Association of Firmicutes/Bacteroidetes Ratio with Body Mass Index in Korean Type 2 Diabetes Mellitus Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Anthropometry and Body Composition
2.3. Nutritional Intake Analysis
2.4. Biochemical Analysis
2.5. Sample Collection and DNA Extraction
2.6. PCR Amplification and NGS Sequencing
2.7. Statistical Analysis
3. Results
3.1. General Characteristic and Anthropometric Parameters
3.2. Nutritional Intakes
3.3. Glycemic Indices and Lipid Profiles
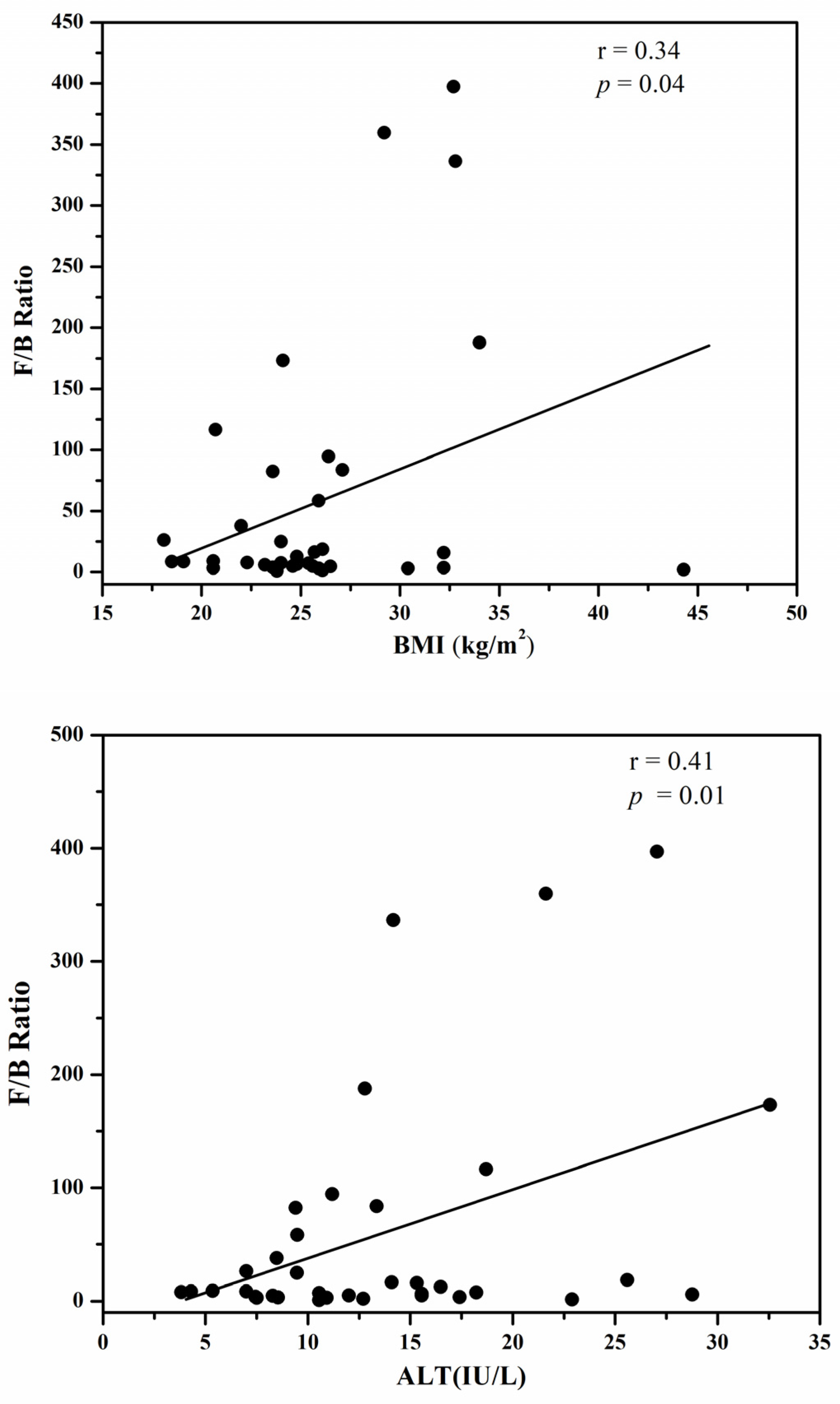
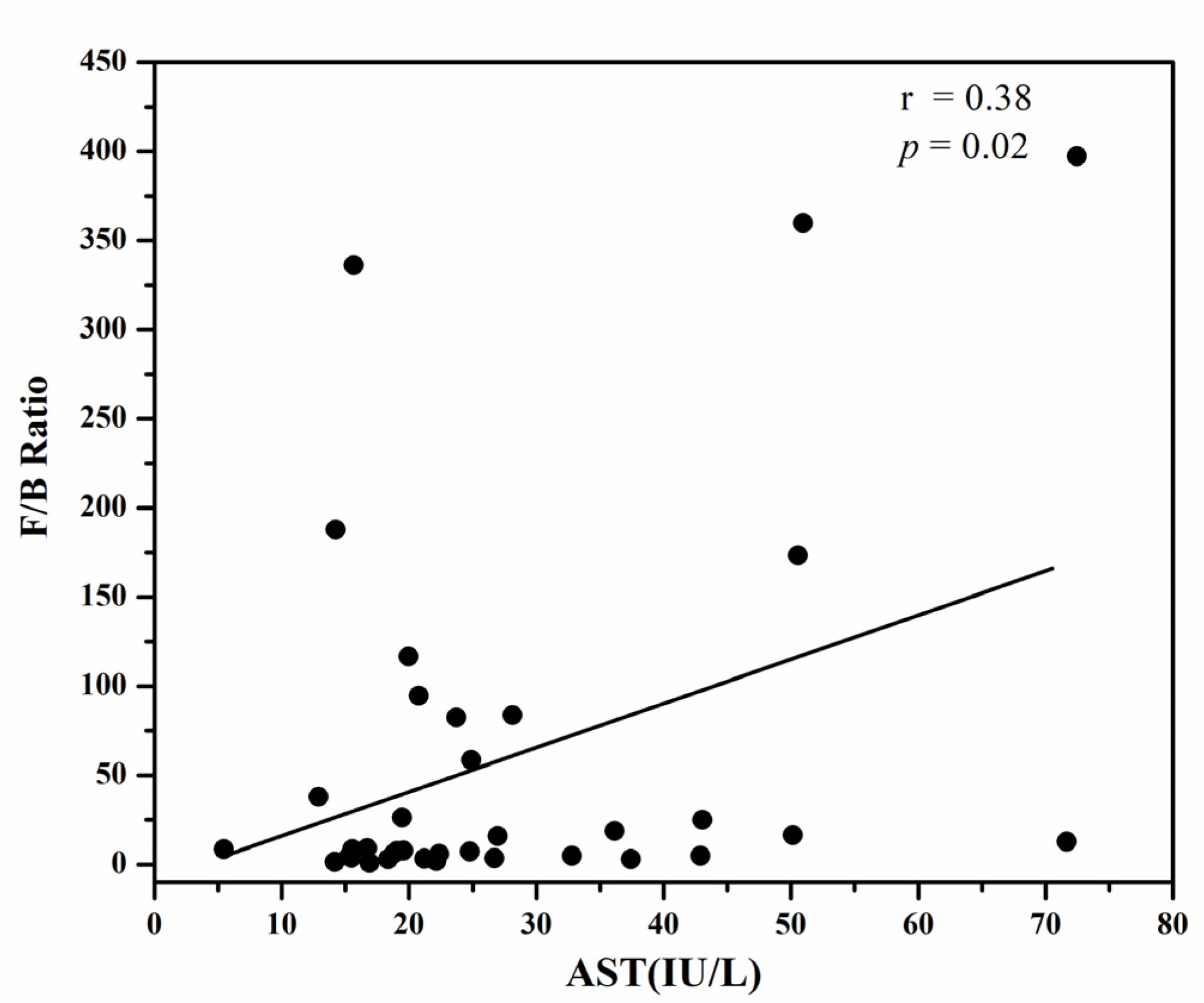
3.4. The Firmicutes/Bacteroidetes Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putignani, L.; Del Chierico, F.; Petrucca, A.; Vernocchi, P.; Dallapiccola, B. The human gut microbiota: A dynamic interplay with the host from birth to senescence settled during childhood. Pediatr. Res. 2014, 76, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lal, R. Survey of (Meta) genomic approaches for understanding microbial community dynamics. Indian J. Microbiol. 2017, 57, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.N.; Young, V.B. Gastrointestinal microbial ecology with perspectives on health and disease. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018; pp. 737–753. [Google Scholar]
- Shapira, M. Gut microbiotas and host evolution: Scaling up symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef]
- Robinson, C.J.; Bohannan, B.J.; Young, V.B. From structure to function: The ecology of host-associated microbial communities. Microbiol. Mol. Biol. Rev. 2010, 74, 453–476. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.A.; Ochman, H.; Hammer, T.J. Systematics. Evolutionary and ecological consequences of gut microbial communities. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 451–475. [Google Scholar] [CrossRef]
- Khan, I.; Yasir, M.; Azhar, E.I.; Kumosani, T.; Barbour, E.K.; Bibi, F.; Kamal, M.A. Implication of gut microbiota in human health. CNS Neurol. Disord. Drug Targets 2014, 13, 1325–1333. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef]
- Fluitman, K.S.; De Clercq, N.C.; Keijser, B.J.; Visser, M.; Nieuwdorp, M.; IJzerman, R.G. The intestinal microbiota, energy balance, and malnutrition: Emphasis on the role of short-chain fatty acids. Expert Rev. Endocrinol. Metab. 2017, 12, 215–226. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Modulation of gut microbiota in the management of metabolic disorders: The prospects and challenges. Int. J. Mol. Sci. 2014, 15, 4158–4188. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Million, M.; Hugon, P.; Armougom, F.; Raoult, D. Human gut microbiota: Repertoire and variations. Front. Cell. Infect. Microbiol. 2012, 2, 136. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Doré, J.; Blottière, H. The influence of diet on the gut microbiota and its consequences for health. Curr. Opin. Biotechnol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053. [Google Scholar] [CrossRef]
- Tyagi, A.M. Mechanism of action of gut microbiota and probiotic Lactobacillus rhamnosus GG on skeletal remodeling in mice. Endocrinol. Diabetes Metab. 2024, 7, e440. [Google Scholar] [CrossRef]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Karami, R.; Kermansaravi, M.; Pishgahroudsari, M.; Talebi, M.; Mohammadzadeh, N.; Pazouki, A. Changes in gut microbial flora after Roux-en-Y gastric bypass and sleeve gastrectomy and their effects on post-operative weight loss. Updates Surg. 2021, 73, 1493–1499. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control. 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Wang, Y.; Beydoun, M.A. The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol. Rev. 2007, 29, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Devaux, M.; Cecchini, M.; Rusticelli, E. The Obesity Epidemic: Analysis of Past and Projected Future Trends in Selected OECD Countries; OECD Health Working Papers, No. 45; OECD Publishing: Paris, Franch, 2009. [Google Scholar]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic syndrome as a risk factor for neurological disorders. Cell. Mol. Life Sci. 2012, 69, 741–762. [Google Scholar] [CrossRef]
- Ahmed, K.; Choi, H.N.; Yim, J.E. The Impact of Taurine on Obesity-Induced Diabetes Mellitus: Mechanisms Underlying Its Effect. Endocrinol. Metab. 2023, 38, 482–492. [Google Scholar] [CrossRef]
- Choudhary, D.; Gupta, P.; Gupta, S. Diabetes Prediction Using Machine Learning Classifiers. In Machine Learning, Image Processing, Network Security and Data Sciences, Proceedings of the 4th International Conference, MIND 2022, Virtual Event, 19–20 January 2023; Springer: Berlin/Heidelberg, Germany, 2023; pp. 140–148. [Google Scholar]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Maiuolo, J.; Bulotta, R.M.; Ruga, S.; Nucera, S.; Macrì, R.; Scarano, F.; Oppedisano, F.; Carresi, C.; Gliozzi, M.; Musolino, V. The Postbiotic Properties of Butyrate in the Modulation of the Gut Microbiota: The Potential of Its Combination with Polyphenols and Dietary Fibers. Int. J. Mol. Sci. 2024, 25, 6971. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Hippe, B.; Zanner, J.; Aumueller, E.; Brath, H.; G Haslberger, A. Gut microbiota of obese, type 2 diabetic individuals is enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after weight loss. Endocr. Metab. Immune Disord. Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2016, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, Y.E.; Esquivel-Hernández, D.A.; Sánchez-Castañeda, J.P.; Neri-Rosario, D.; Guardado-Mendoza, R.; Resendis-Antonio, O. Type 2 diabetes, gut microbiome, and systems biology: A novel perspective for a new era. Gut Microbes 2022, 14, 2111952. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and gut immune system: A good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Cardona, F.; Tinahones, F.J.; Queipo-Ortuño, M. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front. Microbiol. 2014, 5, 190. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Diamant, M.; Blaak, E.; De Vos, W. Do nutrient–gut–microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 2011, 12, 272–281. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe 2019, 26, 252–264.e210. [Google Scholar] [CrossRef]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Worldwide epidemic of obesity. In Obesity and Obstetrics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: Current evidence and perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Companys, J.; Gosalbes, M.J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Pedret, A.; Valls, R.M.; Jiménez-Hernández, N.; Sandoval-Ramirez, B.A.; Del Bas, J.M. Gut microbiota profile and its association with clinical variables and dietary intake in overweight/obese and lean subjects: A cross-sectional study. Nutrients 2021, 13, 2032. [Google Scholar] [CrossRef]
- Dhanda, S.; Kaur, S.; Sandhir, R. Preventive effect of N-acetyl-L-cysteine on oxidative stress and cognitive impairment in hepatic encephalopathy following bile ductligation. Free. Radic. Biol. Med. 2013, 56, 204–215. [Google Scholar] [CrossRef]
| Groups | ||||
|---|---|---|---|---|
| I (N = 8) | II (N = 10) | III (N = 11) | IV (N = 7) | |
| Age (year) | 44.8 ± 11.7 | 50.0 ± 8.6 | 43.3 ± 8.8 | 39.7 ± 12.4 |
| Male (n, %) | 3 (37.5) | 7 (70.0) | 8 (72.7) | 6 (85.7) |
| Female (n, %) | 5 (83.2) | 3 (37.5) | 3 (37.5) | 1 (12.5) |
| BW (kg) | 54.0 ± 6.2 a | 67.4 ± 6.6 b | 75.8 ± 8.7 b | 99.1 ± 19.4 c |
| Height (cm) | 163.1 ± 5.9 | 167.1 ± 7.5 | 169.3 ± 8.8 | 170.0 ± 8.1 |
| BMI (kg/m2) | 20.2 ± 1.5 a | 24.1 ± 0.5 b | 26.4 ± 1.1 b | 34.1 ± 4.6 c |
| BFM (kg) | 13.0 ± 2.8 a | 16.3 ± 2.2 ab | 22.3 ± 4.2 b | 38.1 ± 14.0 c |
| Waist (cm) | 74.1 ± 11.5 a | 84.4 ± 3.2 ab | 90.0 ± 5.2 ab | 106.2 ± 10.0 b |
| Hip (cm) | 85.2 ± 6.8 a | 93.5 ± 4.8 ab | 97.9 ± 4.4 ab | 109.4 ± 11.2 b |
| WHR | 0.91 ± 0.1 | 0.93 ± 0.0 | 0.96 ± 0.0 | 1.02 ± 0.0 |
| Groups | ||||
|---|---|---|---|---|
| I (N = 8) | II (N = 10) | III (N = 11) | IV (N = 7) | |
| Energy (kcal) | 1560.2 ±379.0 | 1665.0 ± 407.2 | 1557.2 ± 289.5 | 2026.5 ± 810.5 |
| Carbohydrate (g) | 208.9 ± 77.7 | 269.0 ± 65.6 | 228.2 ± 48.7 | 248.6 ± 115.5 |
| Protein (g) | 60.8 ± 4.2 a | 63.0 ± 6.4 a | 62.0 ± 4.9 a | 94.2 ± 13.6 b |
| Fat (g) | 51.9 ± 20.1 ab | 38.3 ± 16.6 a | 44.3 ± 14.0 a | 70.6 ± 32.6 b |
| CHO/Pro/Fat (%) | 52.5:16.0:31.2 | 64.9:15.2:20.7 | 58.8:15.8:25.4 | 48.9:19.1:31.7 |
| Fiber (g) | 15.8 ± 7.1 | 22.0 ± 7.5 | 17.7 ± 4.9 | 20.7 ± 11.0 |
| Cholesterol (mg) | 162.7 ± 80.6 | 203.7 ± 146.6 | 160.2 ± 106.3 | 245.5 ± 107.3 |
| Groups | ||||
|---|---|---|---|---|
| I (N = 8) | II (N = 10) | III (N = 11) | IV (N = 7) | |
| Glucose (mg/dL) | 129.4 ± 15.1 | 118.2 ± 5.9 | 134.2 ± 9.7 | 127.4 ± 13.2 |
| Insulin (μlU/mL) | 6.3 ± 1.2 | 9.8 ± 1.8 | 6.2 ± 0.8 | 5.6 ± 0.6 |
| HbA1c (%) | 7.7 ± 1.8 | 7.1 ± 0.7 | 7.5 ± 2.4 | 8.2 ± 2.1 |
| HOMA-IR | 2.1 ± 0.6 | 2.9 ± 0.6 | 1.9 ± 0.2 | 1.7 ± 0.3 |
| TC (mg/dL) | 180.7 ± 30.5 | 186.4 ± 26.6 | 158.3 ± 46.8 | 190.2 ± 20.9 |
| TG (mg/dL) | 59.5 ± 30.0 a | 215.5 ± 150.2 b | 185.7 ± 74.3 b | 168.8 ± 53.4 b |
| HDL-C (mg/dL) | 34.0 ± 11.1 | 35.0 ± 8.8 | 30.8 ± 12.4 | 30.0 ± 7.2 |
| LDL-C (mg/dL) | 55.5 ± 10.1 | 60.2 ± 9.9 | 49.7 ± 15.9 | 62.4 ± 12.7 |
| Atherogenic index | 4.7 ± 1.7 | 4.7 ± 1.8 | 4.5 ± 1.6 | 5.6 ± 1.4 |
| ALT (IU/L) | 7.9 ± 4.7 a | 16.4 ± 8.4 b | 14.2 ± 6.3 ab | 15.8 ± 5.4 b |
| AST (IU/L) | 16.3 ± 5.2 | 32.5 ± 18.7 | 28.8 ± 12.7 | 30.8 ± 20.0 |
| Group | ||||
|---|---|---|---|---|
| I (N = 8) | II (N = 10) | III (N = 11) | IV (N = 7) | |
| Bacteroidetes (count) | 4676.6 ± 4349.2 | 8357.3 ± 9130.2 | 5702.7± 6959.3 | 5805.3 ± 6666.1 |
| Firmicutes (count) | 43,055.9 ± 10,213.7 | 45,515.9 ± 10,314.0 | 42,069.9 ± 16,139.8 | 36,276.3 ± 9154.8 |
| F/B ratio | 27.1 ± 37.9 | 32.2 ± 55.1 | 59.3 ± 105.3 | 135.0 ± 172.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, K.; Choi, H.-N.; Cho, S.-R.; Yim, J.-E. Association of Firmicutes/Bacteroidetes Ratio with Body Mass Index in Korean Type 2 Diabetes Mellitus Patients. Metabolites 2024, 14, 518. https://doi.org/10.3390/metabo14100518
Ahmed K, Choi H-N, Cho S-R, Yim J-E. Association of Firmicutes/Bacteroidetes Ratio with Body Mass Index in Korean Type 2 Diabetes Mellitus Patients. Metabolites. 2024; 14(10):518. https://doi.org/10.3390/metabo14100518
Chicago/Turabian StyleAhmed, Kainat, Ha-Neul Choi, Sung-Rae Cho, and Jung-Eun Yim. 2024. "Association of Firmicutes/Bacteroidetes Ratio with Body Mass Index in Korean Type 2 Diabetes Mellitus Patients" Metabolites 14, no. 10: 518. https://doi.org/10.3390/metabo14100518
APA StyleAhmed, K., Choi, H.-N., Cho, S.-R., & Yim, J.-E. (2024). Association of Firmicutes/Bacteroidetes Ratio with Body Mass Index in Korean Type 2 Diabetes Mellitus Patients. Metabolites, 14(10), 518. https://doi.org/10.3390/metabo14100518







