Beneficial Effects of Bifidobacterium Lactis on Lipid Profile and Cytokines in Patients with Metabolic Syndrome: A Randomized Trial. Effects of Probiotics on Metabolic Syndrome. Nutrition 2016, 32, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Perelman, D.; Topf, M.; Fragiadakis, G.K.; Robinson, J.L.; Sonnenburg, J.L.; Gardner, C.D.; Sonnenburg, E.D. Randomized Controlled Trial Demonstrates Response to a Probiotic Intervention for Metabolic Syndrome That May Correspond to Diet. Gut Microbes 2023, 15, 2178794. [Google Scholar] [CrossRef]
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Jafari, P.; Ebrahimi, M.T.; Amini, M. The Effects of Probiotics and Synbiotic Supplementation on Glucose and Insulin Metabolism in Adults with Prediabetes: A Double-Blind Randomized Clinical Trial. Acta Diabetol. 2018, 55, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal Microbiota Transplantation for the Improvement of Metabolism in Obesity: The FMT-TRIM Double-Blind Placebo-Controlled Pilot Trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef] [PubMed]
- da Ponte Neto, A.M.; Clemente, A.C.O.; Rosa, P.W.; Ribeiro, I.B.; Funari, M.P.; Nunes, G.C.; Moreira, L.; Sparvoli, L.G.; Cortez, R.; Taddei, C.R.; et al. Fecal Microbiota Transplantation in Patients with Metabolic Syndrome and Obesity: A Randomized Controlled Trial. World J. Clin. Cases 2023, 11, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J. Am. Heart Assoc. 2018, 7, 008342. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Hartstra, A.V.; Schüppel, V.; Imangaliyev, S.; Schrantee, A.; Prodan, A.; Collard, D.; Levin, E.; Dallinga-Thie, G.; Ackermans, M.T.; Winkelmeijer, M.; et al. Infusion of Donor Feces Affects the Gut-Brain Axis in Humans with Metabolic Syndrome. Mol. Metab. 2020, 42, 101076. [Google Scholar] [CrossRef]
- Zecheng, L.; Donghai, L.; Runchuan, G.; Yuan, Q.; Qi, J.; Yijia, Z.; Shuaman, R.; Xiaoqi, L.; Yi, W.; Ni, M.; et al. Fecal Microbiota Transplantation in Obesity Metabolism: A Meta Analysis and Systematic Review. Diabetes Res. Clin. Pract. 2023, 202, 110803. [Google Scholar] [CrossRef]
- Pakmehr, A.; Mousavi, S.M.; Ejtahed, H.-S.; Hoseini-Tavassol, Z.; Siadat, S.D.; Hasani-Ranjbar, S.; Larijani, B. The Effect of Fecal Microbiota Transplantation on Cardiometabolic Risk Factors: A Systematic Review and Meta-Analysis. Clin. Ther. 2024, 46, e87–e100. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Cao, M.; He, J.; Zhang, Q.; Chen, Q.; Yan, C.; Lin, A.; Yang, L.; Wu, Z.; Zhu, D.; et al. Gut Bacterial Characteristics of Patients With Type 2 Diabetes Mellitus and the Application Potential. Front. Immunol. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal Mucosa of Patients with Type 1 Diabetes Shows Distinctive Inflammatory Profile and Microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Alkanani, A.K.; Hara, N.; Gottlieb, P.A.; Ir, D.; Robertson, C.E.; Wagner, B.D.; Frank, D.N.; Zipris, D. Alterations in Intestinal Microbiota Correlate with Susceptibility to Type 1 Diabetes. Diabetes 2015, 64, 3510–3520. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut Microbiota in Children with Type 1 Diabetes Differs from That in Healthy Children: A Case-Control Study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef]
- Bajinka, O.; Tan, Y.; Darboe, A.; Ighaede-Edwards, I.G.; Abdelhalim, K.A. The Gut Microbiota Pathway Mechanisms of Diabetes. AMB Express 2023, 13, 16. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut Microbiota in the Pathogenesis and Therapeutic Approaches of Diabetes. EBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Ye, J.; Wu, Z.; Zhao, Y.; Zhang, S.; Liu, W.; Su, Y. Role of Gut Microbiota in the Pathogenesis and Treatment of Diabetes Mullites: Advanced Research-Based Review. Front. Microbiol. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Mokhtari, P.; Metos, J.; Anandh Babu, P.V. Impact of Type 1 Diabetes on the Composition and Functional Potential of Gut Microbiome in Children and Adolescents: Possible Mechanisms, Current Knowledge, and Challenges. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Zhang, C.; Shi, Q.; Zhu, L.; Zhao, S.; Luo, Z.; Long, Y. Effect of Probiotics at Different Intervention Time on Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2024, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Feng, H.; Mao, X.-L.; Deng, Y.-J.; Wang, X.-B.; Zhang, Q.; Guo, Y.; Xiao, S.-M. The Effects of Probiotics Supplementation on Glycaemic Control among Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Transl. Med. 2023, 21, 442. [Google Scholar] [CrossRef]
- Moravejolahkami, A.R.; Shakibaei, M.; Fairley, A.M.; Sharma, M. Probiotics, Prebiotics, and Synbiotics in Type 1 Diabetes Mellitus: A Systematic Review and Meta-analysis of Clinical Trials. Diabetes Metab. Res. Rev. 2024, 40, e3655. [Google Scholar] [CrossRef]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S. Probiotics and Their Role in the Management of Type 2 Diabetes Mellitus (Short-Term Versus Long-Term Effect): A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46741. [Google Scholar] [CrossRef]
- Zhang, C.; jiang, J.; Wang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Meta-Analysis of Randomized Controlled Trials of the Effects of Probiotics on Type 2 Diabetes in Adults. Clin. Nutr. 2022, 41, 365–373. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Ashtary-Larky, D.; Asbaghi, O.; Ghaemi, F.; Pashayee-Khamene, F.; Yari, Z.; de Courten, B. Probiotics and Synbiotics Supplementation Improve Glycemic Control Parameters in Subjects with Prediabetes and Type 2 Diabetes Mellitus: A GRADE-Assessed Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Clinical Trials. Pharmacol. Res. 2022, 184, 106399. [Google Scholar] [CrossRef]
- Kocsis, T.; Molnár, B.; Németh, D.; Hegyi, P.; Szakács, Z.; Bálint, A.; Garami, A.; Soós, A.; Márta, K.; Solymár, M. Probiotics Have Beneficial Metabolic Effects in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Clinical Trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.D.; Chiuve, S.E.; Manson, J.E.; Willett, W.C.; Hu, F.B.; Qi, L. Dietary Phosphatidylcholine Intake and Type 2 Diabetes in Men and Women. Diabetes Care 2015, 38, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide Portends High Mortality Risk Independent of Glycemic Control in Patients with Type 2 Diabetes Mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yuzefpolskaya, M.; Nandakumar, R.; Colombo, P.C.; Demmer, R.T. Plasma Trimethylamine-N-Oxide and Impaired Glucose Regulation: Results from The Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). PLoS ONE 2020, 15, e0227482. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant Intestinal Microbiota in Individuals with Prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef]
- Zhong, H.; Ren, H.; Lu, Y.; Fang, C.; Hou, G.; Yang, Z.; Chen, B.; Yang, F.; Zhao, Y.; Shi, Z.; et al. Distinct Gut Metagenomics and Metaproteomics Signatures in Prediabetics and Treatment-Naïve Type 2 Diabetics. EBioMedicine 2019, 47, 373–383. [Google Scholar] [CrossRef]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: A Systematic Review of Observational Studies. Front. Cell Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Aw, W.; Fukuda, S. Understanding the Role of the Gut Ecosystem in Diabetes Mellitus. J. Diabetes Investig. 2018, 9, 5–12. [Google Scholar] [CrossRef]
- Simon, M.-C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.-H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus Reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans: A Proof of Concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Hariri, M.; Salehi, R.; Feizi, A.; Mirlohi, M.; Ghiasvand, R.; Habibi, N. A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial on Probiotic Soy Milk and Soy Milk: Effects on Epigenetics and Oxidative Stress in Patients with Type II Diabetes. Genes. Nutr. 2015, 10, 52. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berger, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus Rhamnosus CGMCC1.3724 Supplementation on Weight Loss and Maintenance in Obese Men and Women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Churnside, A.A.; Venables, M.C. Probiotic Supplementation Prevents High-Fat, Overfeeding-Induced Insulin Resistance in Human Subjects. Br. J. Nutr. 2015, 113, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of Abdominal Adiposity by Probiotics (Lactobacillus Gasseri SBT2055) in Adults with Obese Tendencies in a Randomized Controlled Trial. Eur. J. Clin. Nutr. 2010, 64, 636–643. [Google Scholar] [CrossRef]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic Effects of Lactobacillus Reuteri DSM 17938 in People with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Obes Metab 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.M.G.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus Acidophilus NCFM on Insulin Sensitivity and the Systemic Inflammatory Response in Human Subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E. Gut Microbiota, Prebiotics, Probiotics, and Synbiotics in Management of Obesity and Prediabetes: Review of Randomized Controlled Trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef]
- Hampe, C.S.; Roth, C.L. Probiotic Strains and Mechanistic Insights for the Treatment of Type 2 Diabetes. Endocrine 2017, 58, 207–227. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Qiu, X.; Wen, Q.; Liu, M.; Zhou, D.; Chen, Q. Probiotics, Pre-Biotics and Synbiotics in the Treatment of Pre-Diabetes: A Systematic Review of Randomized Controlled Trials. Front. Public. Health 2021, 9, 645035. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Wu, L.; Qin, L.; Liu, T. The Effects of Probiotic Administration on Patients with Prediabetes: A Meta-Analysis and Systematic Review. J. Transl. Med. 2022, 20, 498. [Google Scholar] [CrossRef]
- Zeighamy Alamdary, S.; Afifirad, R.; Asgharzadeh, S.; Asadollahi, P.; Mahdizade Ari, M.; Dashtibin, S.; Sabaghan, M.; Shokouhamiri, M.R.; Ghanavati, R.; Darbandi, A. The Influence of Probiotics Consumption on Management of Prediabetic State: A Systematic Review of Clinical Trials. Int. J. Clin. Pract. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of Probiotics on Glucose Metabolism in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Medicina 2016, 52, 28–34. [Google Scholar] [CrossRef]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal Microbiota and Bile Acid Interactions with Systemic and Adipose Tissue Metabolism in Diet-Induced Weight Loss of Obese Postmenopausal Women. J. Transl. Med. 2018, 16, 244. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Gøbel, R.J.; Michaelsen, K.F.; Forssten, S.D.; Lahtinen, S.J.; Jakobsen, M. Effect of Lactobacillus Salivarius Ls-33 on Fecal Microbiota in Obese Adolescents. Clin. Nutr. 2013, 32, 935–940. [Google Scholar] [CrossRef]
- Sharafedtinov, K.K.; Plotnikova, O.A.; Alexeeva, R.I.; Sentsova, T.B.; Songisepp, E.; Stsepetova, J.; Smidt, I.; Mikelsaar, M. Hypocaloric Diet Supplemented with Probiotic Cheese Improves Body Mass Index and Blood Pressure Indices of Obese Hypertensive Patients—A Randomized Double-Blind Placebo-Controlled Pilot Study. Nutr. J. 2013, 12. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Weight Loss during Oligofructose Supplementation Is Associated with Decreased Ghrelin and Increased Peptide YY in Overweight and Obese Adults. Am. J. Clin. Nutr. 2009, 89, 27465. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Farajian, S.; Kelishadi, R.; Mirlohi, M.; Hashemipour, M. The Effects of Synbiotic Supplementation on Some Cardio-Metabolic Risk Factors in Overweight and Obese Children: A Randomized Triple-Masked Controlled Trial. Int. J. Food Sci. Nutr. 2013, 64, 775224. [Google Scholar] [CrossRef]
- Jung, S.-P.; Lee, K.-M.; Kang, J.-H.; Yun, S.-I.; Park, H.-O.; Moon, Y.; Kim, J.-Y. Effect of Lactobacillus Gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial. Korean J. Fam. Med. 2013, 34, 80. [Google Scholar] [CrossRef]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.H.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of Probiotic Yogurt on Fat Distribution and Gene Expression of Proinflammatory Factors in Peripheral Blood Mononuclear Cells in Overweight and Obese People with or without Weight-Loss Diet. J. Am. Coll. Nutr. 2014, 33, 874937. [Google Scholar] [CrossRef]
- Ipar, N.; Aydogdu, S.D.; Yildirim, G.K.; Inal, M.; Gies, I.; Vandenplas, Y.; Dinleyici, E.C. Effects of Synbiotic on Anthropometry, Lipid Profile and Oxidative Stress in Obese Children. Benef. Microbes 2015, 6, 775–782. [Google Scholar] [CrossRef]
- Doria, E.; Buoncore, D.; Michelotti, A.; Nobile, V.; Marzanatico, F. Evaluation of a Phyto-Supplement Efficacy as Adjuvant in Reducing Body Weight and Fat Mass in Overweight Women. Curr. Top. Nutraceutical Res. 2013, 11, 21. [Google Scholar]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The Links between Gut Microbiota and Obesity and Obesity Related Diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The Human Gut Microbiota: Metabolism and Perspective in Obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on Body Weight and Body Fat in Overweight Subjects: A Systematic Review of Randomized Controlled Clinical Trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesæth, J. Effects of Probiotics on Body Weight, Body Mass Index, Fat Mass and Fat Percentage in Subjects with Overweight or Obesity: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef]
- Cao, N.; Zhao, F.; Kwok, L.-Y.; Wang, H.; Sun, Z. Impact of Probiotics on Weight Loss, Glucose and Lipid Metabolism in Overweight or Obese Women: A Meta-Analysis of Randomized Controlled Trials. Curr. Res. Food Sci. 2024, 9, 100810. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Ghalichi, F.; Ahrabi, S.S.; Jamilian, P.; Jamilian, P.; Ghoreishi, Z. Anti-Obesity Properties of Probiotics; a Considerable Medical Nutrition Intervention: Findings from an Umbrella Meta-Analysis. Eur. J. Pharmacol. 2022, 928, 175069. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149–4164. [Google Scholar] [CrossRef]
- Strozzi, P.G.; Mogna, L. Quantification of Folic Acid in Human Feces after Administration of Bifidobacterium Probiotic Strains. J. Clin. Gastroenterol. 2008, 42, 179–184. [Google Scholar] [CrossRef]
- Majewska, K.; Kręgielska-Narożna, M.; Jakubowski, H.; Szulińska, M.; Bogdański, P. The Multispecies Probiotic Effectively Reduces Homocysteine Concentration in Obese Women: A Randomized Double-Blind Placebo-Controlled Study. J. Clin. Med. 2020, 9, 998. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary Intervention Impact on Gut Microbial Gene Richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human Oral, Gut, and Plaque Microbiota in Patients with Atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lian, P.; Liu, H.; Wang, Y.; Zhou, M.; Feng, Z. Causal Associations between Gut Microbiota and Different Types of Dyslipidemia: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 4445. [Google Scholar] [CrossRef]
- Flaig, B.; Garza, R.; Singh, B.; Hamamah, S.; Covasa, M. Treatment of Dyslipidemia through Targeted Therapy of Gut Microbiota. Nutrients 2023, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wu, G.; Zhao, X.; Zhang, H.; Ren, M.; Song, X.; Chang, H.; Jing, Z. Probiotics Combined with Atorvastatin Administration in the Treatment of Hyperlipidemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Medicine 2024, 103, e37883. [Google Scholar] [CrossRef]
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J.; et al. Combined Berberine and Probiotic Treatment as an Effective Regimen for Improving Postprandial Hyperlipidemia in Type 2 Diabetes Patients: A Double Blinded Placebo Controlled Randomized Study. Gut Microbes 2022, 14, 2003176. [Google Scholar] [CrossRef]
- Trotter, R.E.; Vazquez, A.R.; Grubb, D.S.; Freedman, K.E.; Grabos, L.E.; Jones, S.; Gentile, C.L.; Melby, C.L.; Johnson, S.A.; Weir, T.L. Bacillus Subtilis DE111 Intake May Improve Blood Lipids and Endothelial Function in Healthy Adults. Benef. Microbes 2020, 11, 621–630. [Google Scholar] [CrossRef]
- Salamat, S.; Jahan-Mihan, A.; Gharibvand, L.; Reza Tabandeh, M.; Mansoori, A. Multi-Species Synbiotic Supplementation Increased Fecal Short Chain Fatty Acids and Anti-Inflammatory Cytokine Interleukin-10 in Adult Men with Dyslipidemia; A Randomized, Double-Blind, Clinical Trial. Cytokine 2024, 179, 156608. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A Mini-Review of Human Studies on Cholesterol-Lowering Properties of Probiotics. Sci. Pharm. 2019, 87, 26. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Bharathi, M.; Kesika, P.; Suganthy, N.; Chaiyasut, C. The Administration of Probiotics against Hypercholesterolemia: A Systematic Review. Appl. Sci. 2021, 11, 6913. [Google Scholar] [CrossRef]
- Gadelha, C.J.M.U.; Bezerra, A.N. Effects of Probiotics on the Lipid Profile: Systematic Review. J. Vasc. Bras. 2019, 18, e20180124. [Google Scholar] [CrossRef]
- Ettinger, G.; MacDonald, K.; Reid, G.; Burton, J.P. The Influence of the Human Microbiome and Probiotics on Cardiovascular Health. Gut Microbes 2014, 5, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.; Mochizuki, M. Meta-Analysis: Effects of Probiotic Supplementation on Lipid Profiles in Normal to Mildly Hypercholesterolemic Individuals. PLoS ONE 2015, 10, e0139795. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Ponziani, F.R.; Calvani, R.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Primiano, A.; Putignani, L.; Del Chierico, F.; et al. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients 2020, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, X.; Deji, Y.; Gao, S.; Wu, C.; Song, Q.; Shi, Z.; Xiang, X.; Zang, J.; Su, J. Bifidobacterium as a Potential Biomarker of Sarcopenia in Elderly Women. Nutrients 2023, 15, 1266. [Google Scholar] [CrossRef]
- Lee, M.C.; Tu, Y.T.; Lee, C.C.; Tsai, S.C.; Hsu, H.Y.; Tsai, T.Y.; Liu, T.H.; Young, S.L.; Lin, J.S.; Huang, C.C. Lactobacillus Plantarum TWK10 Improves Muscle Mass and Functional Performance in Frail Older Adults: A Randomized, Double-Blind Clinical Trial. Microorganisms 2021, 9, 1466. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Tang, J.; Xu, Z.; Zhou, B.; Liu, Y.; Hu, F.; Zhang, G.; Cheng, R.; Xia, X.; et al. Prevotella Copri Alleviates Sarcopenia via Attenuating Muscle Mass Loss and Function Decline. J. Cachexia Sarcopenia Muscle 2023, 14, 2275–2288. [Google Scholar] [CrossRef]
- Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lane, N.E.; Wu, J.; Yang, T.; Li, J.; He, H.; Wei, J.; Zeng, C.; Lei, G. Population-Based Metagenomics Analysis Reveals Altered Gut Microbiome in Sarcopenia: Data from the Xiangya Sarcopenia Study. J. Cachexia Sarcopenia Muscle 2022, 13, 2340–2351. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in Intestinal Microbiota Diversity, Composition, and Function in Patients with Sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics Supplementation Improves Intestinal Permeability, Obesity Index and Metabolic Biomarkers in Elderly Thai Subjects: A Randomized Controlled Trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef]
- Tominaga, K.; Tsuchiya, A.; Nakano, O.; Kuroki, Y.; Oka, K.; Minemura, A.; Matsumoto, A.; Takahashi, M.; Kadota, Y.; Tochio, T.; et al. Increase in Muscle Mass Associated with the Prebiotic Effects of 1-Kestose in Super-Elderly Patients with Sarcopenia. Biosci. Microbiota Food Health 2021, 40, 150–155. [Google Scholar] [CrossRef]
- Karim, A.; Muhammad, T.; Shah, I.; Khan, J.; Qaisar, R. A Multistrain Probiotic Reduces Sarcopenia by Modulating Wnt Signaling Biomarkers in Patients with Chronic Heart Failure. J. Cardiol. 2022, 80, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Muhammad, T.; Shahid Iqbal, M.; Qaisar, R. A Multistrain Probiotic Improves Handgrip Strength and Functional Capacity in Patients with COPD: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2022, 102, 104721. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Gasparri, C.; Barrile, G.C.; Battaglia, S.; Cavioni, A.; Giusti, R.; Mansueto, F.; Moroni, A.; Nannipieri, F.; Patelli, Z.; et al. Effectiveness of a Novel Food Composed of Leucine, Omega-3 Fatty Acids and Probiotic Lactobacillus Paracasei PS23 for the Treatment of Sarcopenia in Elderly Subjects: A 2-Month Randomized Double-Blind Placebo-Controlled Trial. Nutrients 2022, 14, 4566. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.L.; Nagulesapillai, V.; Piano, A.; Auger, J.; Girard, S.A.; Christman, M.; Tompkins, T.A.; Dahl, W.J. Microbiota Stability and Gastrointestinal Tolerance in Response to a High-Protein Diet with and without a Prebiotic, Probiotic, and Synbiotic: A Randomized, Double-Blind, Placebo-Controlled Trial in Older Women. J. Acad. Nutr. Diet. 2020, 120, 500–516.e10. [Google Scholar] [CrossRef]
- Qaisar, R.; Burki, A.; Karim, A.; Iqbal, M.S.; Ahmad, F. Probiotics Supplements Improve the Sarcopenia-Related Quality of Life in Older Adults with Age-Related Muscle Decline. Calcif. Tissue Int. 2024, 114, 583–591. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-Balance Studies Reveal Associations between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal Microbiota in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef]
- Zhang, C.; Björkman, A.; Cai, K.; Liu, G.; Wang, C.; Li, Y.; Xia, H.; Sun, L.; Kristiansen, K.; Wang, J.; et al. Impact of a 3-Months Vegetarian Diet on the Gut Microbiota and Immune Repertoire. Front. Immunol. 2018, 9, 908. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased Intestinal Permeability and Tight Junction Alterations in Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The Type and Quantity of Dietary Fat and Carbohydrate Alter Faecal Microbiome and Short-Chain Fatty Acid Excretion in a Metabolic Syndrome “at-Risk” Population. Int. J. Obes. 2013, 37, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Fliss Isakov, N.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High Red and Processed Meat Consumption Is Associated with Non-Alcoholic Fatty Liver Disease and Insulin Resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, M.H.; Zyriax, B.-C.; Jagemann, B.; Roth, E.; Windler, E.; Schulze Zur Wiesch, J.; Lohse, A.W.; Kluwe, J. Nonalcoholic Fatty Liver Disease Is Associated with Excessive Calorie Intake Rather than a Distinctive Dietary Pattern. Medicine 2016, 95, e3887. [Google Scholar] [CrossRef]
- Parker, H.M.; Cohn, J.S.; O’Connor, H.T.; Garg, M.L.; Caterson, I.D.; George, J.; Johnson, N.A. Effect of Fish Oil Supplementation on Hepatic and Visceral Fat in Overweight Men: A Randomized Controlled Trial. Nutrients 2019, 11, 475. [Google Scholar] [CrossRef]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef]
- Maestri, M.; Santopaolo, F.; Pompili, M.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota Modulation in Patients with Non-Alcoholic Fatty Liver Disease: Effects of Current Treatments and Future Strategies. Front. Nutr. 2023, 10, 1110536. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Gaborit, B.; Dutour, A.; Clement, K. Gut Microbiota and Non-Alcoholic Fatty Liver Disease: New Insights. Clin. Microbiol. Infect. 2013, 19, 338–348. [Google Scholar] [CrossRef]
- Wieland, A.; Frank, D.N.; Harnke, B.; Bambha, K. Systematic Review: Microbial Dysbiosis and Nonalcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2015, 42, 1051–1063. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Selvakumar, P.C.; Cresci, G.A.M. The Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease. Med. Sci. 2018, 6, 47. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Feng, J.; Ji, J.; Yu, Q.; Li, Y.; Zheng, Y.; Dai, W.; Wu, J.; Guo, C. Crosstalk between PPARs and Gut Microbiota in NAFLD. Biomed. Pharmacother. 2021, 136, 111255. [Google Scholar] [CrossRef]
- Arslan, N. Obesity, Fatty Liver Disease and Intestinal Microbiota. World J. Gastroenterol. 2014, 20, 16452. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Stewart, C.J.; Day, C.P.; Trenell, M. Gut Microbiota and Lifestyle Interventions in NAFLD. Int. J. Mol. Sci. 2016, 17, 447. [Google Scholar] [CrossRef]
- Chang, H.-C.; Huang, C.-N.; Yeh, D.-M.; Wang, S.-J.; Peng, C.-H.; Wang, C.-J. Oat Prevents Obesity and Abdominal Fat Distribution, and Improves Liver Function in Humans. Plant Foods Hum. Nutr. 2013, 68, 18–23. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Therapeutic Implications of the Gut Microbiome and Probiotics in Patients with NAFLD. Diseases 2019, 7, 27. [Google Scholar] [CrossRef]
- Xiao, M.-W.; Lin, S.-X.; Shen, Z.-H.; Luo, W.-W.; Wang, X.-Y. Systematic Review with Meta-Analysis: The Effects of Probiotics in Nonalcoholic Fatty Liver Disease. Gastroenterol. Res. Pract. 2019, 2019, 1484598. [Google Scholar] [CrossRef]
- Loman, B.R.; Hernández-Saavedra, D.; An, R.; Rector, R.S. Prebiotic and Probiotic Treatment of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Nutr. Rev. 2018, 76, 822–839. [Google Scholar] [CrossRef]
- Khan, M.Y.; Mihali, A.B.; Rawala, M.S.; Aslam, A.; Siddiqui, W.J. The Promising Role of Probiotic and Synbiotic Therapy in Aminotransferase Levels and Inflammatory Markers in Patients with Nonalcoholic Fatty Liver Disease—a Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 703–715. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Maraj, B.; Harding-Theobald, E.; Vittinghoff, E.; Terrault, N.A. Gut Microbiome-Targeted Therapies in Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-Analysis, and Meta-Regression. Am. J. Clin. Nutr. 2019, 110, 139–149. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.J. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. The Metabolic Syndrome—A New Worldwide Definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Wortelboer, K.; Nieuwdorp, M.; Herrema, H. Fecal Microbiota Transplantation beyond Clostridioides Difficile Infections. EBioMedicine 2019, 44, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in Inflammatory Bowel Disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Vindigni, S.M.; Surawicz, C.M. Fecal Microbiota Transplantation. Gastroenterol. Clin. N. Am. 2017, 46, 171–185. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Weingarden, A.R.; Unno, T.; Khoruts, A.; Sadowsky, M.J. High-Throughput DNA Sequence Analysis Reveals Stable Engraftment of Gut Microbiota Following Transplantation of Previously Frozen Fecal Bacteria. Gut Microbes 2013, 4, 125–135. [Google Scholar] [CrossRef]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal Microbiota Transplantation for Clostridium Difficile Infection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium Difficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498, quiz 499. [Google Scholar] [CrossRef]
- Proença, I.M.; Allegretti, J.R.; Bernardo, W.M.; de Moura, D.T.H.; Ponte Neto, A.M.; Matsubayashi, C.O.; Flor, M.M.; Kotinda, A.P.S.T.; de Moura, E.G.H. Fecal Microbiota Transplantation Improves Metabolic Syndrome Parameters: Systematic Review with Meta-Analysis Based on Randomized Clinical Trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.T.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of Gut Microbiota during Probiotic-Mediated Attenuation of Metabolic Syndrome in High Fat Diet-Fed Mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Flores-Lopez, A.G.; Aguilar-Lopez, M.; Sanchez-Tapia, M.; Medina-Vera, I.; Dıaz, D.; Tovar, A.R.; Torres, N. Improvement of Lipoprotein Profile and Metabolic Endotoxemia by a Lifestyle Intervention That Modifies the Gut Microbiota in Subjects with Metabolic Syndrome. J. Am. Heart Assoc. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res Clin Pract 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Gea, I.; Sánchez-Alcoholado, L.; Martín-Tejedor, B.; Castellano-Castillo, D.; Moreno-Indias, I.; Urda-Cardona, A.; Tinahones, F.J.; Fernández-García, J.C.; Queipo-Ortuño, M.I. Gut Microbiota Differs in Composition and Functionality between Children with Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 2018, 41, 2385–2395. [Google Scholar] [CrossRef]
- Bjursell, M.; Admyre, T.; Göransson, M.; Marley, A.E.; Smith, D.M.; Oscarsson, J.; Bohlooly-Y, M. Improved Glucose Control and Reduced Body Fat Mass in Free Fatty Acid Receptor 2-Deficient Mice Fed a High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2011, 300, 211–220. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P.-F. Diabetes and Gut Microbiota. World J. Diabetes 2021, 12, 1693–1703. [Google Scholar] [CrossRef]
- Livanos, A.E.; Greiner, T.U.; Vangay, P.; Pathmasiri, W.; Stewart, D.; McRitchie, S.; Li, H.; Chung, J.; Sohn, J.; Kim, S.; et al. Antibiotic-Mediated Gut Microbiome Perturbation Accelerates Development of Type 1 Diabetes in Mice. Nat. Microbiol. 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F.; Weinstock, G.M.; et al. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Qin, S.; Chen, H.H.; Zhao, G.Z.; Li, J.; Zhu, W.Y.; Xu, L.H.; Jiang, J.H.; Li, W.J. Abundant and Diverse Endophytic Actinobacteria Associated with Medicinal Plant Maytenus Austroyunnanensis in Xishuangbanna Tropical Rainforest Revealed by Culture-Dependent and Culture-Independent Methods. Environ. Microbiol. Rep. 2012, 4, 522–531. [Google Scholar] [CrossRef]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the Role of Gut Microbiota in Obesity: Pathogenesis, Mechanisms, and Therapeutic Perspectives. Protein Cell 2018, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the Gut Microbiota to Distant Organs in Physiology and Disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Nieuwdorp, M.; Bäckhed, F. Microbial Modulation of Insulin Sensitivity. Cell Metab. 2014, 20, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Kimura, Y.; Hyogo, H.; Ishitobi, T.; Nabeshima, Y.; Arihiro, K.; Chayama, K. Postprandial Insulin Secretion Pattern Is Associated with Histological Severity in Non-Alcoholic Fatty Liver Disease Patients without Prior Known Diabetes Mellitus. J. Gastroenterol. Hepatol. 2011, 26, 517–522. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Afshani, M.R.; Torfi, E.; Akiash, N.; Jahanshahi, A.; Mohamadi, A.; Sherafat, O. Effect of Empagliflozin on Left Ventricular Volumes in Type 2 Diabetes or Prediabetes Heart Failure Patients with Reduc. Acta Cardiol. 2020, 796–802. [Google Scholar] [CrossRef]
- Smits, M.M.; Fluitman, K.S.; Herrema, H.; Davids, M.; Kramer, M.H.H.; Groen, A.K.; Belzer, C.; de Vos, W.M.; Cahen, D.L.; Nieuwdorp, M.; et al. Liraglutide and Sitagliptin Have No Effect on Intestinal Microbiota Composition: A 12-Week Randomized Placebo-Controlled Trial in Adults with Type 2 Diabetes. Diabetes Metab. 2021, 47, 101223. [Google Scholar] [CrossRef]
- Takewaki, F.; Nakajima, H.; Takewaki, D.; Hashimoto, Y.; Majima, S.; Okada, H.; Senmaru, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Habitual Dietary Intake Affects the Altered Pattern of Gut Microbiome by Acarbose in Patients with Type 2 Diabetes. Nutrients 2021, 13, 2107. [Google Scholar] [CrossRef]
- Bauer, P.V.; Duca, F.A.; Waise, T.M.Z.; Rasmussen, B.A.; Abraham, M.A.; Dranse, H.J.; Puri, A.; O’Brien, C.A.; Lam, T.K.T. Metformin Alters Upper Small Intestinal Microbiota That Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab. 2018, 27, 101–117.e5. [Google Scholar] [CrossRef]
- Lau, K.; Benitez, P.; Ardissone, A.; Wilson, T.D.; Collins, E.L.; Lorca, G.; Li, N.; Sankar, D.; Wasserfall, C.; Neu, J.; et al. Inhibition of Type 1 Diabetes Correlated to a Lactobacillus Johnsonii N6.2-Mediated Th17 Bias. J. Immunol. 2011, 186, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Amyot, J.; Semache, M.; Ferdaoussi, M.; Fontés, G.; Poitout, V. Lipopolysaccharides Impair Insulin Gene Expression in Isolated Islets of Langerhans via Toll-Like Receptor-4 and NF-ΚB Signalling. PLoS ONE 2012, 7, e36200. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota Imbalance Induced by Dietary Sugar Disrupts Immune-Mediated Protection from Metabolic Syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The Endocannabinoid System Links Gut Microbiota to Adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Xu, J.; Xue, C.; Xue, Y.; Wang, Y. Dietary Trimethylamine N-Oxide Exacerbates Impaired Glucose Tolerance in Mice Fed a High Fat Diet. J. Biosci. Bioeng. 2014, 118, 476–481. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Abadia-Molina, F.; Saez-Lara, M.J.; Campaña-Martin, L.; Muñoz-Quezada, S.; Romero, F.; Gil, A.; Fontana, L. Effects of Lactobacillus Paracasei CNCM I-4034, Bifidobacterium Breve CNCM I-4035 and Lactobacillus Rhamnosus CNCM I-4036 on Hepatic Steatosis in Zucker Rats. PLoS ONE 2014, 9, e98401. [Google Scholar] [CrossRef]
- Marazza, J.A.; LeBlanc, J.G.; de Giori, G.S.; Garro, M.S. Soymilk Fermented with Lactobacillus Rhamnosus CRL981 Ameliorates Hyperglycemia, Lipid Profiles and Increases Antioxidant Enzyme Activities in Diabetic Mice. J. Funct. Foods 2013, 5, 1848–1853. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Park, J.-H.; Seok, S.-H.; Baek, M.-W.; Kim, D.-J.; Lee, K.-E.; Paek, K.-S.; Lee, Y.; Park, J.-H. Human Originated Bacteria, Lactobacillus Rhamnosus PL60, Produce Conjugated Linoleic Acid and Show Anti-Obesity Effects in Diet-Induced Obese Mice. Biochim. Biophys. Acta 2006, 1761, 736–744. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Antidiabetic Effect of Lactobacillus Casei CCFM0412 on Mice with Type 2 Diabetes Induced by a High-Fat Diet and Streptozotocin. Nutrition 2014, 30, 1061–1068. [Google Scholar] [CrossRef]
- Tian, P.; Li, B.; He, C.; Song, W.; Hou, A.; Tian, S.; Meng, X.; Li, K.; Shan, Y. Antidiabetic (Type 2) Effects of Lactobacillus G15 and Q14 in Rats through Regulation of Intestinal Permeability and Microbiota. Food Funct. 2016, 7, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Lu, T.-Y.; Tseng, Y.-Y.; Pan, T.-M. The Effects of Lactobacillus-Fermented Milk on Lipid Metabolism in Hamsters Fed on High-Cholesterol Diet. Appl. Microbiol. Biotechnol. 2006, 71, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Tsai, T.-Y.; Pan, T.-M. Anti-Obesity Activity of the Water Extract of Lactobacillus Paracasei Subsp. Paracasei NTU 101 Fermented Soy Milk Products. Food Funct. 2015, 6, 3522–3530. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Yun, S.-I.; Park, M.-H.; Park, J.-H.; Jeong, S.-Y.; Park, H.-O. Anti-Obesity Effect of Lactobacillus Gasseri BNR17 in High-Sucrose Diet-Induced Obese Mice. PLoS ONE 2013, 8, e54617. [Google Scholar] [CrossRef]
- Hsieh, F.-C.; Lee, C.-L.; Chai, C.-Y.; Chen, W.-T.; Lu, Y.-C.; Wu, C.-S. Oral Administration of Lactobacillus Reuteri GMNL-263 Improves Insulin Resistance and Ameliorates Hepatic Steatosis in High Fructose-Fed Rats. Nutr. Metab. 2013, 10, 35. [Google Scholar] [CrossRef]
- Stenman, L.K.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential Probiotic Bifidobacterium Animalis Ssp. Lactis 420 Prevents Weight Gain and Glucose Intolerance in Diet-Induced Obese Mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Tutor, A.W.; Lavie, C.J.; Kachur, S.; Milani, R.V.; Ventura, H.O. Updates on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2023, 78, 2–10. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Vaamonde, J.G.; Álvarez-Món, M.A. Obesity and Overweight. Medicine 2020, 13, 767–776. [Google Scholar] [CrossRef]
- Sheykhsaran, E.; Abbasi, A.; Leylabadlo, H.E.; Sadeghi, J.; Mehri, S.; Mazraeh, F.N.; Feizi, H.; Baghi, H.B. Gut Microbiota and Obesity: An Overview of Microbiota to Microbial-Based Therapies. Postgrad. Med. J. 2023, 99, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review. Eur. J. Clin. Nutr. 2020, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut Microbiota in Obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, M.M.; Rahman, M.A.; Ripon, M.A.R.; Hossain, M.S. Gut Microbiota in Obesity and Related Complications: Unveiling the Complex Interplay. Life Sci. 2023, 334, 122211. [Google Scholar] [CrossRef]
- Shelton, C.D.; Sing, E.; Mo, J.; Shealy, N.G.; Yoo, W.; Thomas, J.; Fitz, G.N.; Castro, P.R.; Hickman, T.T.; Torres, T.P.; et al. An Early-Life Microbiota Metabolite Protects against Obesity by Regulating Intestinal Lipid Metabolism. Cell Host Microbe 2023, 31, 1604–1619.e10. [Google Scholar] [CrossRef]
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and Gut–Microbiota–Brain Axis: A Narrative Review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The Critical Role of Gut Microbiota in Obesity. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Wang, N.; Zuo, Z.; Wei, H.; Zhao, F. A Novel Peptide Protects against Diet-Induced Obesity by Suppressing Appetite and Modulating the Gut Microbiota. Gut 2023, 72. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- González-Lamuño, D.; Arrieta-Blanco, F.J.; Fuentes, E.D.; Forga-Visa, M.T.; Morales-Conejo, M.; Peña-Quintana, L.; Vitoria-Miñana, I. Hyperhomocysteinemia in Adult Patients: A Treatable Metabolic Condition. Nutrients 2023, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Wang, C.; Shang, W.; Lau, C.W.; Luo, J.Y.; Wang, L.; Huang, Y. A High Methionine and Low Folate Diet Alters Glucose Homeostasis and Gut Microbiome. Biochem. Biophys. Rep. 2021, 25, 100921. [Google Scholar] [CrossRef]
- Sugahara, H.; Odamaki, T.; Hashikura, N.; Abe, F.; Xiao, J. Differences in Folate Production by Bifidobacteria of Different Origins. Biosci. Microbiota Food Health 2015, 34, 87–93. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Gong, Z.; Dong, Z.; Yu, F.; Fu, Y.; Jiang, C.; Kong, W. Ablation of the Gut Microbiota Alleviates High-Methionine Diet-Induced Hyperhomocysteinemia and Glucose Intolerance in Mice. NPJ Sci. Food 2023, 7, 36. [Google Scholar] [CrossRef]
- Riedijk, M.A.; Stoll, B.; Chacko, S.; Schierbeek, H.; Sunehag, A.L.; Van Goudoever, J.B.; Burrin, D.G. Methionine Transmethylation and Transsulfuration in the Piglet Gastrointestinal Tract. Proc. Natl. Acad. Sci. USA 2007, 104, 3408–3413. [Google Scholar] [CrossRef]
- Zinno, P.; Motta, V.; Guantario, B.; Natella, F.; Roselli, M.; Bello, C.; Comitato, R.; Carminati, D.; Tidona, F.; Meucci, A.; et al. Supplementation with Dairy Matrices Impacts on Homocysteine Levels and Gut Microbiota Composition of Hyperhomocysteinemic Mice. Eur. J. Nutr. 2020, 59, 345–358. [Google Scholar] [CrossRef]
- Molnar, J.; Mallonee, C.J.; Stanisic, D.; Homme, R.P.; George, A.K.; Singh, M.; Tyagi, S.C. Hidradenitis Suppurativa and 1-Carbon Metabolism: Role of Gut Microbiome, Matrix Metalloproteinases, and Hyperhomocysteinemia. Front. Immunol. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, N.; Zhang, L.; Chen, J.; Liu, X.; Piao, S. Gut Microbiota Is a Potential Goalkeeper of Dyslipidemia. Front. Endocrinol. 2022, 13, 950826. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10−/− Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, V.R.; Hezaveh, R.; Reigstad, C.S.; Gopalacharyulu, P.; Yetukuri, L.; Islam, S.; Felin, J.; Perkins, R.; Borén, J.; Orešič, M.; et al. The Gut Microbiota Modulates Host Energy and Lipid Metabolism in Mice. J. Lipid Res. 2010, 51, 1101. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular Mechanisms and Open Questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of Sarcopenia: Prevalence, Risk Factors, and Consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Bianchi, L.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Corica, F.; Di Bari, M.; Maggio, M.; Manca, G.M.; Rizzo, M.R.; et al. Prevalence and Clinical Correlates of Sarcopenia, Identified According to the EWGSOP Definition and Diagnostic Algorithm, in Hospitalized Older People: The GLISTEN Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1575–1581. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, J.K.; Hu, Y. min Gut Microbiota as a Promising Therapeutic Target for Age-Related Sarcopenia. Ageing Res. Rev. 2022, 81, 101739. [Google Scholar] [CrossRef]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas Gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Alieva, A.; Poluektova, E.; Kudryavtseva, A.; Krasnov, G.; Zharkova, M.; Zharikov, Y. Gut Dysbiosis and Body Composition in Cirrhosis. World J. Hepatol. 2022, 14, 1210–1225. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Picca, A.; Marzetti, E.; Calvani, R.; Conta, G.; Del Chierico, F.; Capuani, G.; Faccia, M.; Fianchi, F.; Funaro, B.; et al. Characterization of the Gut-Liver-Muscle Axis in Cirrhotic Patients with Sarcopenia. Liver International 2021, 41, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Song, S.W.; Jung, S.Y.; Bae, J.; Hwang, N.; Kim, H.N. Sarcopenia in Community-Dwelling Older Adults Is Associated with the Diversity and Composition of the Gut Microbiota. Exp. Gerontol. 2022, 167, 111927. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.A.; Hegazy, S.M.; Aziz, R.K. The Curious Case of Prevotella Copri. Gut Microbes 2023, 15. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediators Inflamm. 2018, 2018. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Anstee, Q.M.; McPherson, S.; Day, C.P. How Big a Problem Is Non-Alcoholic Fatty Liver Disease? BMJ 2011, 343, d3897. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global Incidence and Prevalence of Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of Linoleic Acid by Human Gut Bacteria: Different Routes for Biosynthesis of Conjugated Linoleic Acid. J. Bacteriol. 2007, 189, 2566–2570. [Google Scholar] [CrossRef]
- Baddini Feitoza, A.; Fernandes Pereira, A.; Ferreira da Costa, N.; Gonçalves Ribeiro, B. Conjugated Linoleic Acid (CLA): Effect Modulation of Body Composition and Lipid Profile. Nutr. Hosp. 2009, 24, 422–428. [Google Scholar]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Wostmann, B.S.; Larkin, C.; Moriarty, A.; Bruckner-Kardoss, E. Dietary Intake, Energy Metabolism, and Excretory Losses of Adult Male Germfree Wistar Rats. Lab. Anim. Sci. 1983, 33, 46–50. [Google Scholar] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms Underlying the Resistance to Diet-Induced Obesity in Germ-Free Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-Free C57BL/6J Mice Are Resistant to High-Fat-Diet-Induced Insulin Resistance and Have Altered Cholesterol Metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef]
- Fleissner, C.K.; Huebel, N.; Abd El-Bary, M.M.; Loh, G.; Klaus, S.; Blaut, M. Absence of Intestinal Microbiota Does Not Protect Mice from Diet-Induced Obesity. Br. J. Nutr. 2010, 104, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Swartz, T.D.; Sakar, Y.; Duca, F.A.; Covasa, M. Preserved Adiposity in the Fischer 344 Rat Devoid of Gut Microbiota. FASEB J. 2013, 27, 1701–1710. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the Gut Microbiota on Host Adiposity Are Modulated by the Short-Chain Fatty-Acid Binding G Protein-Coupled Receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef]
- Baes, M.; Gulick, T.; Choi, H.S.; Martinoli, M.G.; Simha, D.; Moore, D.D. A New Orphan Member of the Nuclear Hormone Receptor Superfamily That Interacts with a Subset of Retinoic Acid Response Elements. Mol. Cell Biol. 1994, 14, 1544–1552. [Google Scholar] [CrossRef]
- Björkholm, B.; Bok, C.M.; Lundin, A.; Rafter, J.; Hibberd, M.L.; Pettersson, S. Intestinal Microbiota Regulate Xenobiotic Metabolism in the Liver. PLoS ONE 2009, 4, e6958. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal Microbiota Determines Development of Non-Alcoholic Fatty Liver Disease in Mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.; Schattenberg, J.M.; Leclercq, I.; Yeh, M.M.; Goldin, R.; Teoh, N.; Schuppan, D. Mouse Models of Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2241–2257. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Zocco, M.A.; Cerrito, L.; Gasbarrini, A.; Pompili, M. Bacterial Translocation in Patients with Liver Cirrhosis: Physiology, Clinical Consequences, and Practical Implications. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public. Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like Receptor-4 Signaling and Kupffer Cells Play Pivotal Roles in the Pathogenesis of Non-Alcoholic Steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a Link between Obesity, Metabolic Syndrome and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Moghaddas Sani, H.; Rahbar Saadat, Y.; Barzegari, A.; Omidi, Y. Type 1 Diabetes: Through the Lens of Human Genome and Metagenome Interplay. Biomed. Pharmacother. 2018, 104, 332–342. [Google Scholar] [CrossRef]
- Khalid, Q.; Bailey, I.; Patel, V.B. Non-Alcoholic Fatty Liver Disease: The Effect of Bile Acids and Farnesoid X Receptor Agonists on Pathophysiology and Treatment. Liver Res. Open J. 2015, 1, 32–40. [Google Scholar] [CrossRef]
- Rinella, M.E.; Sanyal, A.J. Genetics, Diagnostics and Therapeutic Advances in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 65–66. [Google Scholar] [CrossRef]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The Role of PH in Determining the Species Composition of the Human Colonic Microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-Chain Fatty Acids Suppress Cholesterol Synthesis in Rat Liver and Intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H.; Parkinson, S.J. You Are What You Eat. Nat. Biotechnol. 2014, 32, 243–245. [Google Scholar] [CrossRef]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D.; et al. Dietary Cholesterol Exacerbates Hepatic Steatosis and Inflammation in Obese LDL Receptor-Deficient Mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef]
- Mao, J.-W.; Tang, H.-Y.; Zhao, T.; Tan, X.-Y.; Bi, J.; Wang, B.-Y.; Wang, Y.-D. Intestinal Mucosal Barrier Dysfunction Participates in the Progress of Nonalcoholic Fatty Liver Disease. Int. J. Clin. Exp. Pathol. 2015, 8, 3648–3658. [Google Scholar]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, P.C.; Bean, E.G.; Ruth Foxwell, A.; Pavli, P. Effects of Bacterial Products on Enterocyte–Macrophage Interactions in Vitro. Biochem. Biophys. Res. Commun. 2011, 413, 336–341. [Google Scholar] [CrossRef]
- Verdam, F.J.; Rensen, S.S.; Driessen, A.; Greve, J.W.; Buurman, W.A. Novel Evidence for Chronic Exposure to Endotoxin in Human Nonalcoholic Steatohepatitis. J. Clin. Gastroenterol. 2011, 45, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Oh, T.G.; Kim, S.M.; Caussy, C.; Fu, T.; Guo, J.; Bassirian, S.; Singh, S.; Madamba, E.V.; Bettencourt, R.; Richards, L.; et al. A Universal Gut-Microbiome-Derived Signature Predicts Cirrhosis. Cell Metab. 2020, 32, 878–888.e6. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.; Day, C.P.; Trenell, M.I. Lifestyle Interventions for the Treatment of Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review. J. Hepatol. 2012, 56, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Ness, E.; Kowdley, K. V Nutritional Approaches to Achieve Weight Loss in Nonalcoholic Fatty Liver Disease. Adv. Nutr. 2017, 8, 253–265. [Google Scholar] [CrossRef]
- He, X.X.; Wu, X.L.; Chen, R.P.; Chen, C.; Liu, X.G.; Wu, B.J.; Huang, Z.M. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0162368. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Venditti, C.; Lee, H.Y.; Darch, M.; Floyd, S.; West, S.; Simon, R. Systematic Review and Meta-Analysis of Controlled Intervention Studies on the Effectiveness of Long-Chain Omega-3 Fatty Acids in Patients with Nonalcoholic Fatty Liver Disease. Nutr. Rev. 2018, 76, 581–602. [Google Scholar] [CrossRef]
- de Velasco, P.; Ferreira, A.; Crovesy, L.; Marine, T.; das Graças Tavares do Carmo, M. Fatty Acids, Gut Microbiota, and the Genesis of Obesity. In Biochemistry and Health Benefits of Fatty Acids; IntechOpen: London, UK, 2018. [Google Scholar]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-Alcoholic Fatty Liver Disease Rats. Probiotics Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef]
- Xin, J.; Zeng, D.; Wang, H.; Ni, X.; Yi, D.; Pan, K.; Jing, B. Preventing Non-Alcoholic Fatty Liver Disease through Lactobacillus Johnsonii BS15 by Attenuating Inflammation and Mitochondrial Injury and Improving Gut Environment in Obese Mice. Appl. Microbiol. Biotechnol. 2014, 98, 6817–6829. [Google Scholar] [CrossRef]
- Velayudham, A.; Dolganiuc, A.; Ellis, M.; Petrasek, J.; Kodys, K.; Mandrekar, P.; Szabo, G. VSL#3 Probiotic Treatment Attenuates Fibrosis without Changes in Steatohepatitis in a Diet-Induced Nonalcoholic Steatohepatitis Model in Mice. Hepatology 2009, 49, 989–997. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.; Diehl, A.M. Probiotics and Antibodies to TNF Inhibit Inflammatory Activity and Improve Nonalcoholic Fatty Liver Disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Possemiers, S.; Verstraete, W.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Dietary Modulation of Clostridial Cluster XIVa Gut Bacteria (Roseburia Spp.) by Chitin-Glucan Fiber Improves Host Metabolic Alterations Induced by High-Fat Diet in Mice. J. Nutr. Biochem. 2012, 23, 51–59. [Google Scholar] [CrossRef]
- You, S.; Hu, X.; Zhao, Q.; Chen, X.; Xu, C. Oat β-Glucan Inhibits Lipopolysaccharide-Induced Nonalcoholic Steatohepatitis in Mice. Food Funct. 2013, 4, 1360–1368. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Nourian, M.; Askari, G.; Maracy, M.R.; Rafiei, R. The Effect of Psyllium on Anthropometric Measurements and Liver Enzymes in Overweight or Obese Adults with Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Adv. Biotechnol. Res. 2015, 33, 1771–1783. [Google Scholar]
- Weitkunat, K.; Schumann, S.; Petzke, K.J.; Blaut, M.; Loh, G.; Klaus, S. Effects of Dietary Inulin on Bacterial Growth, Short-Chain Fatty Acid Production and Hepatic Lipid Metabolism in Gnotobiotic Mice. J. Nutr. Biochem. 2015, 26, 929–937. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Vacante, M.; Antic, T.; Giordano, M.; Chisari, G.; Acquaviva, R.; Mastrojeni, S.; Malaguarnera, G.; Mistretta, A.; Li Volti, G.; et al. Bifidobacterium Longum with Fructo-Oligosaccharides in Patients with Non Alcoholic Steatohepatitis. Dig. Dis. Sci. 2012, 57, 545–553. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Eshraghian, A.; Kani, H.T.; Khannoussi, W.; Lacaze, L.; et al. European Guideline on Obesity Care in Patients with Gastrointestinal and Liver Diseases - Joint ESPEN/UEG Guideline. Clin. Nutr. 2022, 41, 2364–2405. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety Assessment of Probiotics for Human Use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
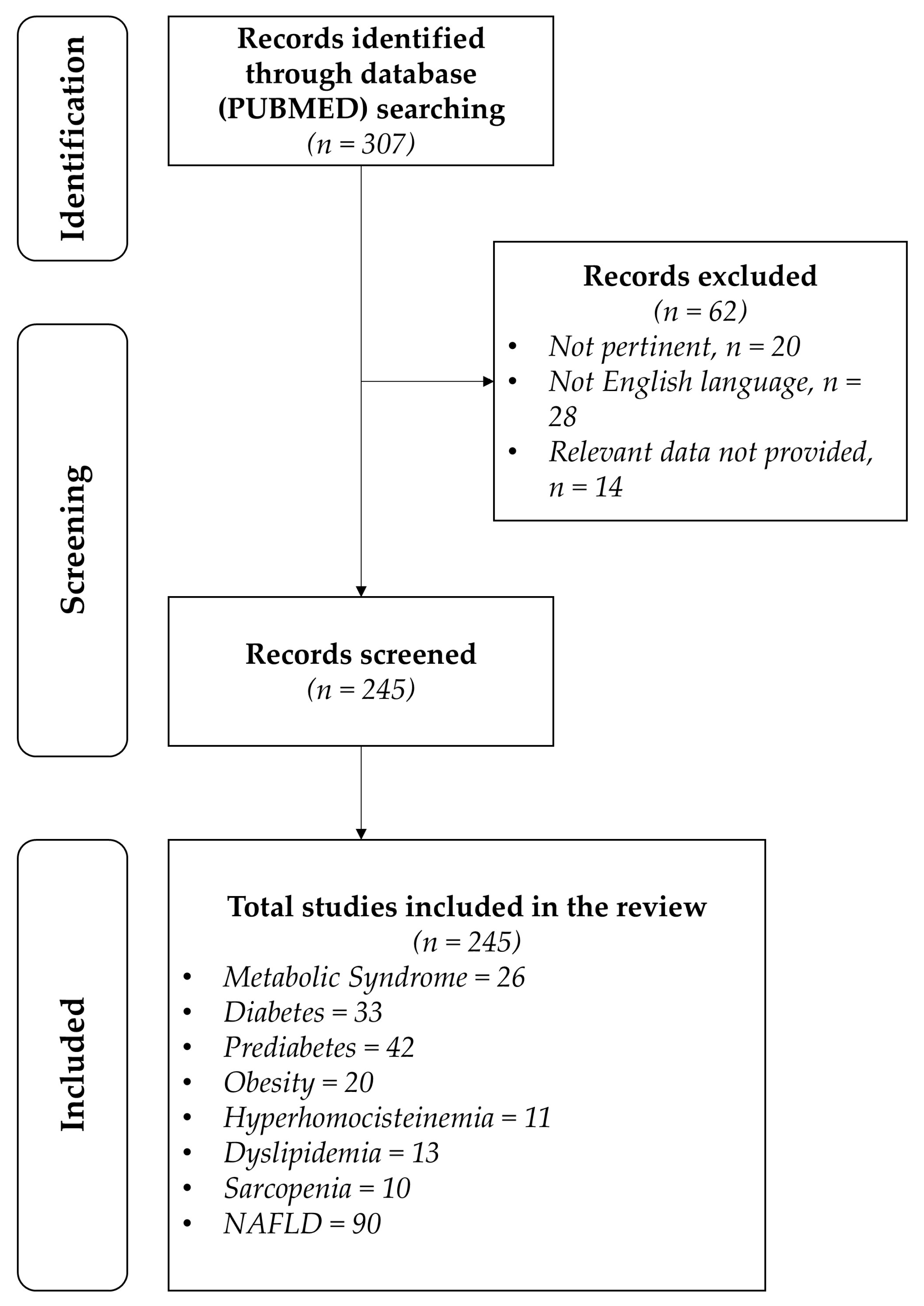
| (A) | ||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subjects | End Point | Results | Conclusion | Strength of Evidence | |
| Portela-Cidade et al. (2015) [32] | Systematic review | 35 of a total of 230 articles were included | - | Access the most recent data about the relevance of intestinal microbiota and Toll-like receptor (TLR) expression in the development of hepatic lesions and metabolic syndrome | Early activation of TLRs and its interactions with a dysbiotic intestinal microbiota play a key role in metabolic syndrome | There is evidence in the literature that suggests that innate immunity and intestinal microbiota may be the hidden link in the metabolic syndrome development mechanisms | High | |
| Cheng et al. (2023) [33] | Systematic review | 11 RCTs | 608 | Effects of supplementation with synbiotics and probiotics on MetS parameters | Lowered BMI, LDL-c levels, and fasting blood glucose was achieved, no effect on blood pressure | The literature supports the possibility of using probiotics to help ameliorate Met-S parameters | High | |
| He M, Shi B (2017) [34] | Narrative review | - | - | Review recent studies concerning the role of the gut microbiota in MS modulation | - | - | Medium | |
| Mallappa RH et al. (2012) [35] | Narrative review | - | - | Analize the effects of prebiotics and probiotics on metabolic syndrome in a “pharmaco-nutritional” approach | - | - | Medium | |
| Horvath (2024) [36] | Scoping review | 13 studies on diet and the gut microbiome in patients with MetS (total of 961 patients; 26 studies testing probiotics as microbiome modulators in obesity, MetS, and diabetes (total of 4403 patients); 15 studies on prebiotics and the gut microbiome in patients with metabolic syndrome (total of 913 patients); 10 studies on fecal microbiome transplantation in patients with metabolic syndrome (total of 483 patients) | - | Metabolic syndrome parameters, weight-related parameters | No significant effects on metabolic parameters | The results do not favor treatment | High | |
| Hadi A et al. (2021) [37] | Meta-analysis | Ten eligible publications (nine RCTs, n = 344 participants) were included | - | Evaluating the effects of pro-/synbiotic consumption and supplementation in adults (≥18 years) with MetS | Supplementation with pro-/synbiotics reduced total cholesterol (TC) in adults with MetS versus placebo (MD: −6.66 mg/dL, 95% CI: −13.25 to −0.07, p = 0.04, I2 = 28.8%, n = 7), without affecting weight, body mass index, waist circumference, fasting blood sugar, homeostasis model assessment for insulin resistance, insulin, triglycerides, low-density lipoprotein cholesterol, or high-density lipoprotein cholesterol (p > 0.05) | Pro-/synbiotic consumption may be beneficial in reducing TC levels in adults with MetS | High | |
| Chen T et al. (2024) [33] | Meta-analysis | 11 RCTs were identified | 608 participants in total included | Analyze the effects of probiotics or synbiotics on cardiovascular factors in adults with MetS | The supplementation with probiotics or synbiotics reduced body mass index (p < 0.0001), low-density lipoprotein (LDL-c) (p = 0.004), and fasting blood glucose (FBG) (p = 0.03), but had no beneficial effect on systolic blood pressure (SBP) (WMD = 1.24, 95% CI = [−2.06, 4.54], p = 0.46, n = 8) in MetS patients | Supplementation with probiotics or synbiotics can reduce BMI, LDL-c, FBG in patients with MetS | High | |
| (B) | ||||||||
| Authors | Type of Studies | Population Characteristics | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of Evidence |
| Leber B et al. (2012) [38] | Randomized controlled trial | Twenty-eight patients and ten healthy controls were included | Supplementation of 3 × 6.5 × 10⁹ CFU L. casei Shirota (probiotic group) per day | 3 months | Investigate the effect of Lactobacillus casei Shirota on gut permeability, presence of endotoxin and neutrophil function in MetS | PCR and LBP levels slightly but significantly increased after 3 months within the probiotic group. Neutrophil function, TLR expression, and LBP and sCD14 levels were not significantly different between the groups. | L. casei Shirota administration in the MetS patients did not have any influence on any parameter tested, possibly due to too-short study duration or underdosing of L. casei Shirota. | High |
| Tripolt NJ et al. (2012) [39] | Randomized controlled trial | 30 subjects with metabolic syndrome | Supplementation with a milk drink containing LcS (3 bottles a day, 65 mL, containing LcS at a concentration of 108/mL) | 12 weeks | Determine the effects of supplementation with Lactobacillus casei Shirota on insulin sensitivity, β-cell function, inflammation, and endothelial dysfunction parameters in subjects with metabolic syndrome | - | No insulin sensitivity index improvements were found, nor in β-cell function. | High |
| Stadlbauer V et al. (2015) [40] | Randomized controlled trial | 28 subjects with metabolic syndrome (13 in treatment group and 15 controls) | Supplementation with a milk drink containing LcS (3 bottles a day, 65 mL, containing LcS at a concentration of 108/mL) | 12 weeks | Investigate the effect of Lactobacillus casei Shirota (LcS) on gut microbiota composition, gut barrier integrity, intestinal inflammation, and serum bile acid profile in metabolic syndrome | Zonulin and calprotectin were increased in metabolic syndrome stool samples but not influenced by LcS supplementation. Serum bile acids were similar to controls and not influenced by LcS supplementation. Metabolic syndrome is associated with a higher Bacteroidetes/Firmicutes ratio and gut barrier dysfunction but LcS was not able to change this. | No effect of LcS on gut barrier integrity markers | High |
| Barreto FM et al. (2013) [41] | Randomized controlled trial | 24 postmenopausal women with METs divided into treatment and placebo group | 80 mL/day of fermented milk with 1.25 × 107 UFC/g of lactobacillus plantarum | 3 months | Evaluate the influence of fermented milk with L. plantarum in the classical parameters related to MetS, as well as in other parameters related to cardiovascular risk in postmenopausal women | A significant reduction in glucose and homocysteine levels was observed in the treatment group compared with the placebo group (p = 0.037 and p = 0.019, respectively) but no significative difference was found for total cholesterol, blood lipids, and inflammatory biomarkers. | L. plantarum supplementation may have a role in the treatment of metabolic syndrome in postmenopausal women. | High |
| Bernini LJ et al. (2016) [42] | Randomized controlled trial | 51 patients with Mets, 25 receiving placebo, the others (n = 26) receiving Bifidobacterium Lactis HN019 supplementation | A daily serving of fermented milk with Bifidobacterium Lactis HN019 (80 mL, each dose with 2.72 × 1010 colony-forming units) | 45 days | Evaluate the effect of consumption of milk containing the probiotic B. lactis HN019 on the classical parameters of MetS and other related cardiovascular risk factors | Treatment group showed significant decrement in body mass index (p = 0.017), total cholesterol (p = 0.009), and low-density lipoprotein (p = 0.008) compared with baseline and control group and a significant reduction in pro-inflammatory cytokines. | B. lactis HN019 may have a role in treating obesity and reducing blood lipids and some inflammatory markers in patients with MetS. | High |
| Wastyk HC et al. [43] | Randomized controlled trial | 39 adults with elevated Met-S parameters, 23 assigned to the intervention arm, 16 to placebo | Probiotic made of a blend of Limosilactobacillus reuteri NCIMB 30242, Lactiplantibacillus plantarum UALp-05™, and Bifidobacterium animalis subsp. lactis B420™ | 18 weeks | Evaluate the effects of the use of probiotics by themselves to lower Met-S parameters | No significant effects on any of the parameters considered. Data possibly explained by there being responders and non-responders. | Hypothesis of some individuals responding to treatments with probiotics while others being resistant to it could be considered. | High |
| Kassaian N et al. (2018) [44] | Randomized controlled trial | 120 adults with impaired glucose tolerance divided into three groups: treatment with probiotics, treatment with probiotics + prebiotics and placebo | 6 g/day of probiotic containing Lactobacillus acidophilus, Bifidobacterium lactis, Bifidobacterium bifidum, and Bifidobacterium longum (1 × 109 for each), or synbiotic comprising the mentioned probiotics with an inulin-based prebiotic, or placebo | 24 weeks | Determine the effects of 6 months of ingestion of probiotic or synbiotic on metabolic syndrome indices and gut micro- biota composition | Compared with the placebo, synbiotic supplementation resulted in a more significant reduction in FPG (−6.5 ± 1.6 vs. −0.82 ± 1.7 mg/dL, p = 0.01), FIL (−2.6 ± 0.9 vs. −0.8 ± 0.8 µIU/mL, p = 0.028), and HOMA-IR (−0.86 ± 0.3 vs. −0.16 ± 0.25, p = 0.007), and a significant elevation in the QUICKI (+0.01 ± 0.003 vs. +0.003 ± 0.002, p = 0.006). In addition, significant decreases in HbA1C were seen following the supplementation of probiotics and synbiotics compared with the placebo (−0.12 ± 0.06 and −0.14 ± 0.05 vs. +0.07 ± 0.06%, p = 0.005 and 0.008, respectively). HOMA-B was not found to be different between or within the three groups. | Glycemic improvement by probiotics and particularly synbiotic supplements in prediabetic individuals has been supported by the current study. However, further studies are required for optimal recommendations in this important area of patient treatment. | High |
| Yu (2020) [45] | Randomized controlled trial | Obese and insulin resistance patients 25 to 60 y.o | FMT (fecal matter transplant) | 12 weeks | Insulin sensitivity, glycemic and lipidic profile | No statistically significant difference | The results do not favor treatment. | High |
| De Moura (2023) [46] | Randomized controlled trial | Obese (BMI 30–40) women with metabolic syndrome from 18 to 70 y.o | FMT | 12 weeks | Insulin sensitivity, glycemic and lipidic profile | No statistically significant difference | The results do not favor treatment. | High |
| Smits (2018) [47] | Randomized controlled trial | Male metabolic syndrome patients from 21 to 69 y.o | FMT | 2 weeks | Vascular injury, glycemic and lipidic profile | No statistically significant difference | The results favor treatment only for glycemic profile at 6 weeks. | High |
| Kootte (2017) [48] | Randomized controlled trial | Male metabolic syndrome patients from 21 to 69 y.o | FMT | 18 weeks | Insulin sensitivity, glycemic and lipidic profile | Insulin sensitivity at 6 weeks: from 25.8 [19.3–34.7] to 28.8 [21.4–36.9] μmol kg−1 min−1, p < 0.05. HbA1c at 6 weeks: 39.5 [36.0–41.0] to 38.0 [34.0–41.0] mmol/mol, p < 0.01. No statistically significant difference at 6 weeks for other parameters or at 18 weeks for any parameter. | The results favor treatment only for glycemic profile at 6 weeks. | High |
| Vrieze (2012) [49] | Randomized controlled trial | Male metabolic syndrome patients | FMT | 6 weeks | Insulin sensitivity, glycemic and lipidic profile, weight | Median rate of glucose disappearance changed from 26.2 to 45.3 μmol/kg/min; p < 0.05. | The results mildly favor treatment for glycemic parameters. | High |
| Hartstra (2020) [50] | Randomized controlled trial | Metabolic syndrome patients, 50–70 y.o | FMT | 4 weeks | Effect on brain dopamine transporter (DAT) and serotonin transporter (SERT) and insulin sensitivity | Increase in brain DAT. No effect on body weight and insulin sensitivity was demonstrated. | The results do not favor treatment. | High |
| (C) | ||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | |
| Zecheng (2023) [51] | Meta-analysis | 10 randomized controlled trials (RCTs) | Obese and metabolic syndrome patients | Metabolic syndrome parameters, weight-related parameters | Significant reduction in cholesterol levels, blood pressure, and triglycerides; no statistically significant difference in other metabolic syndrome parameters or anthropometric parameters. | The results favor treatment only for some parameters of metabolic syndrome. | High | |
| Pakhmer et al. [52] | Meta-analysis | 11 studies:
| Obese and metabolic syndrome patients | Metabolic syndrome parameters, weight-related parameters | No significant long-term effects on metabolic parameters | The results do not favor treatment. | High | |
| (A) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | End Point | Results | Conclusion | Strength of Evidence | ||
| Que et al., 2021 [53] | Original Research | Patients with type 2 diabetes mellitus (T2DM) and healthy controls | 16S rRNA gene sequencing, microbiota composition analysis | Gut bacterial characteristics of T2DM patients and potential therapeutic applications | Significant differences in gut microbiota composition between T2DM patients and healthy controls. Reduced diversity and altered relative abundances of specific bacterial taxa were observed. | Understanding gut bacterial characteristics in T2DM can inform potential therapeutic applications targeting the gut microbiota. | Medium | ||
| Pellegrini et al., 2017 [54] | Case–control study | 19 patients with T1D compared with 16 healthy controls (CTRLs) subjects and 19 patients with celiac disease (CD) as gut inflammatory disease controls | The inflammatory status and microbiome composition were evaluated in biopsies of the duodenal mucosa | Evaluate the gut inflammatory profile and microbiota in patients with T1D compared with healthy control (CTRL) subjects and patients with celiac disease (CD) as gut inflammatory disease controls | An increased expression of CCL13, CCL19, CCL22, CCR2, COX2, IL4R, CD68, PTX3, TNFα, and VEGFA was observed in patients with T1D compared with CTRL subjects and patients with CD. Immunohistochemical analysis confirmed T1D-specific inflammatory status compared with healthy and CD control tissues, mainly characterized by the increase in the monocyte/macrophage lineage infiltration. The T1D duodenal mucosal microbiome results were different from the other groups, with an increase in Firmicutes and Firmicutes/Bacteroidetes ratio and a reduction in Proteobacteria and Bacteroidetes. The expression of genes specific for T1D inflammation was associated with the abundance of specific bacteria in the duodenum. | This study shows that duodenal mucosa in T1D presents disease-specific abnormalities in the inflammatory profile and microbiota | Medium | ||
| Wu et al., 2017 [55] | Clinical study | Individuals with treatment-naive type 2 diabetes | 16S rRNA gene sequencing, metagenomics, blood biochemistry, glucose tolerance test | Impact of metformin on gut microbiota composition and metabolic markers | Metformin treatment significantly altered gut microbiota composition, increasing the abundance of beneficial bacteria like Akkermansia and reducing pathogenic bacteria. Metformin also improved glucose metabolism and insulin sensitivity. | Metformin’s therapeutic effects in type 2 diabetes may be partly mediated by its impact on gut microbiota. Understanding these mechanisms can help optimize diabetes treatment strategies. | Medium | ||
| Alkanani et al., 2015 [56] | Original Research | 35 subjects with islet autoimmunity living in the U.S. | High-throughput sequencing of bacterial 16S rRNA genes | Correlation of intestinal microbiota alterations with susceptibility to type 1 diabetes (T1D) | Significant differences in gut microbiomes of seropositive subjects compared to autoantibody-free relatives, including different levels of Firmicutes and Bacteroidetes genera. No differences in biodiversity between seropositive and seronegative relatives. | Altered intestinal microbiota may be associated with disease susceptibility. Specific bacterial taxa differ in abundance between individuals with and without autoantibodies, suggesting a potential role in T1D progression. | Medium | ||
| David et al., 2014 [57] | Controlled clinical trial | six male and four female American volunteers between the ages of 21 and 33, BMI from 19 to 32 kg/m2 | Each day, subjects logged their food intake and non-invasively sampled their gut microbiota. DNA was extracted from all fecal samples as previously described, sequenced using 16S rRNA- and ITS-specific primers, and analyzed with the Quantitative Insights Into Microbial Ecology (QIIME) software package and custom Python Scripts. SCFA analysis was performed by gas chromatography, and bile-acid analysis used enzymatic assays and mass spectrometry. | Whether dietary interventions in humans can alter gut microbial communities in a rapid, diet-specific manner | The animal-based diet had a greater impact on the gut microbiota than the plant-based diet: there were 22 clusters whose abundance significantly changed while on the animal-based diet, whereas only 3 clusters showed significant abundance changes while on the plant-based diet; the genus Prevotella, one of the leading sources of inter-individual gut microbiota variation and hypothesized to be sensitive to long-term fiber intake, was reduced in our vegetarian subject during consumption of the animal-based diet. We also observed a significant positive correlation between subjects’ fiber intake over the past year and baseline gut Prevotella levels. | Our findings that the human gut microbiome can rapidly switch between herbivorous and carnivorous functional profiles may reflect past selective pressures during human evolution. The microbiota changes on the animal-based diet could be linked to altered fecal bile acid profiles and the potential for human enteric disease. | High | ||
| Murri et al., 2013 [58] | Original Research | Children with type 1 diabetes mellitus (T1DM) and healthy controls | 16S rRNA sequencing, microbiota composition analysis | Comparison of gut microbiota between children with T1DM and healthy controls | Children with T1DM had a different gut microbiota composition compared to healthy controls, with lower diversity and different relative abundances of specific bacterial taxa. | Gut microbiota dysbiosis is associated with T1DM in children. Modulating gut microbiota could be a potential strategy for managing T1DM. | Medium | ||
| (B) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Bajinka et al., 2023 [59] | Review | Various populations with focus on gut microbiota and diabetes | Various laboratory evaluations related to gut microbiota and metabolic markers | Mechanisms and pathways of type 2 diabetes mellitus (T2DM) | Highlights the role of gut microbiota in the development and management of T2DM; discusses inconsistencies in findings | Emphasizes the need for more consistent and robust study designs to better understand the relationship between gut microbiota and diabetes and to develop effective treatments | Medium–high | ||
| Crudele et al., 2023 [60] | Narrative review | Studies include both animal models (mice) and human trials focusing on obesity, metabolic syndrome, and type 2 diabetes | Various trials involve gut microbiota profiling, glucose metabolism, lipid analysis, SCFA concentration analysis, and inflammatory markers | Evaluating the role of gut microbiota in the pathogenesis and potential therapeutic interventions for diabetes and metabolic diseases | Gut microbiota plays a significant role in obesity and diabetes; reduced butyrate-producing species are linked with insulin resistance. Certain probiotics like Lactobacillus show potential in improving insulin sensitivity and decreasing inflammation. | Dysbiosis in gut microbiota contributes to metabolic endotoxemia and chronic inflammation, which can drive the development of insulin resistance and T2D. Probiotic supplementation shows promise as a potential therapy to improve insulin resistance and inflammation in diabetic patients. | Medium | ||
| Ye et al., 2022 [61] | Review | Various studies on human and animal models | Metagenomics, functional studies, microbiota composition analysis | Role of gut microbiota in the pathogenesis and treatment of diabetes mellitus | Gut microbiota dysbiosis is linked to the development of type 1 and type 2 diabetes mellitus. Dysbiosis may cause gut leakiness and immune responses that damage pancreatic β cells or cause metabolic disorders. | Understanding gut microbiota’s role in diabetes pathogenesis can lead to new therapeutic strategies, including microbiological therapies, to improve diabetes management. | Medium–high | ||
| Mokhtari et al., 2021 [62] | Review | Children and adolescents with type 1 diabetes (T1D) and healthy controls | Metagenomics, 16S rRNA sequencing, functional analyses | Impact of T1D on the composition and functional potential of gut microbiome in children and adolescents | Significant alterations in gut microbiota diversity, taxonomic profiles, and functional potential in children with T1D compared to healthy controls. Dysbiosis is associated with increased intestinal permeability, altered immune responses, and chronic inflammation. | Gut microbiota dysbiosis plays a significant role in the pathogenesis of T1D. Understanding these changes can lead to the development of microbiome-based therapeutic strategies for T1D prevention and treatment. | Medium–high | ||
| Zhu and Goodarzi, 2020 [63] | Review | Various studies on human subjects | Metabolomics, gut microbiome analysis, bioinformatics | Linking gut microbiome metabolites with risk for type 2 diabetes | Metabolites produced by gut microbiota, such as short-chain fatty acids (SCFAs), bile acids, and branched-chain amino acids, are associated with insulin resistance and type 2 diabetes. Altered gut microbiota composition leads to increased production of harmful metabolites and decreased production of beneficial metabolites. | Gut microbiota-derived metabolites play a crucial role in the pathogenesis of type 2 diabetes. Targeting these metabolites through dietary interventions, probiotics, and other therapies may offer new strategies for diabetes prevention and treatment. | Medium–high | ||
| (C) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of Evidence |
| David et al., 2014 [57] | Controlled clinical trial | six male and four female American volunteers between the ages of 21 and 33, BMI from 19 to 32 kg/m2 | Each day, subjects logged their food intake and non-invasively sampled their gut microbiota. DNA was extracted from all fecal samples as previously described, sequenced using 16S rRNA- and ITS-specific primers, and analyzed with the Quantitative Insights Into Microbial Ecology (QIIME) software package and custom Python Scripts. SCFA analysis was performed by gas chromatography, and bile-acid analysis used enzymatic assays and mass spectrometry. | Two diets: a ‘plant-based diet’, which was rich in grains, legumes, fruits and vegetables, and an ‘animal-based diet’, which was composed of meats, eggs and cheeses. | 10 days | Whether dietary interventions in humans can alter gut microbial communities in a rapid, diet-specific manner | The animal-based diet had a greater impact on the gut microbiota than the plant-based diet: there were 22 clusters whose abundance significantly changed while on the animal-based diet, whereas only 3 clusters showed significant abundance changes while on the plant-based diet; the genus Prevotella, one of the leading sources of inter-individual gut microbiota variation and hypothesized to be sensitive to long-term fiber intake, was reduced in our vegetarian subject during consumption of the animal-based diet. We also observed a significant positive correlation between subjects’ fiber intake over the past year and baseline gut Prevotella levels. | Our findings that the human gut microbiome can rapidly switch between herbivorous and carnivorous functional profiles may reflect past selective pressures during human evolution. The microbiota changes on the animal-based diet could be linked to altered fecal bile acid profiles and the potential for human enteric disease. | High |
| (D) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Population Characteristics | Type of Laboratory Evaluation | End Point | Results | Conclusion | Strength of Evidence | |
| Wang et al., 2024 [64] | Meta-analysis | 32 RCTs | 1676 patients with DM2 | HbA1c, insulin, HOMA-IR, FBG, BMI | Glycemic control (HbA1c, insulin, HOMA-IR, FBG, BMI) | Probiotics significantly reduced HbA1c (−0.33), insulin (−0.48), and HOMA-IR (−1.36). No effect on BMI. | Probiotics improve glycemic control (HbA1c, insulin, HOMA-IR) in T2DM, especially at 6–8 and 12–24 weeks. | High | |
| Li et al., 2023 [65] | Meta-analysis | 30 RCTs | 1872 patients with DM2 | HbA1c, insulin, HOMA-IR, FBG, BMI | Glycemic control (HbA1c, insulin, HOMA-IR, FBG, BMI) | Probiotics significantly reduced FBG, HbA1c, insulin, and HOMA-IR. Better results in BMI > 30 at baseline and probiotics with Bifidobacterium, while there were no differences with duration and dose of probiotics. | Probiotics improve glycemic control (HbA1c, insulin, HOMA-IR) in T2DM, especially in patients with obesity and probiotics including both Lactobacillus and Bifidobacterium. | High | |
| Moravejolahkami et al., 2023 [66] | Meta-analysis | 5 RCTs | 356 patients with DM2 | FGB, HbA1c, C-peptide, insulin therapy | Glycemic control (FBG, HbA1c) and doses of insulin therapies | Probiotics significantly reduced FBG, while there were no effects on HbA1c, C-peptide, or insulin doses. | Probiotics improve glycemic control in T2DM | High | |
| Ayesha et al., 2023 [67] | Meta-analysis | 22 RCTs | 2218 patients with DM2 | Fasting blood glucose (FBG), HbA1c, HOMA-IR | Glycemic control (FBG, HbA1c, HOMA-IR) | Probiotics significantly reduced HbA1c, FBG, and HOMA-IR compared to placebo. | Probiotics improve glycemic control in T2DM, particularly in long-term interventions (12+ weeks). | High | |
| Zhang et al., 2022 [68] | Meta-analysis | 33 CTRs | 1927 patients with DM2 | HbA1c, FBG, fasting insulin, HOMA-IR | Glycemic control (HbA1c, FBG, fasting insulin, HOMA-IR) | Probiotics significantly reduced HbA1c (−0.19%), FBG (−1.00 mmol/L), and HOMA-IR (−1.00), but not fasting insulin (−5.73 pmol/L). | Probiotics improved glycemic control in T2DM, but reductions in HbA1c were not clinically significant. | High | |
| Naseri et al., 2022 [69] | Meta-analysis | 46 RCTs | 3067 patients with DM2 and prediabetes | FPG, fasting insulin, HbA1c, HOMA-IR, QUICKI | Glycemic control (FPG, HbA1c, fasting insulin, HOMA-IR) | Probiotics and synbiotics significantly reduced FPG (−11.18 mg/dL), HbA1c (−0.35%), insulin (−1.23 µIU/mL), and HOMA-IR (−0.87). No change in OGTT. | Probiotics and synbiotics improve glycemic control in individuals with prediabetes and T2DM. | High | |
| Kocsis et al., 2020 [70] | Meta-analysis | 32 RCTs | 1676 patients with DM2 | HbA1c, FPG, fasting insulin, CRP, blood pressure | Glycemic control, metabolic parameters (cholesterol, triglycerides, BP) | Probiotics significantly reduced HbA1c (−0.33%), FPG (−16.52 mg/dL), fasting insulin (−1.40 µIU/mL), CRP (−0.43 mg/dL), and blood pressure. HDL increased, but no effect on BMI. | Probiotics improve glycemic control and metabolic parameters in T2DM patients, especially HbA1c and FPG. | High | |
| (A) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention (If Applicable) | Period of Intervention | End Point | Results | Conclusion | Strength of Evidence |
| Larsen et al., 2010 [71] | Observational Study | 36 male adults with a broad range of age and body mass indices (BMIs), among which 18 subjects were diagnosed with DM2 | The fecal bacterial composition was investigated by real-time quantitative PCR (qPCR) and in a subgroup of subjects (N = 20) by tag-encoded amplicon pyrosequencing of the V4 region of the 16S rRNA gene. | - | - | To assess the differences between the composition of the intestinal microbiota in humans with DM2 and non-diabetic persons as control | The proportions of phylum Firmicutes and class Clostridia were significantly reduced in the diabetic group compared to the control group. The ratios of Bacteroidetes to Firmicutes as well as the ratios of Bacteroides–Prevotella group to C. coccoides–E. rectale group correlated positively and significantly with plasma glucose concentration but not with BMI. Similarly, class Betaproteobacteria was highly enriched in diabetic compared to non-diabetic persons and positively correlated with plasma glucose. | DM2 in humans is associated with compositional changes in intestinal microbiota. The level of glucose tolerance should be considered when linking microbiota with metabolic diseases such as obesity and developing strategies to control metabolic diseases by modifying the gut microbiota. | Low |
| Qin J. et al., 2012 [2] | Observational Study | 123 patients with metabolic syndrome (MetS) and 304 controls | DNA was extracted from a total of 1770 stool samples using the MagPure Stool DNA KF kit. DNA library construction based on DNA nanospheres (DNB) and shotgun metagenomic sequencing based on combined probe anchoring synthesis (CPAS) were performed on all samples | - | - | To determine if the gut microbiome plays a role in MetS development and progression | MetS patients possessed significantly lower gut microbiome diversity; 28 bacterial species were negatively correlated with waist circumstance, with Alistipes onderdonkii showing the strongest correlation, followed by Bacteroides thetaiotaomicron, Clostridium asparagiforme, C. citroniae, C. scindens, and Roseburia intestinalis. These species were also enriched in controls relative to MetS patients. Pathways involved in the biosynthesis of carbohydrates, fatty acids, and lipids were enriched in the MetS group, indicating that microbial functions related to fermentation may play a role in MetS. Microbiome changes in MetS patients may aggravate inflammation and contribute to MetS diseases by inhibiting the production of short-chain fatty acids (SCFAs). | Results indicate potential utility of beneficial gut microbiota as a potential therapeutic to alleviate MetS. | Low |
| Li Y. et al., 2015 [72] | Observational Study | 203 and 308 men and women from the NHS II and the HPFS, excluding those with diabetes, cardiovascular disease, cancer, or implausible dietary data at baseline | Dietary phosphatidylcholine was estimated by a valid food frequency questionnaire, with approximately 130 food items administered every 2 or 4 years combined with the phosphatidylcholine contents from the U.S. Department of Agriculture database and from values published by Zeisel et al. | - | - | To study the association between dietary phosphatidylcholine and risk of type 2 diabetes (T2D). | Study associated dietary intakes of phosphatidylcholine with incident T2D risk in multiple prospective cohorts with a large sample size, high rates of long-term follow-up, and detailed and repeated assessments of diet and lifestyle | The findings lend support to dietary intervention strategies targeting dietary sources of gut microbiota metabolites in prevention of T2D. | Low |
| Tang WH et al., 2017 [73] | Observational Study | 1216 stable patients with T2DM who underwent elective diagnostic coronary angiography | Quantification of fasting plasma TMAO concentrations was performed utilizing stable isotope dilution liquid chromatography with online tandem mass spectrometry (LC/MS/MS) on an AB Sciex API 5500 triple quadrupole mass spectrometer (SCIEX, Toronto, Canada). | - | - | To study the relation between fasting TMAO and two of its nutrient precursors, choline and betaine, and T2DM and glycemic control | TMAO and choline concentrations were higher in individuals with T2DM vs. healthy controls. Within T2DM patients, higher plasma TMAO was associated with a significant 3.0-fold increased 3-year major adverse cardiac event risk and a 3.6-fold increased 5-year mortality risk. Increased TMAO concentrations remained predictive of both major adverse cardiac events and mortality risks in T2DM patients. | Fasting plasma concentrations of the proatherogenic gut microbe-generated metabolite TMAO are higher in diabetic patients and portend higher major adverse cardiac events and mortality risks independent of traditional risk factors, renal function, and relationship to glycemic control. | Low |
| Roy s. et al., 2020 [74] | Observational Study | Three-hundred diabetes-free men and women (77%) aged 20–55 years (mean = 34 ± 10) were enrolled at baseline and re-examined at 2 years. | Plasma TMAO was measured using Ultra-. Chromatography–Mass Spectrometry. After an overnight fast, FPG was measured longitudinally; HbA1C and insulin were measured only at baseline. Insulin resistance was defined using HOMA-IR. | - | - | To investigate the role of TMAO as an early biomarker of longitudinal glucose increase or prevalent impaired glucose regulation | Multivariable relative risk regressions modeled prevalent prediabetes across TMAO tertiles. Mean values of 2-year longitudinal FPG ± SE across tertiles of TMAO were 86.6 ± 0.9, 86.7 ± 0.9, and 86.4 ± 0.9 (p = 0.98). Trends were null for FPG, HbA1c, and HOMA-IR, cross-sectionally. The prevalence ratios of prediabetes among participants in 2nd and 3rd TMAO tertiles (vs. the 1st) were 1.94 [95%CI 1.09–3.48] and 1.41 [95%CI: 0.76–2.61]. | TMAO levels are associated with increased prevalence of prediabetes in a nonlinear fashion but not with insulin resistance or longitudinal FPG change. | Low |
| Allin KH et. al., 2018 [75] | Case–control Study | 134 Danish adults with prediabetes, overweight, insulin resistance, dyslipidemia and low-grade inflammation and 134 age- and sex-matched individuals with normal glucose regulation | Biochemical analyses were performed on fasting blood samples. Fecal microbiota composition was profiled by sequencing the V4 region of the 16S rRNA gene on an Illumina MiSeq instrument. | - | - | To study if specific gut microbiota profiles are associated with prediabetes and a range of clinical biomarkers of poor metabolic health | Here, 5 bacterial genera and 36 operational taxonomic units (OTUs) were differentially abundant between individuals with prediabetes and those with normal glucose regulation. At the genus level, the abundance of Clostridium was decreased, whereas the abundances of Dorea, [Ruminococcus], Sutterella and Streptococcus were increased. The two OTUs that differed the most were a member of the order Clostridiales (OTU 146564) and Akkermansia muciniphila, which both displayed lower abundance among individuals with prediabetes. Fecal transfer from donors with prediabetes or screen-detected, drug-naive type 2 diabetes to germfree Swiss Webster or conventional C57BL/6 J mice did not induce impaired glucose regulation in recipient mice. | Data show that individuals with prediabetes have aberrant intestinal microbiota characterized by a decreased abundance of the genus Clostridium and the mucin-degrading bacterium A. muciniphila. Our findings are comparable to observations in overt chronic diseases characterized by low-grade inflammation | Low |
| Zhong H. et al., 2019 [76] | Observational Study | Fecal samples from treatment-naïve type 2 diabetic (TN-T2D, n = 77), prediabetic (Pre-DM, n = 80), and normal glucose-tolerant (NGT, n = 97) individuals | A combination of in-depth metagenomics and metaproteomics analyses of fecal samples | - | - | To study differences in the gut microbiome of T2D and prediabetic individuals compared to healthy individuals, without confounding factors such as antidiabetic medication, and identify gut microbial changes in disease development | The study observed distinct differences characterizing the gut microbiota of these three groups and validated several key features in an independent TN-T2D cohort. It also demonstrated that the content of several human antimicrobial peptides and pancreatic enzymes differed in fecal samples between three groups. | These findings suggest a complex, disease stage-dependent interplay between the gut microbiota and the host and point to the value of metaproteomics to gain further insight into interplays between the gut microbiota and the host. | Low |
| (B) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Letchumanan G. et al., 2022 [77] | Systematic Review of Observational Studies | 18 observational studies | 5489 subjects | To summarize the existing evidence related to microbiota composition and diversity in individuals with prediabetes (preDM) and individuals newly diagnosed with T2DM (newDM) in comparison to individuals with normal glucose tolerance (nonDM) | Low gut microbial diversity was generally observed in preDM and newDM when compared to nonDM. Differences in gut microbiota composition between the disease groups and nonDM were inconsistent across the included studies. Four out of the eighteen studies found increased abundance of phylum Firmicutes along with decreased abundance of Bacteroidetes in newDM. At the genus/species levels, decreased abundance of Faecalibacterium prausnitzii, Roseburia, Dialister, Flavonifractor, Alistipes, Haemophilus and Akkermansia muciniphila and increased abundance of Lactobacillus, Streptococcus, Escherichia, Veillonella and Collinsella were observed in the disease groups in at least two studies. Lactobacillus was also found to positively correlate with fasting plasma glucose (FPG), HbA1c and/or homeostatic assessment of insulin resistance (HOMA-IR) in four studies. | There is a need for further investigations on the species/strain-specific role of endogenously present Lactobacillus in glucose regulation mechanism and T2DM disease progression. Differences in dietary intake caused significant variation in specific bacterial abundances. More studies are needed to establish more consistent associations between clinical biomarkers or dietary intake and specific gut bacterial composition in prediabetes and early T2DM. | High | ||
| Gurung M. et al., 2019 [78] | Narrative review | 42 studies (preclinical studies or clinical trials) using treatments with probiotics | - | To summarize the potential role of different bacterial taxa affecting diabetes | The genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia were negatively associated with T2D, while the genera of Ruminococcus, Fusobacterium, and Blautia were positively associated with T2D. | Despite multiple studies supporting the importance of gut microbiota in the pathophysiology of T2D, the field is in an early stage. Some microbial taxa and related molecular mechanisms may be involved in glucose metabolism related to T2D. However, the heterogeneity of T2D and redundancy of gut microbiota do not promise simple interpretations (e.g., low diversity) and easy solutions (such as fecal transplant from non-diabetic/non-obese donor). | Medium | ||
| Aw W. et al., 2018 [79] | Narrative review | - | - | To review the labyrinth encompassing the gut microbiota and gut microbiota-derived metabolites in type 1 diabetes and type 2 diabetes pathogenesis | The studies included in the present review emphasize that diabetes pathogenesis could be a result of specific pathogens, but metabolites produced by gut microbiota, such as bile acids, also play an important part. However so, the exact impacts of gut microbes and their metabolites on the incidence and pathogenesis of diabetes have yet to be clearly elucidated. | As diabetes is multifactorial and can progress to other related metabolic diseases, it is of utmost importance that the delicate interrelationships between gut microbiota and host metabolism are well understood in order to suggest appropriate lifestyle and nutritional interventions by engineering an optimal gut environment towards the prevention and maintenance/remission of diabetes. | Medium | ||
| (C) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of Evidence |
| Simon MC. et al., 2015 [80] | Prospective, double-blind, randomized trial | 21 glucose-tolerant humans (11 lean: age 49 ± 7 years, BMI 23.6 ± 1.7 kg/m2; 10 obese: age 51 ± 7 years, BMI 35.5 ± 4.9 kg/m2) | Oral glucose tolerance and isoglycemic glucose infusion tests were used to assess incretin effect and GLP-1 and GLP-2 secretion, and euglycemic–hyperinsulinemic clamps with [6,6-(2)H2] glucose were used to measure peripheral insulin sensitivity and endogenous glucose production. Muscle and hepatic lipid contents were assessed by (1)H-magnetic resonance spectroscopy, and immune status, cytokines, and endotoxin were measured with specific assays. | Participants ingested 10(10) b.i.d. L. reuteri SD5865 or placebo over 4 weeks. | 4 weeks | The study hypothesized that daily intake of L. reuteri increases insulin sensitivity by changing cytokine release and insulin secretion via modulation of the release of GLP-1 and -2. | In glucose-tolerant volunteers, daily administration of L. reuteri SD5865 increased glucose-stimulated GLP-1 and GLP-2 release by 76% (p < 0.01) and 43% (p < 0.01), respectively, compared with placebo, along with 49% higher insulin (p < 0.05) and 55% higher C-peptide secretion (p < 0.05). However, the intervention did not alter peripheral and hepatic insulin sensitivity, body mass, ectopic fat content, or circulating cytokines. | Enrichment of gut microbiota with L. reuteri increases insulin secretion, possibly due to augmented incretin release, but does not directly affect insulin sensitivity or body fat distribution. This suggests that oral ingestion of one specific strain may serve as a novel therapeutic approach to improve glucose-dependent insulin release. | High |
| M. Hariri et al., 2015 T2D [81] | Randomized, double-blind, placebo-controlled trial | Forty patients with type 2 diabetes mellitus aged 35–68 years were assigned to two groups. | Genomic DNA was extracted from EDTA anticoagulated whole blood and was quantified by a Nano Drop spectrophotometer. Than, the promoter methylation analysis was performed using methylation-specific digestion enzyme and real-time polymerase chain reaction (PCR). | Pts in the intervention group consumed 200 mL/day of probiotic soy milk containing L. plantarum A7, while those in the control group consumed 200 mL/d of conventional soy milk for 8 weeks. | 8 weeks | To discover the effects of probiotic soy milk and soy milk on MLH1 and MSH2 promoter methylation, and oxidative stress among type 2 diabetic patients | Probiotic soy milk significantly decreased promoter methylation in proximal and distal MLH1 promoter region (p < 0.01 and p < 0.0001, respectively) compared with the baseline values, while plasma concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG) decreased significantly compared with soy milk (p < 0.05). In addition, a significant increase in superoxide dismutase (SOD) activity was observed in probiotic soy milk group compared with baseline value (p < 0.01). There were no significant changes from baseline in the promoter methylation of MSH2 within either group (p > 0.05). | The consumption of probiotic soy milk improved antioxidant status in type 2 diabetic patients and may decrease promoter methylation among these patients, indicating that probiotic soy milk is a promising agent for diabetes management. | High |
| M. Sanchez et al., 2014 [82] | Randomized, double-blind, placebo-controlled trial | 153 obese men and women | Anthropometric parameters (body weight, height and waist circumference) and body composition measured by dual-energy X-ray absorptiometry. Biochemical analyses: plasma concentrations of glucose, insulin, leptin, lipids, lipoproteins and inflammatory indicators. Sequence-based microbiota analysis from fecal samples using PCR. | Each subject consumed 2 capsules/d of either a placebo or an LPR formulation (1.6 × 108 colony-forming units of LPR/capsule with oligofructose and inulin). Each group was submitted to moderate energy restriction for the first 12 weeks followed by 12 weeks of weight maintenance. | 24 weeks. | To investigate the impact of a Lactobacillus rhamnosus CGMCC1.3724 (LPR) supplementation on weight loss and maintenance in obese men and women over 24 weeks | After the first 12 weeks and after 24 weeks, mean weight loss was not significantly different between the LPR and placebo groups when all the subjects were considered. However, a significant treatment × sex interaction was observed. The mean weight loss in women in the LPR group was significantly higher than that in women in the placebo group (p = 0.02) after the first 12 weeks, whereas it was similar in men in the two groups (p = 0.53). Women in the LPR group continued to lose body weight and fat mass during the weight maintenance period, whereas opposite changes were observed in the placebo group. Changes in body weight and fat mass during the weight maintenance period were similar in men in both the groups. LPR-induced weight loss in women was associated not only with significant reductions in fat mass and circulating leptin concentrations but also with the relative abundance of bacteria of the Lachnospiraceae family in feces. | The study shows that the Lactobacillus rhamnosus CGMCC1.3724 formulation helps obese women to achieve sustainable weight loss. | High |
| C.J. Hulston et al., 2015 [83] | Randomized controlled trial | Seventeen healthy subjects were randomized to either a probiotic (n = 8) or a control (n = 9) group. | Anthropometric parameters: body weight, height and BMI. Standardized forms and digital kitchen scales to record weighed food intake on 3 d each week during the pre-experimental period (days 1–21). Insulin sensitivity was determined from plasma glucose and serum insulin concentrations during an OGTT. | The probiotic group consumed an LcS-fermented milk drink twice daily for 4 weeks; the control group received no supplementation. Subjects maintained their normal diet for the first 3 weeks of the study, after which they consumed a high-fat (65% of energy), high-energy (50% increase in energy intake) diet for 7 d. | 4 weeks | To determine whether probiotic supplementation (Lactobacillus casei Shirota (LcS)) prevents diet-induced insulin resistance in human subjects | Body mass increased by 0.6 (SE 0.2) kg in the control group (p < 0.05) and by 0.3 (SE 0.2) kg in the probiotic group (p > 0.05). Fasting plasma glucose concentrations increased following 7 d of overeating (control group: 5.3 (SE 0.1) vs. 5.6 (SE 0.2) mmol/L before and after overfeeding, respectively, p < 0.05), whereas fasting serum insulin concentrations were maintained in both groups. Glucose AUC values increased by 10% (from 817 (SE 45) to 899 (SE 39) mmol/L per 120 min, (p < 0.05) and whole-body insulin sensitivity decreased by 27% (from 5.3 (SE 1.4) to 3.9 (SE 0.9), (p < 0.05) in the control group, whereas normal insulin sensitivity was maintained in the probiotic group (4.4 (SE 0.8) and 4.5 (SE 0.9) before and after overeating, respectively (p > 0.05)). | These results suggest that probiotic supplementation may be useful in the prevention of diet-induced metabolic diseases such as type 2 diabetes. | High |
| Y. Kadooka et al., 2010 [84] | Multicenter, double-blind, randomized, placebo-controlled intervention trial | 87 overweight/obese subjects | Measurement of body weight and other body parameters as well as blood pressure, pulse rate, and common blood and urinary tests were performed at each time point in weeks. Abdominal computed tomography scans for the measurement of abdominal fat area were carried out at W0 and W12. Each subject made a daily record of taking the test FM, habitual diet and exercise. A detailed dietary record was also made and analyzed to determine the intake of energy, protein, carbohydrate, fat and calcium. | Subjects were randomly assigned to receive either fermented milk (FM) containing LG2055 (active FM; n = 43) or FM without LG2055 (control FM; n = 44), and were asked to consume 200 g/day of FM for 12 weeks. | 12 weeks | To evaluate the effects of the probiotic Lactobacillus gasseri SBT2055 (LG2055) on abdominal adiposity, body weight and other body measures in adults with obese tendencies | In the active FM group, abdominal visceral and subcutaneous fat areas significantly (p < 0.01) decreased from baseline by an average of 4.6% (mean (confidence interval)) (−5.8 (−10.0, −1.7) cm2) and 3.3% (−7.4 (−11.6, −3.1) cm2), respectively. Body weight and other measures also decreased significantly (p < 0.001) as follows: body weight, 1.4% (−1.1 (−1.5, −0.7) kg); BMI, 1.5% (−0.4 (−0.5, −0.2) kg/m2); waist, 1.8% (−1.7 (−2.1, −1.4) cm); hip, 1.5% (−1.5 (−1.8, −1.1) cm). In the control group, by contrast, none of these parameters decreased significantly. High-molecular weight adiponectin in serum increased significantly (p < 0.01) in the active and control groups by 12.7% (0.17 (0.07, 0.26) microg/mL) and 13.6% (0.23 (0.07, 0.38) microg/mL), respectively. | The probiotic LG2055 showed lowering effects on abdominal adiposity, body weight and other measures, suggesting its beneficial influence on metabolic disorders. | High |
| R. Mobini et al., 2016 [85] | Randomized, double-blind, placebo-controlled trial | 46 people with type 2 diabetes | Biochemical analyses: glycated hemoglobin (HbA1c), serum bile acids and insulin sensitivity (assessed by glucose clamp). Other variables evaluated: liver fat content, body composition, body fat distribution (using MRI and MRS) and fecal microbiota composition (by 16S rRNA-based Illumina MiSeq sequencing). Questionnaires and diaries were used to determine total caloric intake. | 46 people with type 2 diabetes to placebo or a low (108 CFU/d) or high dose (1010 CFU/d) of L. reuteri DSM 17938 for 12 weeks | 12 weeks. | To investigate the metabolic effects of 12-week oral supplementation with Lactobacillus reuteri DSM 17938 in patients with type 2 diabetes on insulin therapy | Supplementation with L. reuteri DSM 17938 for 12 weeks did not affect HbA1c, liver steatosis, adiposity or microbiota composition. Participants who received the highest dose of L. reuteri exhibited increases in insulin sensitivity index (ISI) and serum levels of the secondary bile acid deoxycholic acid (DCA) compared with baseline, but these differences were not significant in the between-group analyses. Post hoc analysis showed that participants who responded with increased ISI after L. reuteri supplementation had higher microbial diversity at baseline and increased serum levels of DCA after supplementation. In addition, increases in DCA levels correlated with improvement in insulin sensitivity in the probiotic recipients. | Intake of L. reuteri DSM 17938 for 12 weeks did not affect HbA1c in people with type 2 diabetes on insulin therapy; however, L. reuteri improved insulin sensitivity in a subset of participants and we propose that high diversity of the gut microbiota at baseline may be important. | High |
| A.S. Andreasen et al., 2010 [86] | Randomized, double-blind, placebo-controlled trial | Forty-five males with type 2 diabetes, impaired or normal glucose tolerance | OGTT. Plasma levels of TNF-α, IL-6 and IL-1 receptor antagonist (IL-1ra), C-reactive protein. Detection of Lactobacillus acidophilus in stool samples. | Treatment course with either L. acidophilus NCFM, one capsule/d (1 g; about 1010 colony-forming units), or placebo | 4 weeks | To evaluate the effects of oral supplementation with the probiotic bacterium Lactobacillus acidophilus NCFM on insulin sensitivity and the inflammatory response | Insulin sensitivity was preserved among volunteers in the L. acidophilus NCFM group, whereas it decreased in the placebo group. Both baseline inflammatory markers and the systemic inflammatory response were, however, unaffected by the intervention | Intake of L. acidophilus NCFM for 4 weeks preserved insulin sensitivity compared with placebo, but did not affect the systemic inflammatory response. | High |
| L.J. Bernini et al., 2016 [42] | Randomized controlled trial | Fifty-one patients with MetS were selected and divided into a control group (n = 25) and a probiotic group (n = 26). | Anthropometric measurements: waist circumference (WC), body weight, and height and BMI. Biochemical analyses: glucose, total cholesterol, HDL-C, LDL-C), TGs. Insulin, TNF-α, IL-6. | The probiotic group consumed 80 mL of fermented milk with probiotics (with 2.72 × 1010 colony-forming units of B. lactis HN019) daily over the course of 45 d. | 45 days | To evaluate the effect of consumption of milk containing the probiotic B. lactis HN019 on the classical parameters of MetS and other related cardiovascular risk factors | Daily ingestion of 80 mL fermented milk with 2.72 × 1010 colony-forming units of B. lactis HN019 showed significant reduction in body mass index (p = 0.017), total cholesterol (p = 0.009), and low-density lipoprotein (p = 0.008) compared with baseline and control group values. Furthermore, a significant decrease in tumor necrosis factor-α (p = 0.033) and interleukin-6 (p = 0.044) pro-inflammatory cytokines was observed. | These data showed potential effects of B. lactis HN019 in reducing obesity, blood lipids, and some inflammatory markers, which may reduce cardiovascular risk in patients with MetS. | High |
| (D) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subjects | End Point | Results | Conclusion | Strength of Evidence | ||
| Barengolts E. et al., 2016 [87] | Review of randomized controlled trials | Randomized controlled trials | - | To review the data from randomized controlled trials (RCTs) for the roles of microbiota and pre-, pro-, and synbiotics in metabolic conditions (obesity, prediabetes, and diabetes mellitus type 2 [DM2]) | Microbiota could increase harvesting of energy from food and cause subclinical inflammation seen in metabolic disorders. Diet-related interventions including prebiotics, probiotics, and synbiotics may benefit metabolic conditions. Results of RCTs of prebiotics suggested a neutral effect on body weight, decreased fasting and postprandial glucose, and improved insulin sensitivity and lipid profile. Some inflammation markers were reduced, sometimes substantially (20–30%). RCTs for probiotics demonstrated significant but small effects on body weight (<3%) and metabolic parameters. The effect was seen mostly with fermented milk or yogurt compared to capsule form, consumption for at least 8 weeks, and use of multiple rather than a single bacterial strain. Changes in microbiota were seen at times with both pre- and probiotics. Pickled and fermented foods, particularly vegetables and beans, could serve as a dietary source of pre-, pro-, and synbiotics. These foods showed possible benefits for morbidity and mortality in prospective cohort studies. | Pre-, pro-, and synbiotics could prove useful, but further research is needed to clarify their clinical relevance for the prevention and management of metabolic disease. | High | ||
| Hampe, C.S. et al., 2017 [88] | Narrative review | - | - | To discuss the mechanisms employed by specific probiotic strains of Lactobacillus and Bifidobacterium genuses, which showed efficacy in the treatment of obesity and T2D | Some probiotic strains employ recurring beneficial effects, including the production of antimicrobial lactic acid, while other strains display highly unique features, such as hydrolysis of tannins. | A major obstacle in the evaluation of probiotic strains lays in the great number of strains, differences in detection methodology and measured outcome parameters. The understanding of further research should be directed towards the development of standardized evaluation methods to facilitate the comparison of different studies. | Medium | ||
| Wang X. et al., 2021 [89]. | Systematic review of randomized controlled trials | 8 RCTs | 391 subjects | To review the data from randomized controlled trials (RCTs) and identify evidence for microbiota’s role and the use of probiotics, prebiotics, or synbiotics in prediabetes | The gut microbiota influences host metabolic disorders via the modulation of metabolites, including short-chain fatty acids (SCFAs), the endotoxin lipopolysaccharide (LPS), bile acids (BAs) and trimethylamine N-oxide (TMAO), as well as mediating the interaction between the gastrointestinal system and other organs. Due to the limited sources of studies, there are inconsistent outcomes between included studies. Probiotics can decrease glycated hemoglobin (HbA1c) and have the potential to improve post-load glucose levels. The supplementation of probiotics can suppress the rise in blood cholesterol, but the improvement cannot be verified. Prebiotics are failed to show an evident improvement in glycemic control, but their use caused changes in the composition of gut microbiota. A combination of probiotics and prebiotics in synbiotic supplementation is more effective than probiotics alone in glycemic control. | Using probiotics, prebiotics or synbiotics for the treatment of prediabetes, the benefits of modulating the abundance of gut microbiota were partially demonstrated. However, there is insufficient evidence to show significant benefits on glucose metabolism, lipid metabolism and body composition. | High | ||
| Li Y. et al., 2022 [90] | Meta-analysis and systematic review | 7 randomized controlled trials | 460 patients | To examine the effects of probiotics on eight factors in the prediabetic population by meta-analysis, namely, fasting blood glucose (FBG), HbA1c, homeostatic model assessment of insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), and the mechanisms of action are summarized from the existing studies. | Probiotics were able to significantly decrease the levels of HbA1c (WMD, −0.07; 95% CI −0.11, −0.03; p = 0.001), QUICKI (WMD, 0.01; 95% CI 0.00, 0.02; p = 0.04), TC (SMD, −0.28; 95% CI −0.53, −0.22; p = 0.03), TG (SMD, −0.26; 95% CI −0.52, −0.01; p = 0.04), and LDL-C (WMD, −8.94; 95% CI −14.91, −2.97; p = 0.003) compared to levels in the placebo group. The effects on FBG (WMD, −0.53; 95% CI −2.31, 1.25; p = 0.56), HOMA-IR (WMD, −0.21; 95% CI −0.45, 0.04; p = 0.10), and HDL-C (WMD, 2.05; 95% CI −0.28, 4.38; p = 0.08) were not different from those of the placebo group. | Probiotics may fulfill an important role in the regulation of HbA1c, QUICKI, TC, TG and LDL-C in patients with prediabetes. In addition, based on existing studies, we concluded that probiotics may regulate blood glucose homeostasis in a variety of ways. | High | ||
| Zeighamy Alamdary S. et al., 2022 [91] | Systematic review of randomized controlled trials | 15 randomized controlled trials | 1116 subjects | To compile the results of clinical trials investigating the effects of pro-/pre-/synbiotics on prediabetic subjects from 2010 to 2020 | Positive and significant effects of probiotics in the reduction in hyperglycemia, insulin concentration levels, lipid profile, and BMI (body mass index). Administration of probiotics may provide beneficial and healthful effects in the clinical management of patients with prediabetes and metabolic syndrome. | Different probiotic compositions have shown beneficial and noticeable effects on glucose homeostasis, lipid profiles, BMI, and inflammatory markers in subjects with prediabetes and metabolic syndrome and healthy individuals and could be advantageous in recomposing the gut microbiota back into the normal state during the prediabetic state. | High | ||
| Zhang Q. et al., 2015 [92] | Meta-analysis of randomized controlled trials | 7 randomized controlled trials | 438 subjects | To investigate the effects of probiotics on glucose metabolism in patients with type 2 diabetes mellitus | Probiotic consumption significantly changed fasting plasma glucose (FPG) by −15.92 mg/dL and glycosylated hemoglobin (HbA1c) by −0.54% compared with control groups. Meta-analysis of trials with multiple species of probiotics found a significant reduction in FPG. The duration of intervention for ≥8 weeks resulted in a significant reduction in FPG. Subgroup analysis of trials with species of probiotics did not result in a significant meta-analysis effect. Furthermore, the duration of intervention < 8 weeks did not result in a significant reduction in FPG. The results also showed that probiotic therapy significantly decreased homeostasis model assessment of insulin resistance (HOMA-IR) and insulin concentration. | This meta-analysis suggests that consuming probiotics may improve glucose metabolism by a modest degree, with a potentially greater effect when the duration of intervention is ≥8 weeks, or multiple species of probiotics are consumed. | High | ||
| (A) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention (If Applicable) | Period of Intervention | End Point | Results | Conclusion | Strength of Evidence |
| Alemán JO et al., 2018 [93] | Interventional study | Obese postmenopausal women | VLCD, 16S rRNA sequencing, metabolomics, transcriptomics | Diet with 800 kcal/day (time constrained by the goal of losing 10% of body weight) | 46.2 ± 15.3 days | Weight loss, microbiota composition, metabolic changes | VLCD-induced weight loss led to changes in gut microbiota composition and associated metabolic benefits | VLCD dietary intervention in obese women changed the composition of several fecal microbial populations while preserving the core fecal microbiome. Changes in individual microbial taxa and their functions correlated with variations in plasma metabolomes, fecal bile acid composition, and adipose tissue transcriptome. | Medium |
| (B) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Intervention | Duration | End Point | Type of Laboratory Evaluation | Results | Conclusion | Strength of Evidence |
| Larsen N. et al., 2013 [94] | Randomized control trials | Obese adolescents, 50 subjects, ages 12–15 | Intake of Lactobacillus salivarius Ls-33 or placebo | 12 weeks | Impact on fecal microbiota | Real-time quantitative PCR, gas chromatography for short-chain fatty acids | Significant increase in ratios of Bacteroidetes, Prevotella, and Porphyromonas, a group to Firmicutes. No significant change in cell numbers of fecal bacteria and short-chain fatty acids. | Lactobacillus salivarius Ls-33 might modify fecal microbiota in a way not related to metabolic syndrome. | High |
| Sharafedtinov KK et al., 2013 [95] | Randomized double-blind placebo-controlled pilot study | Obese hypertensive patients, 40 subjects, ages 30–69 | Hypocaloric diet supplemented with probiotic cheese (Lactobacillus casei group) or control cheese | 3 weeks | Body mass index (BMI) and blood pressure (BP) | Molecular methods, gas chromatography for polyamines, standard lab methods for blood and urine analysis | Significant reduction in BMI and BP in the probiotic cheese group. Higher intestinal Lactobacilli associated with higher BMI and urinary putrescine content. | Hypocaloric diet supplemented with probiotic cheese helps reduce BMI and BP values, recognized symptoms of metabolic syndrome. | High |
| Parnell J.A. et al., 2009 [96] | Randomized, double-blind, placebo-controlled trial | 48 overweight and obese adults, BMI > 25, ages 20–70 | 21 g of oligofructose per day vs. placebo for 12 weeks | 12 weeks | Body weight, satiety hormone concentrations, glucose regulation | Dual-energy X-ray absorptiometry, meal tolerance tests, plasma analysis for glucose, insulin, ghrelin, GLP-1, PYY | Reduction in body weight by 1.03 ± 0.43 kg in oligofructose group, increase of 0.45 ± 0.31 kg in control group. Lower ghrelin and higher PYY levels. Improved glucose regulation. | Oligofructose supplementation promotes weight loss and improves glucose regulation independent of other lifestyle changes. | High |
| Sanchez M. et al., 2014 [82] | Randomized, double-blind, placebo-controlled trial | 125 obese men and women, ages 18–55, BMI ~29–41 kg/m² | Two capsules per day of Lactobacillus casei CGMC1.3724 or placebo, combined with reduced energy consumption | 24 weeks | Body weight and plasma markers | Dual-energy X-ray absorptiometry, biochemical assays for plasma markers, 16S ribosomal RNA gene sequencing for microbiota | Significant weight loss in women taking LPR compared to placebo, but no significant difference in men. Women continued to lose weight during maintenance phase. Reductions in fat mass and leptin concentration in women. | Lactobacillus casei CGMC1.3724 formulation supports sustainable weight loss in obese women. | High |
| Safavi M et al., 2013 [97] | Randomized triple-masked controlled trial | 70 overweight and obese children and adolescents, ages 6–18, BMI ≥ 85th percentile | Synbiotic supplementation (probiotics and prebiotics) or placebo for 8 weeks | 8 weeks | BMI Z-score, waist circumference, waist-to-hip ratio, serum triglycerides, total cholesterol, LDL cholesterol | Biochemical assays for serum markers, stool examination for bacterial count | Significant reduction in BMI Z-score, waist circumference, waist-to-hip ratio, serum triglycerides, total cholesterol, and LDL cholesterol in synbiotic group. | Synbiotic supplementation helps control excess weight and improve cardiometabolic risk factors in children and adolescents. | High |
| Jung SP 2013 [98]. | Randomized, double-blind, placebo-controlled clinical trial | 62 overweight and obese adults, ages 19–60, BMI ≥ 23 kg/m², fasting blood sugar ≥ 100 mg/dL | Daily supplementation of 1010 CFU Lactobacillus gasseri BNR17 or placebo for 12 weeks | 12 weeks | Body weight, body fat, waist and hip circumference, biochemical parameters | Bioelectrical impedance analysis, computed tomography, blood test for metabolic markers | Slight reduction in body weight in BNR17 group. Significant decrease in waist and hip circumference compared to placebo. No significant changes in visceral adipose tissue. | Lactobacillus gasseri BNR17 may reduce weight and waist and hip circumference without dietary changes. | High |
| Zarrati M. 2014 [99] | Randomized controlled trial | Overweight and obese individuals, ages 18–50, BMI 25–40 kg/m² | Probiotic yogurt vs. conventional yogurt, with or without weight-loss diet | 8 weeks | Fat distribution, gene expression of pro-inflammatory factors in peripheral blood mononuclear cells | Anthropometric measurements, gene expression analysis, blood tests | Probiotic yogurt significantly reduced body weight, BMI, waist circumference, and fat mass. Reduced expression of TNF-α and IL-6 genes in the probiotic group compared to control. | Probiotic yogurt consumption, with or without a weight-loss diet, improves body composition and reduces pro-inflammatory gene expression in overweight and obese individuals. | High |
| Ipar N. et al., 2015 [100] | Randomized controlled trial | Obese children, ages 7–18 | Synbiotic supplementation vs. placebo | 12 weeks | Anthropometric measurements, lipid profile, oxidative stress markers | Anthropometric measurements, blood tests for lipid profile and oxidative stress markers | Significant improvements in BMI, waist circumference, and lipid profile in the synbiotic group. Reduction in oxidative stress markers compared to placebo. | Synbiotic supplementation improves anthropometric measures and lipid profile and reduces oxidative stress in obese children. | High |
| Doria E. et al., 2013 [101] | Randomized, double-blinded, placebo-controlled trial | 40 slightly overweight women aged 30 to 54 | Hypocaloric diet supplemented with phyto-supplement (Re-Code®) containing phloridzin, isoflavones, and probiotics | 90 days (with measurements at T30, T60, T90) | Body weight, fat mass, waist, thigh, and buttock circumference | Anthropometric measurements (body weight, height, BMI, circumferences), ultrasound skinfold thickness, statistical analysis | Significant reduction in body weight, fat mass, and waist, thigh, and buttock circumference in the treatment group compared to placebo | Phyto-supplementation, combined with a mild hypocaloric diet and moderate physical activity, is effective in reducing body weight and fat mass in overweight women | High |
| (C) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Geng J. et al., 2022 [102] | Review | Literature review, data synthesis | Obese individuals, various populations | Role of gut microbiota in obesity and related diseases | Gut microbiota composition and metabolites play a critical role in obesity and related diseases. Potential therapeutic treatments include probiotics, prebiotics, dietary interventions, and FMT. | There is overwhelming evidence that the composition of the gut microbiota and metabolites impact the progression of obesity and obesity-related diseases. | Medium | ||
| Gomes et al., 2018 [103] | Review | 22 studies (literature review, data synthesis) | Obese individuals | Role of gut microbiota in obesity and metabolism | Gut microbiota dysbiosis in obese individuals is linked to increased Firmicutes, Clostridium, and several other species, contributing to inflammation and altered satiety signaling. | Obesity was characterized by the presence of intestinal dysbiosis. The resulting dysbiosis could change the functioning of the intestinal barrier and the GALT. Intestinal dysbiosis could alter the production of gastrointestinal peptides related to satiety. | Medium | ||
| Crovesy L. et al., 2017 [104] | Systematic review | 14 studies (observational studies and clinical trials) | Obese and lean adults | Gut microbiota composition differences between obese and lean individuals | Obese individuals have higher Firmicutes/Bacteroidetes ratio, increased Firmicutes, Fusobacteria, Proteobacteria, and Lactobacillus, and decreased Verrucomicrobia. | Probiotics have the potential to help in weight loss and fat mass loss in overweight subjects. The probiotics that aid weight loss include Lactobacillus gasseri, L. casei, L. rhamnosus, L. acidophilus, and L. plantarum. | High | ||
| Borgeraas et al., 2017 [105] | Systematic review and meta-analysis | 15 randomized controlled trials (RCTs) | Overweight and obese individuals | Effect of probiotics on weight, BMI, fat mass, and body fat percentage | Reduction in body weight (−0.60 kg), BMI (−0.27 kg/m²), and body fat percentage (−0.60%). However, the effect on fat mass was not always significant. | Probiotic supplementation may slightly reduce body weight, BMI, and body fat percentage, offering a potential approach for obesity management. | Moderate for fat mass and fat percentage due to smaller study sample sizes and variability in probiotic strains used. | ||
| Cao N. et al., 2024 [106] | Meta-analysis or randomized controlled trials (RCT) | 11 randomized controlled trials (RCTs) | Overweight or obese women treated with probiotics | Weight loss, glucose metabolism (insulin, fasting blood glucose), lipid metabolism | Probiotics significantly reduced waist circumference (WC), insulin levels, and LDL cholesterol, but had no significant effect on weight, BMI, or fat mass. | Probiotics can effectively reduce some metabolic parameters, especially WC and LDL-C, in overweight and obese women, but have limited effect on weight loss and BMI. | Moderate: Results varied by duration of intervention and inclusion of dietary/exercise factors, but consistent reductions in LDL-C and WC were noted. | ||
| Musazadeh V et al., 2022 [107] | Meta-analyses (umbrella review) | 29 meta-analyses | Effects of probiotic supplementation on obesity | Evaluate the effect of probiotics on BMI, body weight, and waist circumference (WC) | Probiotics significantly reduced BMI (ES = −0.21), body weight (ES = −0.38), and waist circumference (ES = −0.60). | Probiotics can be an effective intervention for managing obesity by reducing BMI, weight, and waist circumference. | Moderate in 83% of studies; low or very low in 17% of studies. | ||
| (A) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Kaye et al. (2020) [108]. | Systematic review | 5 studies: 3 RCTs, 2 meta-analyses | 75,541 | Daily supplementation with 0.5–5.0 mg of folic acid typically lowers plasma Hcy levels by approximately 25% | Hyperhomocysteinemia is a known risk factor for coronary artery disease. In this regard, elevated levels of Hcy have been found in the majority of patients with vascular disease. | Folic acid supplementation should be recommended to any patient who has an elevated Hcy level | High | ||
| (B) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of Evidence |
| Strozzi et al. (2008) [109] | Pilot study controlled clinical trial | 23 healthy volunteers | Strain effectiveness was evaluated by determination of the folate concentration in feces evacuated within 48 h before and after administration of the probiotics. | Volunteers were randomly assigned to 1 of 3 groups for treatment with a specific probiotic strain (5 × 109 colony-forming units/d), Bifidobacterium adolescentis DSM 18350, B. adolescentis DSM 18352, and Bifidobacterium pseudocatenulatum DSM 18353, to produce folates in the human intestine. | 2 weeks | If the probiotic treatments would cause a significant increase in folic acid concentration in human feces in all treated groups | Ingestion of these probiotic strains resulted in a significant increase in folic acid concentration in human feces in all treated groups | There was an increase in Faecalibaculum and Dubosiella phyla. The demonstrated ability of the probiotic microorganisms B. adolescentis DSM 18350, B. adolescentis DSM 18352, and B. pseudocatenulatum DSM 18353 to synthesize and secrete folates in the human intestinal environment may provide a complementary endogenous source of such molecules. | Medium |
| Majewska et al. (2020) [110] | Randomized double-blind placebo-controlled trial | 50 obese women (aged 45–70 years) | Blood tests | Subjects were randomly assigned to take either a multispecies probiotic supplement (n = 25) or placebo (n = 25) for 12 weeks | 12-week supplementation with a multispecies probiotic | The purpose was to assess if supplementation with probiotics can potentially be a natural therapeutic method for metabolic disorders. | At the end of the study, a significant decrease in Hcy, tumor necrosis factor α (TNF-α), total cholesterol (TC), low-density lipoprotein cholesterol (LDL) and triglyceride (TG) levels were observed in the probiotic group. | These multidirectional effects can potentially reduce cardiometabolic risks. | High |
| (A) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention | Period of Intervention | End Point | Results | Conclusion | Strength of Evidence |
| Cotillard et al., 2013 [111] | Randomized clinical trial | 38 obese and 11 overweight people, including 8 men and 41 women, without chronic diseases | At 0, 6 and 12 weeks, blood (total cholesterol, HDL, triglycerides, insulin, glucose, and inflammatory markers) and fecal samples were collected and anthropometric measurements were performed (body composition). | Low-calorie high-protein diet for 6 weeks, followed by maintenance diet for another 6 weeks | 12 weeks | Investigating temporal relationships between food intake, gut microbiota, and metabolic and inflammatory phenotypes, to assess possible association between microbiota and fat metabolism | A 35% reduction in energy intake after the first 6 weeks was associated with a reduction in fat mass and adipocyte diameter and improvements in insulin sensitivity and markers of metabolism and inflammation. Quantitative metagenomic analysis of the gut microbiome revealed the existence of a high proportion of individuals (23–40%) with low microbial richness, who had dyslipidemia associated with adiposity, increased insulin resistance, and low-grade inflammation. | The concomitant improvement in gut microbiome gene richness and bioclinical variables by dietary intervention suggests the possibility of moving from risk identification to risk reduction, on the assumption that less-rich microbiota are also less healthy | High |
| Koren et al., 2011 [112] | Randomized cross-sectional study | 15 patients aged 45 to 47 years with atherosclerosis and as many healthy controls matched for age and sex | 454 pyrosequencing of 16S rRNA genes was used to examine the bacterial diversity of atherosclerotic plaques, oral samples from swabs, and fecal samples from stool collection. | These studies were based on screening examinations of randomly selected population cohorts. | / | To study the association between microbiota composition and pathophysiological conditions associated with dyslipidemia and ectopic fat deposition, such as atherosclerosis and hepatic steatosis | Shared OTUs were observed among all three sites (oral, gut, and atherosclerotic plaques), consistent with the possibility that the oral and gastrointestinal microbiota are involved in the inflammatory processes responsible for atherosclerosis and that the atherosclerotic plaque microbiota may derive from the oral cavity and/or the gut. In addition, specific components of the oral/intestinal microbiota correlate with markers of disease: Streptococcus correlates with HDL, Fusobacterium correlates with LDL and total cholesterol, members of the families Erysipelotrichaceae and Lachnospiraceae in the gut correlate with LDL and total cholesterol. | Bacteria in the oral cavity and perhaps the gut may be correlated with disease markers of atherosclerosis. | High |
| Zhou et al., 2023 [113] | Two-sample Mendelian randomization study | Individuals of European descent | // | // | // | Causal association between gut microbiota and dyslipidemia | The families Lachnospiraceae and Lactobacillaceae are of notable importance and should be recognized as crucial microbiota in ameliorating dyslipidemia. The Bacillota phylum emerges as the most influential regulator of body lipid levels. | These findings demonstrate a causal link between gut microbiota and dyslipidemia within humans. The families Lachnospiraceae and Lactobacillaceae assume a noteworthy role in ameliorating lipid metabolism abnormalities. | High |
| (B) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Flaig et al., 2023 [114] | Narrative review | // | // | Association between gut microbiota, dysbiosis, and altered lipid metabolism; effects of key gut microbial metabolites on the development and progression of dyslipidemia; how diet impacts changes in the gut microbiota and the resulting influences on lipid metabolism | Improved dietary intake through the MD; statin therapy exerts its positive effects by increasing SCFA-producing bacteria like Lactobacillus, Eubacterium, Faecalibacterium, Bifidobacterium and Akkermansia, which are key in maintaining gut barrier integrity. Prebiotics, probiotics, synbiotics, FMT, and next-generation probiotics provide a simple, effective treatment modality for dyslipidemia by enriching the gastrointestinal tract of affected individuals with beneficial microbial species. | Substantial evidence supports the involvement of gut microbiota and states of dysbiosis in the development and progression of metabolic diseases, such as dyslipidemia. | Medium | ||
| (C) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of evidence |
| Tian Y et al., 2024 [115] | Randomized, double-blind, placebo-controlled clinical trial | 33 patients with hyperlipidemia, divided into a probiotic group (n = 18) and a control group (n = 15) | 16S rRNA gene pyrosequencing to examine bacterial diversity, from serum and stool samples | The probiotic group was given probiotics (2 g/day) and atorvastatin, 20 mg/day, while the control group was given placebo (2 g/day) and atorvastatin 20 mg/day. | 3 months | To evaluate the role of probiotics (Lactobacillus casei Zhang, Bifidobactetium animalis subsp. lactis V9 and Lactobacillus plantarum P-8) in the treatment of hyperlipidemia, in combination with atorvastatin administration | Significant effect on levels of total cholesterol, triglycerides, and LDL cholesterol in the probiotic and control groups (p < 0.05). Gut microbial abundance in the probiotic group was significantly higher than that in the control group after 3 months (p < 0.05). At the phylum level, probiotics increased the abundance of Tenericutes and decreased that of Proteobacteria. At the genus level, probiotics increased the abundance of Bifidobacterium, Lactobacillus, and Akkermansia and decreased that of Escherichia, Eggerthella, and Sutterella. | Probiotics optimize the structure of the gut microbiota and decrease the amount of harmful bacteria in patients with hyperlipidemia. | High |
| Wang S et al., 2022 [116] | Multicenter randomized, placebo- controlled clinical trial | 365 participants with T2D divided into 4 groups at 1:1:1:1 ratio | HbA1c, serum insulin, and C peptide were analyzed. Pyrosequencing of 16S rRNA genes to examine bacterial diversity. | The 4 groups were divided as follows: BBR (0.6 g per 6 pills, 2 v/day before a meal) + probiotics (4 g per 2 powder strips, 1 v/day at bedtime) (Prob + BBR); probiotics + placebo (Prob); BBR + placebo (BBR); or placebo + placebo (Plac). | 12 weeks | To evaluate whether therapy combining probiotics and berberine, combined with an antidiabetic and hypolipidemic regimen, could reduce postprandial lipidemia in T2D | Prob + BBR was superior to BBR or Prob alone in improving postprandial col tot and LDL col levels with a decrease in multiple species of postprandial lipidomic metabolites | BBR and Prob may exert a synergistic hypolipidemic effect on PL, acting as a reservoir of intestinal lipids to achieve better control of lipidemia and CV risk in T2D. | High |
| Trotter RE et al., 2020 [117] | Randomized, double-blind, placebo-controlled study. | 94 men and women aged 18–65 years with BMI between 20 and 34.9 | Blood samples (100 mL) were collected from the antecubital vein in a lithium heparin tube and analyzed for total chol, HDL-c, triglycerides, non-HDL cholesterol, total/HDL cholesterol ratio, LDL-c and VLDL-c using a lipid panel reagent disc on the Piccolo Xpress Chemistry blood analyzer (Abaxis, Union City, CA, USA). Blood pressure and pulse wave analysis were also evaluated. | Subjects were given 15 mg/day supplementation of B. subtilis for 4 weeks. | 4 weeks | To evaluate how Bacillus subtilis DE 111 supplementation may be helpful in improving the dyslipidemia picture and consequently CV risk factors | Supplementation with 15 mg daily resulted in a significant reduction in total cholesterol compared with baseline measurements, as well as LDL cholesterol. In addition, modest improvements in endothelial function and significant changes in several plasma lipids were observed | B. subtilis supplementation may be useful in improving risk factors associated with CVD. | High |
| Salamat et al., 2024 [118] | The double-blind randomized controlled trial | 56 adult men aged 60 years or younger with dyslipidemia with TG of 200–400 mg/dL and LDL of 130–160 mg/dL randomly assigned to intervention and control groups | Blood and stool samples were collected at baseline and at the end of the study. Food intake, physical activity, anthropometric measures, serum IL-10 and fecal SCFAs were assessed before and after the intervention. | Subjects received synbiotic powder or placebo twice daily for 12 weeks. | 12 weeks | To investigate the effects of multispecies synbiotic supplementation on serum interleukin 10 and fecal short-chain fatty acids (SCFAd) in patients with dyslipidemia | Serum IL-10 increased in the synbiotic group. Synbiotic supplementation increased the fecal concentrations of acetate, butyrate, propionate, and valerate. | A significant increase in fecal abundance of Lactobacillus and Bifidobacterium and serum HDL was observed in the synbiotic group. | High |
| (D) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Sivamaruthi et al., 2019 [119] | Systematic review | / | Hypercholesterolemic subjects, healthy subjects, diabetes patients | To analyze the ability of probiotics (Enterococcus faecium CRL 183 and Lactobacillus helveticus 416) fermented with isoflavone-containing soy products to reduce cholesterol levels in hypercholesterolemic subjects | Supplementation of the soy product with 50 mg isoflavone daily for 42 days significantly improved total and LDL cholesterol while HDL levels remained unchanged. A 12-week supplementation with a mixture of L. plantarum strains (10 CFU/day) significantly increased HDL levels and reduced LDL, col tot, triglycerides, LDL/HDL ratio, and LDL levels. In dyslipidemic children, supplementation of a mixture of Bifidobacterium strains for three months improved the concentration of serum levels of col tot, HDL, TG and LDL. Consumption of a single probiotic strain (E. faecium) and selenium (50 μg) for one year did not alter HDL-C and TG levels, while it reduced TC and LDL-C in elderly people. | Probiotic consumption significantly improved the health status of hypercholesteremic patients by reducing LDL, total cholesterol and triglyceride levels and increasing HDL cholesterol. | High | ||
| Sivamaruthi et al., 2021 [120] | Systematic review | seventeen (Lactobacillus in nine studies, Bifidobacterium in eight studies and Enterococcus in two studies) | Hypercholesterolemic subjects | To analyze dietary interventions with probiotics in humans and their effects on cardiovascular risk factors and hypercholesterolemia | Supplementation of B. longum and red yeast rice in 33 patients with low CVD risk and no CVD risk led to a reduction in LDL-c levels; administration of L. plantarum, along with regular diet, to 23 people with hypercholesterolemia led to a reduction in LDL-c level. Administration of a soy product supplemented with isoflavones and fermented with E. faecium and L. helveticus to 17 patients led to a reduction in LDL-c level of up to 14.8 percent. Probiotic mixture consisting of B. lactis MB, B. bifidum and B. longum showed a significant reduction in TC, LDL-c and TG levels and an increase in HDL-c levels in dyslipidemic children. Probiotic mixture supplementation of three strains of L. plantarum significantly reduced TC (13.6%), LDL-c and LDL-c levels in hypercholesterolemic adults. | Probiotics have the propensity to become dietary supplements in moderate/severe hypercholesterolemic patients, which significantly reduces the CVD risk. | High | ||
| Gadelha CJMU et al., 2019 [121] | Systematic review | 14 clinical studies | Subjects older than 18 years, predominantly with dyslipidemia | To examine the effects of probiotic supplementation on the prevention and treatment of dyslipidemia | Probiotic supplementation significantly reduced total cholesterol, LDL, and triglycerides and increased HDL values, especially when combined with other treatments (statins). The group with col tot > 200 mg/dL had the best response to probiotic treatment. Some benefits were also observed on anthropometric variables, glycemic control, oxidative stress, inflammatory markers, and immune system. | This study has shown that probiotic supplementation should be indicated as an additional treatment for lipid profile alterations. | High | ||
| Ettinger G et al., 2014 [122] | Meta-analysis | // | 485 participants with “high”, “borderline”, and “normal” serum cholesterol levels | Examine the role of the microbiome in the prevention and treatment of cardiovascular disease | Several specific probiotic strains have been identified as effective in the management of hypercholesterolemia. The most effective probiotic strain clinically proven to reduce LDL-C levels by about 11.6 percent in hypercholesterolemic adults is Lactobacillus reuteri NCIMB 30242. | Probiotic consumption significantly reduced LDL-C and total cholesterol levels in all categories, compared with control. | High | ||
| Shimizu M et al., 2015 [123] | Meta-analysis | 11 randomized controlled clinical trials describing data on differences before and after intervention in serum lipids, (col tot, LDL, HDL and TG) | The participants were healthy or hypercholesterolemic individuals of all ages (from infants to elderly people). | Demonstrate that probiotic supplementation may be useful in the primary prevention of hypercholesterolemia by leading to reduced risk factors for cardiovascular disease | Changes in col tot and LDL. Triglyceride and HDL levels did not differ significantly between the probiotic and control groups. Reductions in col tot and LDL levels with the probiotic intervention were greater in mildly hypercholesterolemic subjects. A subanalysis determined that the long-term (>4 weeks) probiotic intervention was statistically more effective in reducing TC and LDL-C than the short-term intervention, and high-dose probiotics more effectively reduced LDL-C levels than low-dose probiotics. | Lactobacillus acidophilus and Gaius reduced TC and LDL levels to a greater extent than the other bacterial strains. | High | ||
| (A) | ||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | End Point | Results | Conclusion | Strength of Evidence | |
| Picca et al., 2020 [124] | Case–control study | N = 18 sarcopenic patients N = 17 healthy patients | Microbiota analysis on fecal samples | Microbiota composition | ↑: Bifidobacteriacee, Peptostreptococcacee in sarcopenic group | Sarcopenic patients show modifications in microbiota composition | Medium | |
| Wang et al., 2023 [125] | Case–control study | N = 50 sarcopenic patients N = 50 healthy controls | Microbiota analysis | Microbiota composition | ↓: Bifidobacterium longum, Prevotella coprii in sarcopenic group | Sarcopenic patients show modifications in microbiota composition | Medium | |
| Lee et al., 2020 [126] | Case–control study | N = 27 sarcopenic patients N = 33 healthy controls | Microbiota analysis | Microbiota composition | ↓: Prevotella copri in sarcopenia group ↑: Anaerotruncus in sarcopenia group | Sarcopenic patients show modifications in microbiota composition | Medium | |
| Liu et al., 2023 [127] | Case–control study | N = 141 sarcopenic patients N = 142 healthy controls | Microbiota analysis | Microbiota composition | ↓: Prevotella coprii; BCAA metabolism in sarcopenia group ↑: Bifidobacteria in sarcopenia group | Sarcopenic patients show modifications in microbiota composition and less BCAA metabolism | Medium | |
| Ticinesi et al., 2020 [128] | Case–control study | N = 5 sarcopenic patients N = 12 healthy controls | Shotgun sequencing on fecal samples | Microbiota composition | ↓: SCFA production, Faecalibacterium prausnizii; Roseburia inulinivorans, Alistipes shahii in sarcopenia group | Sarcopenic patients show modifications in microbiota composition and less production of SCFA | Medium | |
| Wang et al., 2022 [129] | Cross-sectional study | N = 141 sarcopenic patients N = 1276 healthy controls | Shotgun sequencing on fecal samples | Microbiota composition | ↓: beta-diversity in sarcopenia group ↑: Clostridium | Sarcopenic patients show less beta-diversity in microbiota composition | Medium | |
| Kang et al., 2021 [130] | Longitudinal study | N = 27 sarcopenic patients N = 60 healthy controls | Sequencing on fecal samples | Changes in microbiota composition | ↓: alpha-diversity, beta-diversity, SCFA production, Firmicutes in sarcopenia patients ↑: Porphyromondanaceae, Lactobacillaceae in sarcopenia patients | Sarcopenic patients show modifications in microbiota composition | Medium | |
| (B) | ||||||||
| Authors | Type of Studies | Population Characteristics | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of Evidence |
| Lee et al., 2018 [126] | Randomized, double-blind study | Elderly patients with frailty | Administration of lactobacillus plantarum TWK10 | >6 weeks | Muscle mass (kg) HGS (kg) | ↑: muscle strength, endurance, HGS, gait speed ↓: sarcopenia, muscle weakness | Treatment with probiotics ameliorates muscle function | High |
| Chaiyasut et al., 2022 [131] | Randomized, double-blind study | Healthy older adults | Administration mixture of probiotics (2.0 × 1010 CFU of L. paracasei HII01; 2.0 × 1010 CFU of B. breve; 1.0 × 1010 CFU of B. longum) (Lactomason Co., Ltd., Jinju-si, Republic of Korea) | 12 weeks | Muscle mass (%) | ↑: HDL-c; muscle % ↓: VAT %, body fat % | Treatment with probiotics ameliorates body composition and lipid profile | High |
| Tominaga et al., 2021 [132] | Not controlled, experimental study | Patients with frailty | Administration of 20 g of prebiotic 1-kestose daily | 8 weeks | Fecal microbiota composition | ↑: increase in Bifidobacterium longum population | Treatment with probiotics modifies microbiota composition | Medium |
| Karim et al., 2022 [133] | Randomized, double-blind study | Patients with chronic heart failure and sarcopenia | Administration of probiotic with 112 billion UFC | 12 weeks | ASM (kg) HGS (kg) | ↑: improvement in HGS, SPPB and gait speed in probiotic group | Treatment with probiotics ameliorates muscle function | High |
| Karim et al., 2022 [134] | Randomized double-blind study | Patients with COPD and sarcopenia | Administration of probiotic with 112 billion UFC | 16 weeks | ASM (kg) HGS (kg) | ↑: improvement in handgrip; SPPB, gait speed in probiotic group | Treatment with probiotics ameliorates muscle function | High |
| Rondanelli et al., 2022 [135] | Randomized double-blind study | Patients with sarcopenia | Administration of novel food composed of Leucine, Omega-3 fatty acids and probiotic Lactobacillus paracasei PS23 | 2 months | ASM (kg) HGS (kg) | ↑: improvement in ALM and plasma amino acids in probiotic group ↓: decrease in visceral adiposity in probiotic group | Treatment with probiotics ameliorates body composition and increases plasma amino acids | High |
| Ford et al., 2020 [136] | Placebo-controlled, double-blind, cross-sectional study | Elderly woman | 1.54 × 109 Bifidobacterium bifidum HA-132, 4.62 × 109 Bifidobacterium Breve HA-129, 4.62 × 109 Bifidobacterium longum HA-135, 4.62 × 109 Lactobacillus acidophilus HA-122, and 4.62 × 109 Lactobacillus plantarum HA-119 | 2-week periods with 2-week diet washout | ASM (kg) | ↑: increase in fat-free mass | Treatment with probiotics ameliorates body composition | High |
| Qaisar et al., 2024 [137] | Randomized, controlled study | Elderly man with sarcopenia | B. longum DSM 24736, B. breve DSM 24732, DSM 24737, Streptococcus thermophilus DSM 24731, e lactobacilli (DSM 24735, DSM 24730, DSM 24733, L. delbrueckii subsp. bulgaricus DSM 24734 | 16 weeks | Sar-QoL scores, fecal zonulin; gait speed (%), HGS (%) | ↑: gait speed, HGS | Treatment with probiotics ameliorates muscle function | High |
| (A) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention (If Applicable) | Duration | End Point | Results | Conclusion | Strength of Evidence |
| Jumpertz, R. et al., 2011 [138] | Observational study | 12 lean and 9 obese individuals | Pyrosequencing bacterial 16S ribosomal RNA (rRNA) genes present in the feces of participants and measuring ingested and stool calories with the use of bomb calorimetry | - | - | To evaluate changes in gut microbiota during diets that varied in caloric content (2400 compared with 3400 kcal/d). To study how gut bacterial community structure is affected by altering the nutrient load in lean and obese individuals and whether their microbiota are correlated with the efficiency of dietary energy harvest. | The alteration of the nutrient load induced rapid changes in the gut microbiota. These changes were directly correlated with stool energy loss in lean individuals such that a 20% increase in Firmicutes and a corresponding decrease in Bacteroidetes were associated with an increased energy harvest of ≈150 kcal. A high degree of overfeeding in lean individuals was accompanied by a greater fractional decrease in stool energy loss. | These results show that the nutrient load is a key variable that can influence the gut (fecal) bacterial community structure over short time scales. Furthermore, the observed associations between gut microbes and nutrient absorption indicate a possible role of the human gut microbiota in the regulation of the nutrient harvest. | Low |
| Mouzaki, M. et al., 2013 [139] | Prospective, cross-sectional study | 50 subjects included: 11 with simple steatosis (SS), 22 with non-alcoholic steatohepatitis (NASH), and 17 living liver donors as healthy controls (HCs) | One stool sample was collected from each participant. Quantitative real-time polymerase chain reaction was used to measure total bacterial counts, Bacteroides/Prevotella (herein referred to as Bacteroidetes), Clostridium leptum, C. coccoides, bifidobacteria, Escherichia coli and Archaea in stool. Clinical and laboratory data, food records, and activity logs were collected. | - | - | To identify differences in intestinal microbiota between adults with biopsy-proven NAFLD (SS or NASH) and HCs | Patients with NASH had a lower percentage of Bacteroidetes (Bacteroidetes to total bacteria counts) compared to both SS and HC (p = 0.006) and higher fecal C. coccoides compared to those with SS (p = 0.04). There were no differences in the remaining microorganisms. As body mass index (BMI) and dietary fat intake differed between the groups (p < 0.05), we performed linear regression adjusting for these variables. The difference in C. coccoides was no longer significant after adjusting for BMI and fat intake. However, there continued to be a significant association between the presence of NASH and lower percentages of Bacteroidetes even after adjusting for these variables (p = 0.002; 95% confidence interval = −0.06 to −0.02). | There is an inverse and diet-/BMI-independent association between the presence of NASH and percentage Bacteroidetes in the stool, suggesting that the IM may play a role in the development of NAFLD. | Low |
| Zhang C. et al., 2018 [140] | Observational study | 15 healthy volunteers, who normally consume an omnivorous diet (study group), 7 healthy omnivorous volunteers (control group 1) and 7 healthy long-term vegetarians (lacto-ovo-vegetarian diet, control group 2) | Blood and fecal samples were collected and weight was measured days 0 and 91 for all individuals to determine the composition of their microbiota. | Subjects in study group changed to a lacto-ovo-vegetarian diet for 3 months. The participants had not taken antibiotics 3 months before the study nor during the study period. Blood and fecal samples were collected and weight was measured days 0 and 91 for all individuals. | 3 months | To investigate the effect of a 3-month lacto-ovo-vegetarian diet on the diversity of gut microbiota and the immune system in healthy omnivorous volunteers. | The short-term vegetarian diet did not have any major effect on the diversity of the immune system and the overall composition of the metagenome. The prevalence of bacterial genera/species with known beneficial effects on the intestine, including butyrate producers and probiotic species, and the balance of autoimmune-related variable genes/families were, however, altered in the short-term vegetarians. A number of bacterial species that are associated with the expression level of IgA, a key immunoglobulin class that protects the gastrointestinal mucosal system, were also identified. Furthermore, a lower diversity of T-cell repertoire and expression level of IgE, as well as a reduced abundance of inflammation-related genes in the gut microbiota, were potentially associated with a control group with long-term vegetarians. | Thus, the composition and duration of the diet may have an impact on the balance of pro-/anti-inflammatory factors in the gut microbiota and immune system. | Low |
| Miele, L. et al., 2009 [141] | Observational study | 35 consecutive patients with biopsy-proven NAFLD, 27 with untreated celiac disease (as a model of intestinal hyperpermeability) and 24 healthy volunteers | Assessment of the presence of SIBO by glucose breath testing (GBT), intestinal permeability by means of urinary excretion of (51)Cr-ethylene diamine tetraacetate ((51)Cr-EDTA) test, and the integrity of tight junctions within the gut by immunohistochemical analysis of zona occludens-1 (ZO-1) expression in duodenal biopsy specimens | - | - | To investigate intestinal permeability in patients with NAFLD and evaluated the correlations between this phenomenon and the stage of the disease, the integrity of tight junctions within the small intestine, and prevalence of small intestinal bacterial overgrowth (SIBO) | Patients with NAFLD had significantly increased gut permeability (compared with healthy subjects; p < 0.001) and a higher prevalence of SIBO, although both were lower than in the untreated celiac patients. In patients with NAFLD, both gut permeability and the prevalence of SIBO correlated with the severity of steatosis but not with presence of NASH. | NAFLD in humans is associated with increased gut permeability and this abnormality is related to the increased prevalence of SIBO in these patients. The increased permeability appears to be caused by disruption of intercellular tight junctions in the intestine, and it may play an important role in the pathogenesis of hepatic fat deposition. | Low |
| Fava, F. et al., 2013 [142] | Randomized, controlled, single-blind, parallel design | 88 subjects at increased MetS risk | High monounsaturated fat (MUFA)/high glycemic index (GI) (HM/HGI); high MUFA/low GI (HM/LGI); high carbohydrate (CHO)/high GI (HC/HGI); and high CHO/low GI (HC/LGI). Dietary intakes, MetS biomarkers, fecal bacteriology and SCFA concentrations were monitored. | Subjects were fed a high-saturated-fat diet (HS) for 4 weeks (baseline), then randomized onto one of the five experimental diets for 24 weeks: HS; high monounsaturated fat (MUFA)/high glycemic index (GI) (HM/HGI); high MUFA/low GI (HM/LGI); high carbohydrate (CHO)/high GI (HC/HGI); and high CHO/low GI (HC/LGI). | 28 weeks | To determine the effect of the amount and type of dietary fat and carbohydrate on fecal bacteria and short-chain fatty acid (SCFA) concentrations in people ‘at risk’ of MetS. | High-MUFA diets did not affect individual bacterial population numbers but reduced total bacteria and plasma total and LDL cholesterol. The low-fat, HC diets increased fecal Bifidobacterium (p = 0.005, for HC/HGI; p = 0.052, for HC/LGI) and reduced fasting glucose and cholesterol compared to baseline. HC/HGI also increased fecal Bacteroides (p = 0.038), whereas HC/LGI and HS increased Faecalibacterium prausnitzii (p = 0.022 for HC/HGI and p = 0.018, for HS). Importantly, changes in fecal Bacteroides numbers correlated inversely with body weight (r = −0.64). A total bacterial reduction was observed for high-fat diets HM/HGI and HM/LGI (p = 0.023 and p = 0.005, respectively) and HS increased fecal SCFA concentrations (p < 0.01). | This study provides new evidence from a large-scale dietary intervention study that HC diets, irrespective of GI, can modulate human fecal saccharolytic bacteria, including Bacteroides and bifidobacteria. Conversely, high-fat diets reduced bacterial numbers, and in the HS diet, there was increased excretion of SCFAs, which may suggest a compensatory mechanism to eliminate excess dietary energy. | High |
| Zelber-Sagi, S. et al., 2018 [143] | Cross-sectional study | 789 individuals, 40–70 years old, who underwent screening colonoscopy between 2013 and 2015 in a single center in Israel | NAFLD and IR were evaluated by ultrasonography and homeostasis model assessment. | To test the association of meat type and cooking method with NAFLD and insulin resistance (IR) | High consumption of total, red and/or processed meat was independently associated with higher odds of NAFLD and IR, respectively, when adjusted for body mass index, physical activity, smoking, and alcohol, energy, saturated fat and cholesterol intake. High intake of meat cooked using unhealthy methods and heterocyclic amines (formed by cooking meat at high temperatures for a long duration) were independently associated with higher odds of IR. | High consumption of red and/or processed meat is associated with both NAFLD and IR. High HCA intake is associated with IR. If confirmed in prospective studies, limiting the consumption of unhealthy meat types and improving preparation methods may be considered as part of NAFLD lifestyle treatment. | Medium | ||
| Wehmeyer, M.H et al., 2016 [144] | Observational study | 55 consecutive patients diagnosed with NAFLD compared to an age- and gender-matched cohort of 88 healthy individuals by univariate analysis | Biochemical data: AST, ALT, GGT, gluco-lipid pattern. Hepatic fibrosis was evaluated by a transient elastography of the liver. | The efficacy of the dietary intervention was assessed in a subgroup of 24 NAFLD patients 6 months after receiving dietary advice. | To assess the dietary patterns associated with non-alcoholic fatty liver disease (NAFLD) and the efficacy of dietary interventions in a real-life setting | NAFLD patients consumed more calories per day as compared with healthy controls (p < 0.001). The absolute amounts of most nutritional components ingested by NAFLD patients were higher than those of the controls. However, there were no significant differences with regard to the relative consumption of carbohydrates (p = 0.359), fat (p = 0.416), and fructose (p = 0.353) per 1000 kcal energy intake. NAFLD patients displayed a higher intake of glucose/1000 kcal (p = 0.041) and protein/1000 kcal (p = 0.009) but a lower intake of fibers/1000 kcal (p < 0.001) and mineral nutrients/1000 kcal (p = 0.001) than healthy controls. In the longitudinal analysis, patients significantly reduced their caloric intake, and their ALT levels improved 6 months after the dietary counseling (p < 0.001). | The study demonstrates that dietary patterns of patients with NAFLD display great variability and little disease specificity, while the most distinctive feature compared with healthy controls was higher energy intake in NAFLD patients. | Low | |
| Cotillard, A. et al., 2013 [111] | Clinical trial | 38 obese and 11 overweight individuals. | Anthropometric markers. Biochemical parameters: plasma glucose and lipid homeostasis, inflammatory markers (PCR, IL-6). Subcutaneous abdominal adipose tissue samples were obtained at all time points by needle biopsy from the periumbilical area under local anesthesia (1% xylocaine) to measure the adipocyte diameter and for immunohistochemical studies. | Diet-induced weight-loss and weight-stabilization interventions | 12 weeks | To investigate the temporal relationships between food intake, gut microbiota and metabolic and inflammatory phenotypes | Individuals with reduced microbial gene richness (40%) present more pronounced dys-metabolism and low-grade inflammation. | Dietary intervention improves low gene richness and clinical phenotypes, but seems to be less efficient for inflammation variables in individuals with lower gene richness. Low gene richness may therefore have predictive potential for the efficacy of intervention. | Medium |
| Parker, H.M et al., 2019 [145] | Double-blind randomized controlled trial | Fifty apparently healthy overweight men (BMI 25.0–29.9 kg/m2; waist > 94 cm) randomly allocated to consume fish oil or placebo (olive oil capsules). | Anthropometric parameters: standing height, weight, waist circumference. Body composition measured using BIA. Biochemical parameters: serum aminotransferases (ALT, AST, GGT) and triglycerides (TG), omega-3 index testing. Dietary and physical activity habits. MRI and 1H-MRS methods for quantifying abdominal visceral (VAT) and subcutaneous (SAT) adipose tissue, liver fat (intrahepatic lipid; IHL) concentration and composition. | Treatment group: total daily dose: 1728 mg marine triglycerides, of which 588 mg EPA and 412 mg DHA, combined with 200 mg antioxidant (coenzyme Q10). Placebo group: olive oil capsules daily for 12 weeks | 12 weeks | To evaluate effect of fish oil supplementation on liver fat | No significant time or group × time effect for fish oil versus placebo for liver fat, liver enzymes, anthropometry, or body composition including VAT, with similar finding for sub-analysis of participants with NAFLD. | Omega-3 PUFA did not appear to be an effective agent for reducing liver fat in overweight men. | High |
| Šmíd V et al., 2022 [146] | Double-blind placebo-controlled trial | Sixty patients with metabolic syndrome and NAFLD were randomized in a double-blind placebo-controlled trial | Biochemical parameters: AST, ALT, GGT, gluco-lipid assessment. Liver stiffness was evaluated by ultrasonography. The 1H MRS was used for visceral and liver fat determination. Plasma lipidomics was determined using UPLC-HR-MS. Genomic DNA was isolated from peripheral blood white cells by the standard salting-out procedure. The specific variants of the PNPLA3 (rs738409 and rs738408), TM6SF2 (rs58542926), and MBOAT7 (rs641738) genes were typed by PCR. | Intervention group assumed 3.6 g/day n-3-PUFA for one year vs. placebo. | 1 year | To assess the effects of n-3-PUFA administration on lipid metabolism and the progression of NAFLD in patients with metabolic syndrome | After 12 months of n-3-PUFA administration, a significant decrease in serum GGT activity was recorded compared with the placebo group (2.03 ± 2.8 vs. 1.43 ± 1.6; p < 0.05). Although no significant changes in anthropometric parameters were recorded, a significant correlation between the reduction in liver fat after 12 months of treatment and weight reduction was observed; furthermore, this effect was clearly potentiated by n-3-PUFA treatment (p < 0.005). In addition, n-3-PUFA treatment resulted in substantial changes in the plasma lipidome, with n-3-PUFA-enriched triacylglycerols and phospholipids being the most expressed lipid signatures. | Twelve months of n-3-PUFA treatment of patients with NAFLD patients was associated with a significant decrease in GGT activity, the liver fat reduction in those who reduced their weight, and beneficial changes in the plasma lipid profile. | High |
| (B) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Maestri M. et al., 2023 [147] | Narrative Review | - | - | To investigate the impact of the gut microbiota on the molecular mechanisms underlying NAFLD; to understand how current therapeutic approaches used to treat NAFLD/MAFLD and its associated comorbidities may influence the natural history of the disease through gut microbiota modulation. Finally, to point the view to what may become future therapeutic weapons in NAFLD/MAFLD, acting on the gut microbiota. | The gut–liver axis has a strong impact in the promotion of NAFLD and in the progression of the wide spectrum of its manifestations. Western diet negatively affects intestinal permeability and the gut microbiota composition and function, selecting pathobionts, whereas Mediterranean diet fosters health-promoting bacteria, with a favorable impact on lipid and glucose metabolism and liver inflammation. Drugs for the treatment of type 2 diabetes mellitus (T2DM), such as metformin, glucagon-like peptide-1 (GLP-1) agonists, and sodium-glucose cotransporter (SGLT) inhibitors, are not only effective in the regulation of glucose homeostasis, but also in the reduction in liver fat content and inflammation, and they are associated with a shift in the gut microbiota composition towards a healthy phenotype. Even bariatric surgery significantly changes the gut microbiota, mostly due to the modification of the gastrointestinal anatomy, with a parallel improvement in histological features of NAFLD. | Mediterranean diet, drugs for the treatment of type 2 diabetes mellitus, and bariatric surgery have positive impacts on gut microbiota and NAFLD. Other options with promising effects in reprogramming the gut–liver axis, such as fecal microbial transplantation (FMT) and next-generation probiotics, deserve further investigation for future inclusion in the therapeutic armamentarium of NAFLD. | Medium | ||
| Aron-Wisnewsky J. et al., 2013 [148] | Narrative review | - | - | To summarizes what is currently known of microbiota composition in obesity and the physiopathogenesis of NAFLD in that context | Gut microflora may stimulate hepatic fat deposition and promote NASH though several mechanisms: it promotes obesity by improving energy yield from food, it regulates gut permeability, low-grade inflammation and immune balance, it modulates dietary choline metabolism, it regulates bile acid metabolism, and finally it increases endogenous ethanol production by bacteria. | The gut microbiota is involved in gut permeability, low-grade inflammation and immune balance; it modulates dietary choline metabolism, regulates bile acid metabolism and produces endogenous ethanol. All of these factors are molecular mechanisms by which the microbiota can induce NAFLD or its progression toward overt non-alcoholic steatohepatitis. | Medium | ||
| Wieland, A et al., 2015 [149] | Systematic review | Nine studies (five human and four animal models) | 226 humans | To perform a comprehensive review of the medical literature investigating associations between intestinal dysbiosis and NAFLD, with a particular emphasis on studies that characterize the microbiome in NAFLD. | Because of the anatomical links between the intestines and the liver, dysbiosis may also disrupt hepatic function and thereby contribute to the pathogenesis of non-alcoholic fatty liver disease (NAFLD) through these mechanisms:
| Investigations in humans and animals demonstrate associations between intestinal dysbiosis and NAFLD; however, causality has not been proven and mechanistic links require further delineation. | High | ||
| Roychowdhury, S. et al., 2018 [150] | Narrative review | - | - | To review the progression of NAFLD, discussing the mechanistic modes of hepatocyte injury and the potential role for manipulation of the gut microbiome as a therapeutic strategy in the prevention and treatment of NAFLD | There is no concrete evidence to support probiotics as monotherapy for NAFLD, which has a multi-hit pathophysiology. Nonetheless, manipulation of the gut microbiome using probiotics may be used as combination therapy, in addition to lifestyle interventions and other available natural or pharmacologic options, especially in patients who are struggling with compliance. | Only diet and lifestyle change have been demonstrated to improve obesity and NAFLD, but patient compliance is problematic. As more is being discovered about the gut microbiome and its role in obesity and liver disease, future directions in targeting the gut microbiome as a therapeutic option for NAFLD is warranted. However, several questions remain unanswered, such as the actual mechanism of action of probiotics in NAFLD, the differences in efficacy between children and adults with NAFLD, the comparison of efficacy of available probiotics, the specific targets of each probiotic, and long-term outcomes. | Medium | ||
| Aron-Wisnewsky J. et al., 2020 [148] | Narrative review | - | - | To provide a broad insight into microbiome signatures for human NAFLD and to explore issues with disentangling these signatures from underlying metabolic disorders. | Whilst animal studies have demonstrated a potential causal role of gut microbiota in non-alcoholic fatty liver disease (NAFLD), human studies have only just started to describe microbiome signatures in NAFLD. Proteobacteria are consistently enriched in steatosis and non-alcoholic steatohepatitis. Bacterial signatures (Clostridium and Lactobacillus) overlap between NAFLD and metabolic diseases (type 2 diabetes mellitus). | Discrepant microbiome signatures across studies could be linked to the heterogeneity of geographical regions, ethnicity, population characteristics, microbiome sequencing tools, NAFLD diagnostic tools, disease spectrum, drug consumption and circadian rhythm. | Medium | ||
| Albillos A. et al., 2020 [151] | Narrative review | - | - | To find out the pathophysiological basis for therapy of liver diseases based on the gut–liver axis | Growing evidence indicates the pathogenetic role of microbe-derived metabolites, such as trimethylamine, secondary bile acids, short-chain fatty acids and ethanol, in the pathogenesis of non-alcoholic fatty liver disease. The identification of the elements of the gut–liver axis primarily damaged in each chronic liver disease offers possibilities for intervention. | Beyond antibiotics, upcoming therapies centered on the gut include new generations of probiotics, bacterial metabolites (postbiotics), fecal microbial transplantation, and carbon nanoparticles. FXR agonists target both the gut and the liver and are currently being tested in different liver diseases. Finally, synthetic biotic medicines, phages that target specific bacteria, and therapies that create physical barriers between the gut and the liver offer new therapeutic approaches. | Medium | ||
| Wu L. et al., 2021 [152] | Narrative review | - | - | To integrate related articles on gut microbiota, PPARs and NAFLD, and present a balanced overview on how the microbiota can possibly influence the development of NAFLD through PPARs | Clinical studies have shown that gut microbiome signatures in NAFLD may serve as diagnostic biomarkers for liver disease. In connection with this, PPARs are transcription factors involved in the regulation of lipid metabolism, energy balance, and inflammation, thus playing a decisive role in various metabolic diseases. | Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily and can regulate multiple pathways involved in metabolism, and serve as effective targets for the treatment of many types of metabolic syndromes, including NAFLD. | Medium | ||
| Arslan, N. et al., 2014 [153] | Narrative review | - | - | To review the relationship between intestinal microbial changes and obesity and its complications, including insulin resistance and NAFLD | Given that the gut and liver are connected by the portal venous system, it makes the liver more vulnerable to translocation of bacteria, bacterial products, endotoxins or secreted cytokines. Altered intestinal microbiota (dysbiosis) may stimulate hepatic fat deposition through several mechanisms: regulation of gut permeability, increasing low-grade inflammation, modulation of dietary choline metabolism, regulation of bile acid metabolism and producing endogenous ethanol. | High-energy diets alter intestinal microbiota and induce gut dysfunction, which subsequently result in visceral fat inflammation and systemic metabolic dysregulation. An obesogenic microbiota can alternate liver function by stimulating hepatic triglycerides and by modulating systemic lipid metabolism, which indirectly impact the storage of fatty acids in the liver. Several studies suggested that intestinal microbiota might also play an important part in the progression of NAFLD to NASH. Modulation of gut microbiota by diet modifications or by using probiotics, prebiotics and synbiotics as a treatment for obesity and fatty liver disease might be a topic of further investigations. | Medium | ||
| Houghton D. et al., 2016 [154] | Narrative review | - | - | To review the effects of lifestyle interventions (diet and physical activity/exercise) on gut microbiota and how this impacts NAFLD prognosis | The Western diet (high in fat and carbohydrates) is associated with an altered gut microbiota and increased risk of developing obesity and NAFLD. Fructans are the most extensively studied prebiotics and have been linked with modulation of the gut microbiota, resulting in positive health benefits. Probiotics have been suggested as a potential treatment for patients with NAFLD, due to their apparent ability to modulate the gut microbiota and impact metabolic control, inflammation, lipid profile and intestinal permeability. Exercise does appear to be able to modulate the gut microbiota and reduce the risk of NAFLD with different potential mechanisms: increased butyrate production, which is linked with colonic epithelial cell proliferation and modulation of mucosal immunity and exclusion of pathogens; increased primary bile acid secretion and cholesterol turnover; growth of beneficial bacteria; increased core body temperature; and reduced blood flow to the GI system, reducing gut transit time and substrate delivery to the microbiota. | This review reveals that diet, pre/probiotics, and exercise play a significant role in the function and diversity of the gut microflora. To date, studies have predominantly focused on preclinical models, which have limitations in the transferability of their data to humans. Although much is known, there are significant questions about how lifestyle therapies may influence the gut microbiota as a therapeutic target for NAFLD care. | Medium | ||
| (C) | |||||||||
| Authors | Type of Studies | Population Characteristics | Type of Laboratory Evaluation | Type of Intervention | Duration | End Point | Results | Conclusion | Strength of Evidence |
| Chang HC et al., 2013 [155] | Double-blinded, randomized clinical trial | 40 subjects with BMI ≥27 aged 18–65 randomly divided into a control (n = 18) and an oat-treated (n = 16) group | Serum parameters (glucose, TG, cholesterol, LDL-C, HDL-C, FFA, AST, ALT, GGT, creatinine, blood urea nitrogen, albumin, uric acid,), BMI, waist-to-hip ratio, body fat | Oat group taking beta-glucan-containing oat cereal and the other a placebo (with a similar composition but without beta-glucan). One cereal pack (37.5 g) was prescribed to be mixed with 250 mL hot water and replace some staple foods in meals twice daily for 12 weeks. | 12 weeks | To verify if oat, rich in beta-glucan, had a metabolic-regulating and liver-protecting effect in humans | Consumption of oat reduced body weight, BMI, body fat and the waist-to-hip ratio. Profiles of hepatic function, including AST, but especially ALT, were useful resources to help in the evaluation of the liver, since both showed decrements in patients with oat consumption. Nevertheless, anatomic changes were still not observed by ultrasonic image analysis. Ingestion of oat was well tolerated and there was no adverse effect during the trial. | Consumption of oat reduced obesity, abdominal fat, and improved lipid profiles and liver functions. Taken as a daily supplement, oat could act as an adjuvant therapy for metabolic disorders. | High |
| (D) | |||||||||
| Authors | Type of Studies | Number of Studies and Type of Study | Subject | End Point | Results | Conclusion | Strength of Evidence | ||
| Perumpail, B.J. et al., 2019 [156] | Narrative review | - | - | To deepen probiotic supplementation as a potential treatment method for NAFLD due to its ability to retard and/or reverse dysbiosis and restore normal gut flora | All studies reviewed indicate that probiotics had a beneficial effect in patients with NAFLD and its subset NASH. Results varied between studies, but there was evidence demonstrating improvement in liver enzymes, hepatic inflammation, hepatic steatosis, and hepatic fibrosis. No major adverse effects were noted. Currently, there are no guidelines addressing the use of probiotics in the setting of NAFLD. | Probiotics appear to be a promising option in the treatment of NAFLD. Future research is necessary to assess the efficacy of probiotics in patients with NAFLD. | Medium | ||
| Xiao, M.-W et al., 2020 [157] | Systematic review and meta-analysis | 28 RCTs | 1555 proven NAFLD patients | To verify if probiotics can be considered as a potential therapy for non-alcoholic fatty liver disease (NAFLD) | The use of probiotics was evaluated from 4 to 28 weeks. Overall, probiotic therapy had beneficial effects on body mass index (WMD: −1.46, 95% CI: [−2.44, −0.48]), alanine aminotransferase (WMD: −13.40, 95% CI: [−17.03, −9.77]), aspartate transaminase (WMD: −13.54, 95% CI: [−17.86, −9.22]), gamma-glutamyl transpeptidase (WMD: −9.88, 95% CI: [−17.77, −1.99]), insulin (WMD: −1.32, 95% CI: [−2.43, −0.21]), homeostasis model assessment insulin resistance (WMD: −0.42, 95% CI: [−0.73, −0.12]), and total cholesterol (WMD: −15.38, 95% CI: [−26.50, −4.25]), but not fasting blood sugar, lipid profiles, or tumor necrosis factor-alpha. | The systematic review and meta-analysis support that probiotics are superior to placebo in NAFLD patients and could be utilized as a common complementary therapeutic approach. | High | ||
| Loman, B.R et al., 2018 [158] | Systematic review and meta-analysis | 25 RCTs | 1309 patients | To systemically review and quantitatively synthesize evidence on prebiotic, probiotic, and synbiotic therapies for patients with NAFLD in randomized controlled trials | Meta-analysis indicated that microbial therapies significantly reduced BMI (−0.37 kg/m2; 95% confidence interval [CI], −0.46 to −0.28; p < 0.001), hepatic enzymes (ALT, −6.9 U/L [95%CI, −9.4 to −4.3]; AST, −4.6 U/L [95%CI, −6.6 to −2.7]; γ-GT, −7.9 U/L [95%CI, −11.4 to −4.4]; p < 0.001), serum cholesterol (−10.1 mg/dL 95%CI, −13.6 to −6.6; p < 0.001), LDL-c (−4.5 mg/dL; 95%CI, −8.9 to −0.17; p < 0.001), and TAG (−10.1 mg/dL; 95%CI, −18.0 to −2.3; p < 0.001), but not inflammation (TNF-α, −2.0 ng/mL; [95%CI, −4.7 to 0.61]; CRP, −0.74 mg/L [95%CI, −1.9 to 0.37]). Subgroup analysis by treatment category indicated similar effects of prebiotics and probiotics on BMI and liver enzymes but not total cholesterol, HDL-c, and LDL-c. | This meta-analysis supports the potential use of microbial therapies in the treatment of NAFLD and sheds light on their potential mode of action. Further research into these treatments should consider the limitations of biomarkers currently used for the diagnosis and progression of NAFLD, in addition to the inherent challenges of personalized microbial-based therapies. | High | ||
| Khan, M.Y. et al., 2019 [159] | Systematic review and meta-analysis | 12 randomized controlled trials | 624 subjects | To study the effect of probiotics/synbiotics on various laboratory and radiographic parameters in NAFLD management | The intervention arm, which comprising the probiotic and/or the synbiotic arm, showed a significant improvement in alanine aminotransferase levels (MD = −13.93, confidence interval (CI) = −20.20 to −7.66, p value of less than 0.0001, I = 92%) and aspartate aminotransferase levels (MD = −11.45, CI = −15.15 to −7.74, p value of less than 0.00001, I = 91%). There was a reduction in high-sensitivity C-reactive protein levels in the intervention arm (SMD = −0.68, CI = −1.10 to −0.26, p value of 0.001, I = 0%). The liver fibrosis score improved in the intervention arm (MD = −0.71, CI = −0.81 to −0.61, p value less than 0.00001, I = 0%). | Probiotic/synbiotic use improves aminotransaminase levels and reduces pro-inflammatory marker high-sensitivity C-reactive protein and liver fibrosis in NAFLD patients. | High | ||
| Sharpton, S.R. et al., 2019 [160] | Systematic review and meta-analysis | 21 RCTs | 1252 subjects | To evaluate the most current evidence for liver-specific and metabolic effects of microbiome-targeted therapies (MTTs) in persons with NAFLD | Probiotics/synbiotics were associated with a significant reduction in alanine aminotransferase activity [ALT, weighted mean difference (WMD): −11.23 IU/L; 95% CI: −15.02, −7.44 IU/L] and liver stiffness measurement (LSM) by elastography (reflecting inflammation and fibrosis) (WMD: −0.70 kPa; 95% CI: −1.00, −0.40 kPa), although analyses showed heterogeneity (I2 = 90.6% and I2 = 93.4%, respectively). Probiotics/synbiotics were also associated with increased odds of improvement in hepatic steatosis, as graded by ultrasound (OR: 2.40; 95% CI: 1.50, 3.84; I2 = 22.4%). No RCTs examined sequential liver biopsy findings. Probiotics (WMD: −1.84; 95% CI: −3.30, −0.38; I2 = 23.6%), but not synbiotics (WMD: −0.85; 95% CI: −2.17, 0.47; I2 = 96.6%), were associated with a significant reduction in body mass index. | The use of probiotics/synbiotics was associated with improvement in liver-specific markers of hepatic inflammation, LSM, and steatosis in persons with NAFLD. Although promising, given the heterogeneity in pooled analyses, additional well-designed RCTs are needed to define the efficacy of probiotics/synbiotics for treatment of NAFLD. | High | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Borromeo, S.; Cavioni, A.; Gasparri, C.; Gattone, I.; Genovese, E.; Lazzarotti, A.; Minonne, L.; Moroni, A.; Patelli, Z.; et al. Therapeutic Strategies to Modulate Gut Microbial Health: Approaches for Chronic Metabolic Disorder Management. Metabolites 2025, 15, 127. https://doi.org/10.3390/metabo15020127
Rondanelli M, Borromeo S, Cavioni A, Gasparri C, Gattone I, Genovese E, Lazzarotti A, Minonne L, Moroni A, Patelli Z, et al. Therapeutic Strategies to Modulate Gut Microbial Health: Approaches for Chronic Metabolic Disorder Management. Metabolites. 2025; 15(2):127. https://doi.org/10.3390/metabo15020127
Chicago/Turabian StyleRondanelli, Mariangela, Sara Borromeo, Alessandro Cavioni, Clara Gasparri, Ilaria Gattone, Elisa Genovese, Alessandro Lazzarotti, Leonardo Minonne, Alessia Moroni, Zaira Patelli, and et al. 2025. "Therapeutic Strategies to Modulate Gut Microbial Health: Approaches for Chronic Metabolic Disorder Management" Metabolites 15, no. 2: 127. https://doi.org/10.3390/metabo15020127
APA StyleRondanelli, M., Borromeo, S., Cavioni, A., Gasparri, C., Gattone, I., Genovese, E., Lazzarotti, A., Minonne, L., Moroni, A., Patelli, Z., Razza, C., Sivieri, C., Valentini, E. M., & Barrile, G. C. (2025). Therapeutic Strategies to Modulate Gut Microbial Health: Approaches for Chronic Metabolic Disorder Management. Metabolites, 15(2), 127. https://doi.org/10.3390/metabo15020127








