The Impact of Negative Energy Balance in Holstein-Friesian Cows on the Blood Concentrations of Interleukin-6 and Plasminogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing Conditions and Health of Cows
2.2. Control of the Diet and Body Conditions of Cows
2.3. Collection of Material for Analysis
2.4. Analytical Procedures
2.5. Statistical Analysis
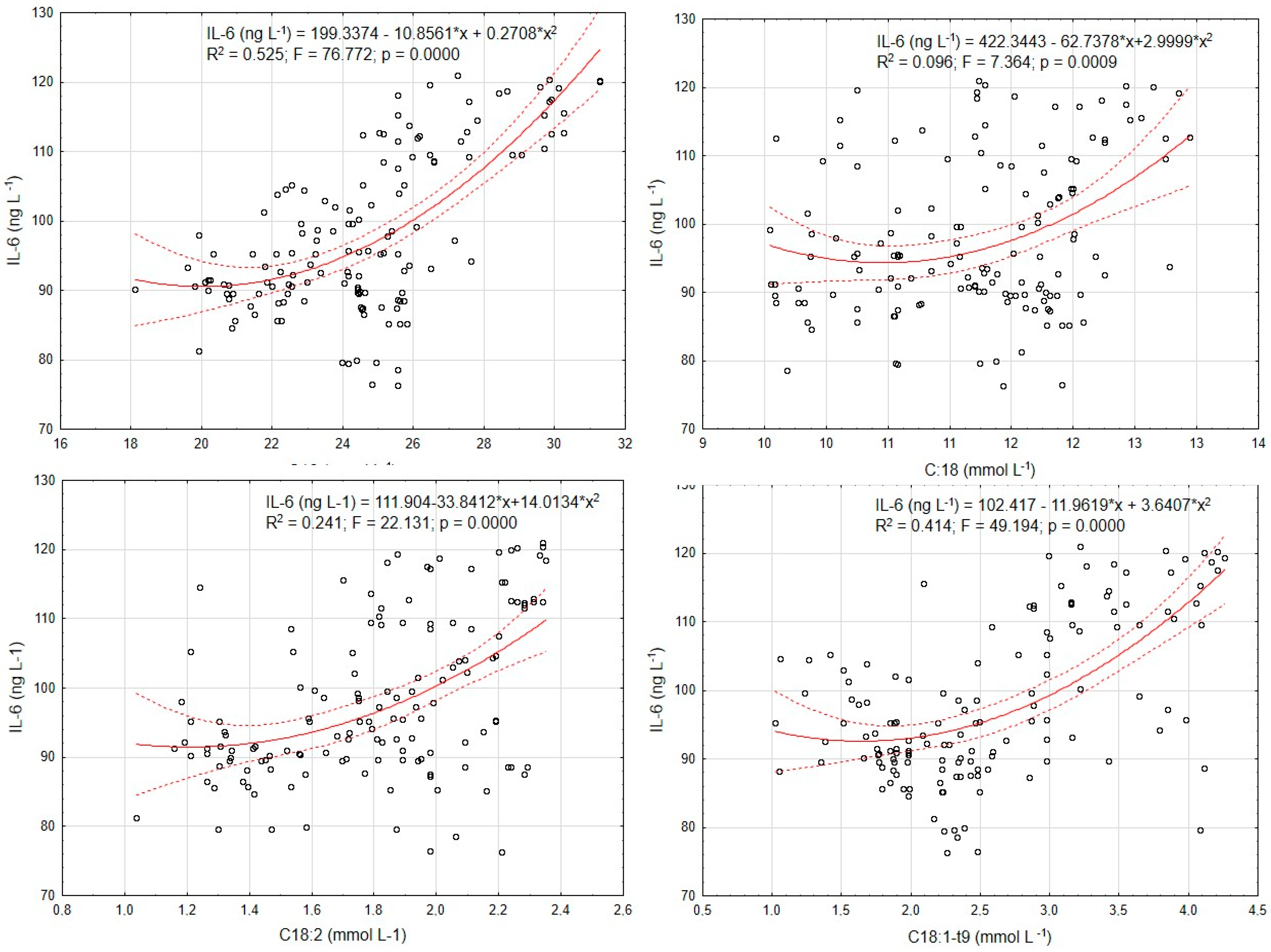
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EB | energy balance |
| AT | adipose tissue |
| BCS | body condition of cows |
| BCS% | rate of loss of body condition |
| BHBA | β-hydroxybutyrate |
| DMI | dry matter intake |
| DMP | daily milk production |
| FA | fatty acids |
| GLU | glucose |
| HF | Holstein-Friesian cows |
| IL-6 | interleukin 6 |
| LEP | leptin |
| LSM | least squares method |
| NEB | negative energy balance |
| NEFAs | non-esterified fatty acids |
| PL | plasminogen |
| SEM | standard error of the mean |
| SL | stages of lactation |
| TMR | total mixed ration |
References
- Adewuyi, A.A.; Gruys, E.; van Eerdenburg, F.J. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet. Q. 2005, 27, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Olsen, J.R.; Jeffress, E.J.; Moore, D.A.; Kastelic, J.P. Associations among serum pro-and anti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reprod. Biol. Endocrinol. 2013, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Breukink, H.J.; Wensing, T. Pathophysiology of the liver in high yielding dairy cows and its consequences for health and production. Bov. Pract. 1998, 52, 73–78. [Google Scholar] [CrossRef]
- Loor, J.J.; Everts, R.E.; Bionaz, M.; Dann, H.M.; Morin, D.E.; Oliveira, R.; Rodriguez-Zas, S.L.; Drackley, J.K.; Lewin, H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007, 32, 105–116. [Google Scholar] [CrossRef]
- Gordon, J.L.; Leblanc, S.J.; Duffield, T.F. Ketosis treatment in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 433–445. [Google Scholar] [CrossRef]
- Kuhla, B.; Metges, C.C.; Hammon, H.M. Endogenous and dietary lipids influencing feed intake and energy metabolism of periparturient dairy cows. Domest. Anim. Endocrinol. 2016, 56, S2–S10. [Google Scholar] [CrossRef]
- Turk, R.; Juretić, D.; Geres, D.; Svetina, A.; Turk, N.; Flegar-Mestrić, Z. Influence of oxidative stress and metabolic adaptation on PON1 activity and MDA level in transition dairy cows. Anim. Reprod. Sci. 2008, 108, 98–106. [Google Scholar] [CrossRef]
- Keane, C.J.; Hanlon, A.J.; Roche, J.F.; Burton, J.L.; Mee, J.F.; O’Doherty, J.V.; Sweeney, T. Short communication: A potential antiapoptotic phenotype in neutrophils of cows milked once daily in early lactation. J. Dairy Sci. 2006, 89, 1024–1027. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- van Knegsel, A.T.; de Vries Reilingh, G.; Meulenberg, S.; van den Brand, H.; Dijkstra, J.; Kemp, B.; Parmentier, H.K. Natural antibodies related to energy balance in early lactation dairy cows. J. Dairy Sci. 2007, 90, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–920. [Google Scholar] [CrossRef]
- Loskutoff, D.J.; van Mourik, J.A.; Erickson, L.A.; Lawrence, D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc. Natl. Acad. Sci. USA 1983, 80, 2956–2960. [Google Scholar] [CrossRef] [PubMed]
- Zorio, E.; Gilabert-Estellés, J.; España, F.; Ramón, L.A.; Cosín, R.; Estellés, A. Fibrinolysis: The key to new pathogenetic mechanisms. Curr. Med. Chem. 2008, 15, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Wankhade, P.R.; Manimaran, A.; Kumaresan, A.; Jeyakumar, S.; Sejian, V.; Rajendran, D.; Bagath, M.; Sivaram, M.; Ramesha, K.P.; Varghese, M.R. Active immune system and dry matter intake during the transition period are associated with postpartum fertility in lactating Zebu cows. Livest. Sci. 2019, 228, 18–24. [Google Scholar] [CrossRef]
- Keragala, C.B.; Medcalf, R.L. Plasminogen: An enigmatic zymogen. Blood 2021, 137, 2881–2889. [Google Scholar] [CrossRef]
- Chana-Muñoz, A.; Jendroszek, A.; Sønnichsen, M.; Wang, T.; Ploug, M.; Jensen, J.K.; Andreasen, P.A.; Bendixen, C.; Panitz, F. Origin and diversification of the plasminogen activation system among chordates. BMC Evol. Biol. 2019, 19, 27. [Google Scholar] [CrossRef]
- Barthel, D.; Schindler, S.; Zipfel, P.F. Plasminogen is a complement inhibitor. J. Biol. Chem. 2012, 287, 18831–18842. [Google Scholar] [CrossRef]
- Baker, S.K.; Strickland, S. A critical role for plasminogen in inflammation. J. Exp. Med. 2020, 217, e20191865. [Google Scholar] [CrossRef]
- Faty, A.; Ferré, P.; Commans, S. The acute phase protein Serum Amyloid A induces lipolysis and inflammation in human adipocytes through distinct pathways. PLoS ONE 2012, 7, e34031. [Google Scholar] [CrossRef] [PubMed]
- Rega, G.; Kaun, C.; Weiss, T.W.; Demyanets, S.; Zorn, G.; Kastl, S.P.; Steiner, S.; Seidinger, D.; Kopp, C.W.; Frey, M.; et al. Inflammatory cytokines interleukin-6 and oncostatin m induce plasminogen activator inhibitor-1 in human adipose tissue. Circulation 2005, 111, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Contreras, G.A.; Strieder-Barboza, C.; De Koster, J. Symposium review: Modulating adipose tissue lipolysis and remodeling to improve immune function during the transition period and early lactation of dairy cows. J. Dairy Sci. 2018, 101, 2737–2752. [Google Scholar] [CrossRef]
- Karis, P.; Jaakson, H.; Ling, K.; Bruckmaier, R.M.; Gross, J.J.; Pärn, P.; Kaart, T.; Ots, M. Body condition and insulin resistance interactions with periparturient gene expression in adipose tissue and lipid metabolism in dairy cows. J. Dairy Sci. 2020, 103, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Sabzikar, Z.; Mohri, M.; Seifi, H.A. Variations of some adipokines, pro-inflammatory cytokines, oxidative stress biomarkers, and energy characteristics during the transition period in dairy cows. Vet. Res. Forum Int. Q. J. 2023, 14, 87–95. [Google Scholar] [CrossRef]
- Kruithof, E.K. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb. Haemost. 2008, 100, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Loskutoff, D.J.; Samad, F. The adipocyte and hemostatic balance in obesity: Studies of PAI-1. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-Reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Zachut, M. Defining the Adipose Tissue Proteome of Dairy Cows to Reveal Biomarkers Related to Peripartum Insulin Resistance and Metabolic Status. J. Proteome Res. 2015, 14, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Strzetelski, J.E.D. The Nutritional Value of French and National Feed fur Ruminants; Instytut Zootechniki PIB.; National Research Institute of Animal Production INRA Norm of Ruminant Nutrition: Kraków, Poland, 2009.
- Sjaunja, L.O.; Baevre, L.; Junkkarinen, L.; Pedersen, J.; Setälä, J.A. Nordic proposal for an energy corrected milk (ECM) formula. In Proceedings of the 7th Session International Committee for Recording and Productivity of Milk Animals, Paris, France, 2–6 July 1990. [Google Scholar]
- Thompson, A.; Taylor, B. Guide for the Use of the International System of Units (SI); Special Publication (NIST SP); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008. [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th rev. ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Wildman, E.E.; Jones, G.M.; Wagner, P.E.; Boman, R.; Troutt, H.F.; Lesch, T.N. A Dairy Cow Body Condition Scoring System and Its Relationship to Selected Production Characteristics. J. Dairy Sci. 1982, 65, 495–501. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kessel, S.; Stroehl, M.; Meyer, H.H.D.; Hiss, S.; Sauerwein, H.; Schwarz, F.J.; Bruckmaier, R.M. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J. Anim. Sci. 2008, 86, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.; Overton, T.; Douglas, G. Adaptations of Glucose and Long-Chain Fatty Acid Metabolism in Liver of Dairy Cows during the Periparturient Period. J. Dairy Sci. 2001, 84, 110–112. [Google Scholar] [CrossRef]
- Schoenberg, K.M.; Overton, T.R. Effects of plane of nutrition and 2,4-thiazolidinedione on insulin responses and adipose tissue gene expression in dairy cattle during late gestation. J. Dairy Sci. 2011, 94, 6021–6035. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.E.; LeBlanc, S.J.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.; Overton, M.W.; Duffield, T.F. The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. J. Dairy Sci. 2012, 95, 1301–1309. [Google Scholar] [CrossRef]
- Greenfield, R.B.; Cecava, M.J.; Donkin, S.S. Changes in mRNA expression for gluconeogenic enzymes in liver of dairy cattle during the transition to lactation. J. Dairy Sci. 2000, 83, 1228–1236. [Google Scholar] [CrossRef]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Sauerwein, H.; Bruckmaier, R.M.; Becker, F.; Kanitz, W.; et al. Hepatic gene expression involved in glucose and lipid metabolism in transition cows: Effects of fat mobilization during early lactation in relation to milk performance and metabolic changes. J. Dairy Sci. 2013, 96, 5670–5681. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. Int. Union Biochem. Mol. Biol. Life 2010, 62, 869–877. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Çolakoğlu, H.E.; Polat, İ.M.; Vural, M.R.; Kuplulu, S.; Pekcan, M.; Yazlık, M.O.; Baklaci, C.U. Associations between leptin, body condition score, and energy metabolites in Holstein primiparous and multiparous cows from 2 to 8 weeks postpartum. Rev. Médecine Vétérinaire 2017, 168, 93–101. [Google Scholar]
- Roh, S.G.; Suzuki, Y.; Gotoh, T.; Tatsumi, R.; Katoh, K. Physiological Roles of Adipokines, Hepatokines, and Myokines in Ruminants. Asian-Australas. J. Anim. Sci. 2016, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kabara, E.; Sordillo, L.M.; Holcombe, S.; Contreras, G.A. Adiponectin links adipose tissue function and monocyte inflammatory responses during bovine metabolic stress. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, J. Regulation of energy balance by inflammation: Common theme in physiology and pathology. Rev. Endocr. Metab. Disord. 2015, 16, 47–54. [Google Scholar] [CrossRef]
- Ye, J.; McGuinness, O.P. Inflammation during obesity is not all bad: Evidence from animal and human studies. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 466–477. [Google Scholar] [CrossRef]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75–79. [Google Scholar] [CrossRef]
| Parameter | F1 | F2 | F3 |
|---|---|---|---|
| Number of cows | 15 | 18 | 22 |
| Dry matter | 43.1 | 42.2 | 42.9 |
| Protein | 16.4 | 16.9 | 17.1 |
| Fibre | 19.2 | 18.9 | 19.4 |
| Fat | 2.5 | 2.4 | 2.6 |
| Ash | 8.0 | 7.8 | 8.3 |
| Starch | 22.7 | 23.1 | 22.8 |
| Acid detergent fibre—ADF (%) | 22.8 | 22.4 | 22.6 |
| Neutral detergent fibre—NDF (%) | 39.5 | 38.6 | 38.9 |
| Physically effective NDF—peNDF (%) | 30.6 | 30.8 | 31.1 |
| UFL | 21.5 | 20.5 | 21.3 |
| PDIN (g) | 2459 | 2318 | 2473 |
| PDIE (g) | 2201 | 2197 | 2185 |
| Energy (MJ NEL): | |||
| Requirement | 151.7 | 145.3 | 149.8 |
| Intake | 153.2 | 147.4 | 151.6 |
| Balance | +1.5 | +2.1 | +1.8 |
| Dry matter intake—DMI (kg/day) | 23.1 | 24.4 | 23.5 |
| Trait | Stage of Lactation (SL) | SEM | Correlation DL x | |||
|---|---|---|---|---|---|---|
| SL1 | SL2 | SL3 | SL4 | |||
| Days of lactation (DL) | 22.6 d | 45.4 c | 67.2 b | 152.9 a | 9.2 | − |
| EB (MJNEL/d) | −7.7 c | −11.3 b | −13.6 a | 2.3 d | 3.8 | 0.658 * |
| DMP (kg) | 23.5 d | 32.4 b | 36.9 a | 29.8 c | 0.6 | −0.467 * |
| Contents in milk (%): | ||||||
| Protein | 3.42 a | 3.28 b | 3.27 b | 3.44 a | 0.22 | −0.438 * |
| Fat | 4.31 a | 4.12 c | 4.14 c | 4.25 b | 0.34 | −0.345 * |
| Lactose | 4.91 b | 5.12 a | 5.03 a | 4.88 b | 0.19 | −0.294 * |
| BHBA (mmol L−1) | 0.638 c | 0.998 b | 1.424 a | 0.968 b | 0.026 | 0.375 * |
| GLU (mmol L) | 2.70 a | 2.37 b | 2.21 c | 2.36 b | 0.12 | −0.345 * |
| LEP (ng mL−1) | 2.57 c | 2.82 b | 2.92 a | 2.55 c | 0.09 | −0.344 * |
| Parameter | Stage of Lactation (SL) | SEM | Correlation DL x | |||
|---|---|---|---|---|---|---|
| SL1 | SL2 | SL3 | SL4 | |||
| BCS | 2.61 a | 2.34 b | 1.97 c | 2.09 c | 0.12 | 0.361 * |
| BCS% | −4.75 c | −10.36 b | −15.67 a | +6.49 c | 0.19 | −0.553 * |
| NEFA: (mmol L−1) | ||||||
| C16:0 | 23.21 c | 26.43 b | 28.24 a | 24.95 c | 0.23 | −0.435 * |
| C18:0 | 11.35 a | 11.39 a | 11.42 a | 10.72 b | 0.07 | −0.458 * |
| C18:1-t9 | 1.81 d | 3.16 b | 3.58 a | 2.36 c | 0.11 | −0.451 * |
| C18:2 | 1.68 c | 1.92 b | 2.10 a | 1.89 b | 0.09 | −0.432 * |
| IL-6 (ng L−1) | 94.46 c | 105.04 b | 111.58 a | 108.89 b | 0.94 | −0.257 * |
| PL (ng L−1) | 2.04 c | 2.50 b | 2.79 a | 2.53 b | 0.05 | −0.395 * |
| Parameter | SL1—3 (DL) | SL4 (DL) | GLU | LEP | IL-6 | EB |
|---|---|---|---|---|---|---|
| BCS% | 0.521 * | −0.621 * | −0.341 * | 0.395 * | 0.473 * | 0.762 * |
| DMP (kg) | 0.447 * | −0.343 * | −0.826 * | 0.534 * | 0.518 * | 0.643 * |
| NEFAs: (mmol L−1) | ||||||
| C16:0 | 0.447 * | −0.328 * | −0.536 * | 0.643 * | 0.638 * | 0.636 * |
| C18:0 | 0.628 * | −0.455 * | −0.327 * | 0.387 * | 0.386 * | 0.263 * |
| C18:1-t9 | 0.521 * | −0.334 * | −0.533 * | 0.432 * | 0.431 * | 0.519 * |
| C18:2 | 0.487 * | −0.434 * | −0.519 * | 0.392 * | 0.572 * | 0.378 * |
| BHBA (mmol L−1) | 0.411 * | −0.224 * | −0.648 * | 0.524 * | 0.535 * | 0.493 * |
| Glucose (mmol L−1) | −0.422 * | 0.358 * | − | −0.487 * | −0.414 * | −0.356 * |
| Leptin (ng mL−1) | 0.527 * | −0.425 * | −0.589 * | − | 0.447 * | 0.397 * |
| IL-6 (ng L−1) | 0.428 * | −0.331 * | −0.486 * | 0.257 * | − | 0.288 * |
| PL (ng L−1) | 0.398 * | −0.253 * | −0.619 * | 0.359 * | 0.528 | 0,428 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnorowska, K.; Młynek, K.; Puppel, K. The Impact of Negative Energy Balance in Holstein-Friesian Cows on the Blood Concentrations of Interleukin-6 and Plasminogen. Metabolites 2024, 14, 548. https://doi.org/10.3390/metabo14100548
Wnorowska K, Młynek K, Puppel K. The Impact of Negative Energy Balance in Holstein-Friesian Cows on the Blood Concentrations of Interleukin-6 and Plasminogen. Metabolites. 2024; 14(10):548. https://doi.org/10.3390/metabo14100548
Chicago/Turabian StyleWnorowska, Kalina, Krzysztof Młynek, and Kamila Puppel. 2024. "The Impact of Negative Energy Balance in Holstein-Friesian Cows on the Blood Concentrations of Interleukin-6 and Plasminogen" Metabolites 14, no. 10: 548. https://doi.org/10.3390/metabo14100548
APA StyleWnorowska, K., Młynek, K., & Puppel, K. (2024). The Impact of Negative Energy Balance in Holstein-Friesian Cows on the Blood Concentrations of Interleukin-6 and Plasminogen. Metabolites, 14(10), 548. https://doi.org/10.3390/metabo14100548








