Synergistic Fermentation of Pichia kluyveri and Saccharomyces cerevisiae Integrated with Two-Step Sugar-Supplement for Preparing High-Alcohol Kiwifruit Wine
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Isolation and Purification of Wild Yeast Strain
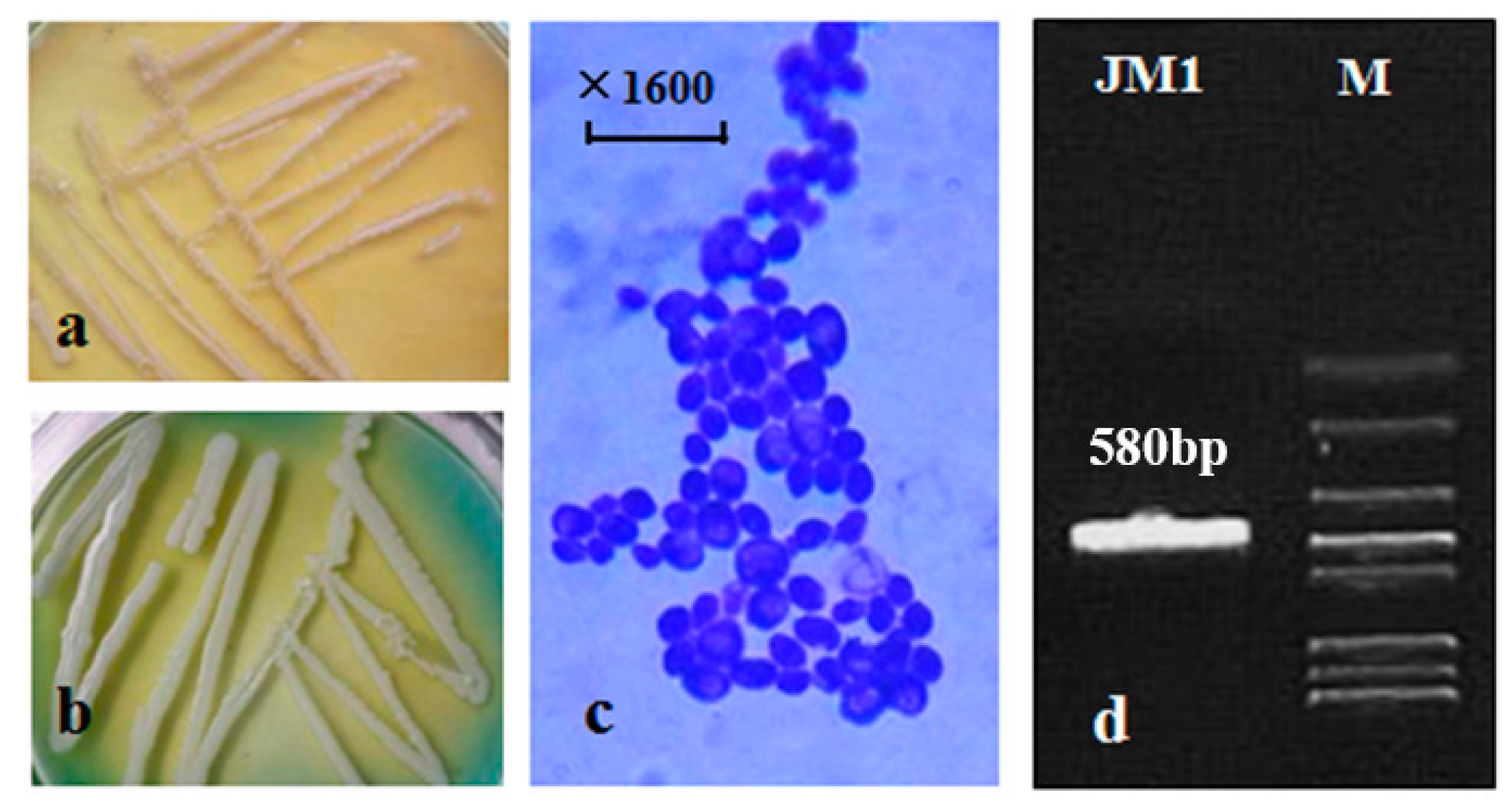
2.3. Identification of the Purified JM1 Strain
2.4. Two-Step Sugar Supplementation Combined with Synergistic Fermentation Process
2.4.1. Single-Factor Test
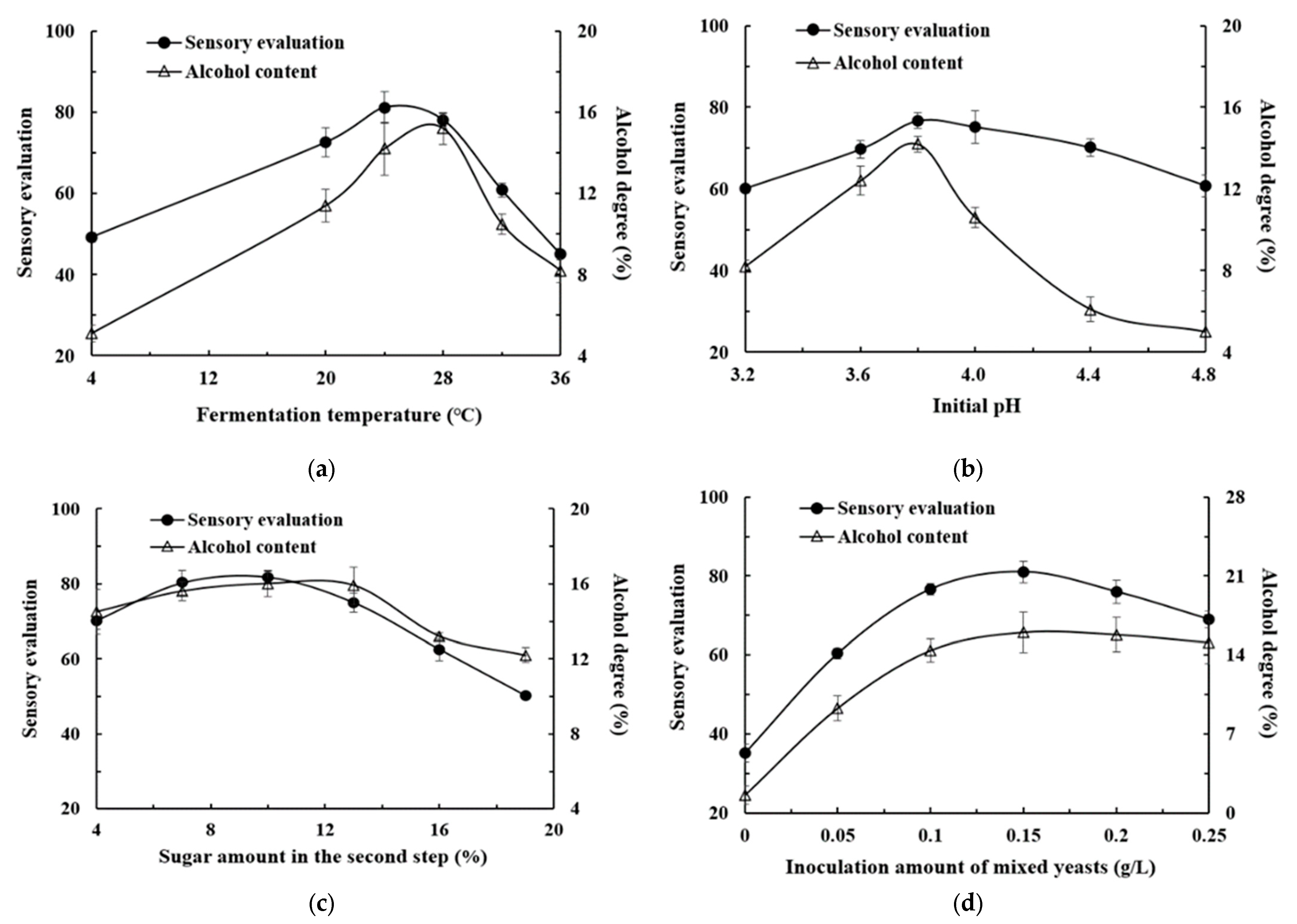
2.4.2. Different Fermentation Temperature
2.4.3. Different Initial pH
2.4.4. Different Supplement Amount of the Second-Step Sugar Supplement
2.4.5. Different Inoculation Amount of Wild Yeasts
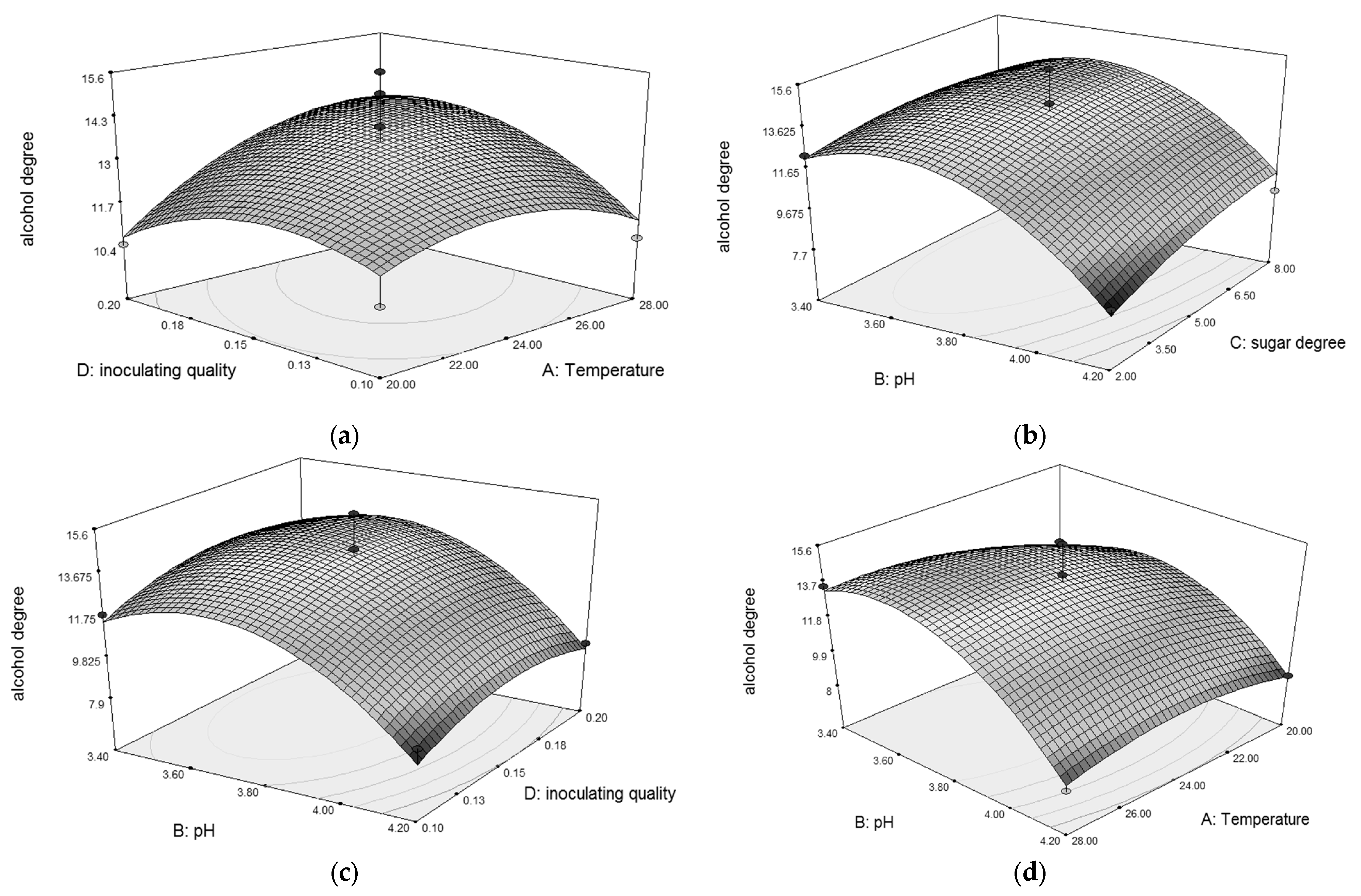
2.4.6. Response Surface Test
3. Results and Discussion
3.1. Identification of JM1 Strain Purified from Wild Kiwifruit
3.2. Effects of Different Fermentation Conditions on the Quality of Kiwifruit Wine
3.3. Optimization of Synergistic Fermentation process of Pichia kluyveri and Saccharomyces cerevisiae
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Hu, J.; Zhang, Y.; Xie, B.X.; He, B. Study on the Brewing Technology of Kiwifruit “Jinshi1” Wine. Food Ferment. Sci. Technol. 2015, 51, 84–87. [Google Scholar]
- Hafezi, F.; Rad, H.E.; Naghibzadeh, B.; Nouhi, A.H.; Naghibzadeh, G. Actinidia Aeliciosa (Kiwifruit), a New Drug for Enzymatic Debridement of Acute Burn Wounds. Burns 2010, 36, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Zhou, F.; Wang, S.; Wang, R.R.; Zhu, J. Screening and Tolerance of Superior Lactic Acid Bacteria for Wild Kiwifruit Wine Malolactic Fermentation. China Brew. 2019, 38, 56–59. [Google Scholar]
- Wang, R.R.; Liu, K.F.; Zhu, J.; Wang, M.N. Yeast Selection and Its Fermentation Characteristics for Wild Kiwi Wine. Sci. Tech. Food Ind. 2018, 39, 115–119. [Google Scholar]
- Hao, Y.; Wang, T.; Li, W.; Li, T.; Yuan, H. Screening and Identification of Yeast for Production of Wine from Selenium-Enriched Kiwifruits. Food Sci. 2014, 35, 175–179. [Google Scholar]
- Wu, Q.; Peng, J.J.; Yu, Y.G.; Wan, C.; Xiao, C.Y.; Wang, M.L. Isolation, Identification and Tolerance of Yeasts from Wild Kiwifruit Pulpa in Western Hunan. China Brew. 2018, 37, 83–87. [Google Scholar]
- Wu, Q.; Wan, C.; Peng, J.J.; Zeng, H.; Yu, Y.G. Isolation and Identification of Saccharomyces cerevisiae of Fermentation Liquor of Wild Kiwifruit from the Western of Hunan and the Relative ACE Inhibitory Activity. Food Mach. 2018, 34, 135–138. [Google Scholar]
- Tsegay, Z.T.; Sathyanarayana, C.B.; Lemma, S.M. Optimization of Cactus Pear Fruit Fermentation Process for Wine Production. Foods 2018, 7, 121. [Google Scholar] [CrossRef]
- Shi, T.X.; He, Y.C.; Liu, J.; Shu, J.B.; Zhou, J. Optimization of Chitinase Production by Serratia Marcescens with Two-Step Fermentation. J. Huaqiao Univ. 2011, 32, 67–71. [Google Scholar]
- Lu, B.S.; Li, X.S.; Fan, P. Study on the Production of Microbial Oils Fermented by Two-Step Fermentation with Distiller’s Grains. Biotechnology 2012, 22, 79–82. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Zamora, A.; Gutiérrez-Avendaño, D.O.; Arellano-Plaza, M.; De la Torre González, F.J.; Barrera-Martínez, I.; Gschaedler Mathis, A.; Casas-Godoy, L. The non-Saccharomyces yeast Pichia kluyveri for the production of aromatic volatile compounds in alcoholic fermentation. FEMS Yeast Res. 2021, 20, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.Y.; Ge, J.P.; Song, G.; Chen, L.; Ling, H.Z.; Ping, W.X. Isolation, Screening of Yeast and Identification of Functional Yeast from the Traditional Fermented Soybean Paste. J. Chin. Inst. Food Sci. Technol. 2013, 13, 183–188. [Google Scholar]
- Kong, X.C.; Chen, J.; Li, X.C.; Wu, X.Y. Optimization Test of Yeast Activity by Magnolia Staining. Beer Sci. Technol. 2006, 12, 50–52. [Google Scholar]
- Cheng, L.; Li, Z.; Wang, J. Study on Yeasts During the Spontaneous Fermentation of Grapevine. J. Chin. Inst. Food Sci. Technol. 2010, 10, 131–137. [Google Scholar]
- Niu, G.C.; Zhu, D.; Wang, J.; Fan, Z.J.; Li, Z.J. Screening and Molecular Identification of Superior Yeasts for Hippophae rhamnoides L. Wine. J. Chin. Inst. Food Sci. Technol. 2009, 9, 60–65. [Google Scholar]
- Yu, Y.G.; Zhang, W.W.; Cao, L.; Xu, C.H.; Ma, L.Q.; Yang, Z.L. Comparison of Fermentation Performance and Cellar mud Characteristics between Aging Cellar and Normal Cellar. Food Mach. 2015, 31, 2–5. [Google Scholar]
- Yang, H.; Huang, L.M.; Luo, J.H. Selection and Optimization of Fermentation by Box-Behnken Design of Yeast for Haihong Wine Brewing. Sci. Technol. Food Ind. 2014, 35, 221–225. [Google Scholar]
- Tan, C.D.; Chen, W.X.; Liao, Y.Z.; Zhu, X.L. Screening and Optimization of Fermentation Conditions of a High-yield Isoamyl Acetate Strain. Chin. Condiment 2015, 40, 30–34. [Google Scholar]
- Rao, Y.; Chang, W.; Gong, L.; Li, R.; Tang, J.; Che, Z.M. Isolation and Identification of Spoilage Microorganisms from Sichuan pickle. Food Ferment. Technol. 2013, 49, 19–22. [Google Scholar]
- Li, X.C.; Tang, M.; Cao, L.; Xing, Y.G.; Ma, L. Effect of Different Yeasts on Physicochemical Properties, Antioxidant Ability and Sensory of Kiwifruit Wine. Food Sci. Technol. 2016, 41, 16–20. [Google Scholar]
- Chen, Y.F.; Wang, Z.X.; Wang, C.X.; Fang, H.Y.; Zhu, G.J. Preliminary Study on Isolation, Identification and Ethanol Fermentation of Thermotolerant Yeast. Microbiology 2003, 30, 24–27. [Google Scholar]
- Xie, W.C.; Yin, C.; Song, L.; Xu, Z.Y.; Jia, J.T.; Zhang, J.Y.; Li, Y.J.; Lian, X.; Yang, X.H. Optimization of Rapid Fermentation Processing of Paste Produced with Shrimp Head by Adding Mixed Strains. Trans. Chin. So. Agric. Eng. 2018, 34, 306–312. [Google Scholar]
- Zhang, Q.G.; Sun, T.L.; Jin, Y.Y.; Wang, Y.; Zhang, Y.; Hu, J.J. Process Optimization of Bio-Hydrogen Production by Anaerobic Fermentation of Enzymatic Hydrolysate Supernatants of Corn Stalk. Trans. Chin. So. Agric. Eng. 2016, 32, 233–238. [Google Scholar]
- Zhou, Y.; Zhang, Q.; Fu, H.F.; Ben, H. Yeast Screening and Fermentation Process Optimization of Kiwifruit Wine. J. Northwest A F Univ. 2014, 42, 151–160. [Google Scholar]
- Li, X.Z.; Liu, C.J. Research of Fermenting Process of Actinidia arguta Wine. Sci. Technol. Food Ind. 2014, 35, 207–210. [Google Scholar]
- Luo, Q.; Sun, Q.; Ye, X.; Hu, Y.; Ran, X. Study on the Brewing Technology of Red Kiwifruit Wine. Food Ind. 2014, 35, 144–147. [Google Scholar]
- Wang, W.J.; Chen, J.; Zhang, R.; Wang, H.Z.; Long, H.; Tang, J.N. Optimization of the Primary Fermentation Technology of ‘Jinyan’ Kiwifruit Wine. Sci. Technol. Food Ind. 2017, 38, 216–219. [Google Scholar]
- Tang, X.; Cao, N.; Zhou, J.R. Production of Guichang Kiwifruit Wine. Liq. Mak. Sci. Technol. 2017, 12, 50–54. [Google Scholar]
- Zou, Y.Y.; Chen, W.J.; Li, Z.X.; Zhang, J.Y.; Wang, Z.D. Optimization of Fermentation Process for Red Kiwi Fruit Wine by Response Surface Methodology. Food Ind. 2017, 38, 113–116. [Google Scholar]
- Chen, Z.J.; Yang, X.C.; Zhao, J.; Hu, J.X.; Yuan, W.Y. Breeding of Saccharomyces cerevisiae with High Ethanol Tolerance and Its Application in Kiwi Wine. Sci. Technol. Food Ind. 2018, 39, 141–145. [Google Scholar]
- Zhang, J.; Zuo, Y.; Xie, G.J.; Zhang, X.; Sun, S.G.; Qu, J.J. Effect of Complex Bacteria Fermentation on Wild Kiwi Fruit Wine. Sci. Technol. Food Ind. 2017, 38, 213–217. [Google Scholar]

| Project | Sensory Description | Score |
|---|---|---|
| Color | Golden color, transparent, and clear | 20–30 |
| Light yellow color, not bright enough | 10–19 | |
| Yellowish brown, not clear enough | 5–9 | |
| Grayish brown, not clear | 0–4 | |
| Fragrant | Strong alcohol scent, fragrant kiwifruit aroma | 20–30 |
| Lighter alcohol scent, slight kiwifruit aroma | 10–19 | |
| Very light alcohol scent, slight kiwifruit aroma | 5–9 | |
| No obvious alcohol scent | 0–4 | |
| Taste | Rich wine astringency, light sour and sweet taste | 30–40 |
| Slightly lighter alcohol taste, balanced acidity and wine taste | 20–29 | |
| Very light alcohol taste, obvious sourness | 10–19 | |
| Strong sour taste, no alcoholic taste | 0–9 | |
| Total score | -- | 100 |
| Project | Sensory Description | Score |
|---|---|---|
| Color | Golden color, transparent, and clear | 20–30 |
| Light yellow color, not bright enough | 10–19 | |
| Yellowish brown, not clear enough | 5–9 | |
| Grayish brown, not clear | 0–4 | |
| Fragrant | Strong alcohol scent, fragrant kiwifruit aroma | 20–30 |
| Lighter alcohol scent, slight kiwifruit aroma | 10–19 | |
| Very light alcohol scent, slight kiwifruit aroma | 5–9 | |
| No obvious alcohol scent | 0–4 | |
| Taste | Rich wine astringency, light sour and sweet taste | 30–40 |
| Slightly lighter alcohol taste, balanced acidity and wine taste | 20–29 | |
| Very light alcohol taste, obvious sourness | 10–19 | |
| Strong sour taste, no alcoholic taste | 0–9 | |
| Total score | -- | 100 |
| No. | A | B | C | D | Y/vol% | No. | A | B | C | D | Y/vol% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 3.4 | 8 | 0.15 | 11.3 | 16 | 24 | 3.8 | 8 | 0.15 | 13.0 |
| 2 | 24 | 3.4 | 8 | 0.10 | 11.8 | 17 | 20 | 3.8 | 6 | 0.15 | 11.1 |
| 3 | 28 | 3.8 | 10 | 0.15 | 14.3 | 18 | 20 | 3.8 | 8 | 0.10 | 10.7 |
| 4 | 20 | 3.8 | 8 | 0.20 | 10.4 | 19 | 24 | 4.2 | 10 | 0.15 | 8.9 |
| 5 | 28 | 4.2 | 8 | 0.15 | 8.0 | 20 | 24 | 3.4 | 10 | 0.15 | 11.6 |
| 6 | 24 | 3.4 | 6 | 0.15 | 12.3 | 21 | 24 | 3.8 | 10 | 0.10 | 12.3 |
| 7 | 24 | 4.2 | 8 | 0.20 | 8.8 | 22 | 24 | 3.8 | 8 | 0.15 | 14.0 |
| 8 | 24 | 3.8 | 8 | 0.15 | 15.6 | 23 | 24 | 3.8 | 8 | 0.15 | 12.8 |
| 9 | 24 | 3.4 | 8 | 0.20 | 12.2 | 24 | 24 | 3.8 | 8 | 0.15 | 13.0 |
| 10 | 24 | 4.2 | 8 | 0.10 | 8.7 | 25 | 28 | 3.4 | 8 | 0.15 | 13.5 |
| 11 | 24 | 3.8 | 10 | 0.20 | 14.3 | 26 | 20 | 3.8 | 10 | 0.15 | 12.7 |
| 12 | 20 | 4.2 | 8 | 0.15 | 8.4 | 27 | 24 | 4.2 | 6 | 0.15 | 8.0 |
| 13 | 28 | 3.8 | 8 | 0.20 | 13.6 | 28 | 24 | 3.8 | 6 | 0.20 | 10.8 |
| 14 | 24 | 3.8 | 6 | 0.10 | 11.8 | 29 | 28 | 3.8 | 6 | 0.15 | 12.1 |
| 15 | 28 | 3.8 | 8 | 0.10 | 10.6 |
| Source of Variation | Sum of Square | Degrees of Freedom | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 104.83 | 14 | 7.49 | 9.29 | <0.0001 | |
| Improper | 5.80 | 10 | 0.58 | 0.42 | 0.8772 | |
| error | 5.49 | 4 | 1.37 | |||
| sum | 116.12 | 28 | ||||
| R2 = 0.9028 | ||||||
| RAdj = 0.8056 | ||||||
| A | 4.69 | 1 | 4.69 | 5.81 | 0.0302 | * |
| B | 39.97 | 1 | 39.97 | 49.57 | <0.0001 | *** |
| C | 5.33 | 1 | 5.33 | 6.62 | 0.0222 | * |
| D | 1.47 | 1 | 1.47 | 1.82 | 0.1983 | - |
| AB | 1.69 | 1 | 1.69 | 2.10 | 0.1697 | - |
| AC | 0.09 | 1 | 0.09 | 0.11 | 0.7432 | - |
| AD | 2.72 | 1 | 2.72 | 3.38 | 0.0874 | - |
| BC | 0.64 | 1 | 0.64 | 0.79 | 0.3880 | - |
| BD | 0.02 | 1 | 0.02 | 0.03 | 0.8697 | - |
| CD | 2.25 | 1 | 2.25 | 2.79 | 0.1170 | - |
| A2 | 5.58 | 1 | 5.58 | 6.92 | 0.0198 | * |
| B2 | 43.09 | 1 | 43.09 | 53.45 | <0.0001 | *** |
| C2 | 1.56 | 1 | 1.56 | 1.93 | 0.1863 | - |
| D2 | 6.68 | 1 | 6.68 | 8.29 | 0.0121 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Yuan, Q.; Wang, X.; Chen, L.; Yi, S.; Huang, X.; Wang, J.; Wang, X. Synergistic Fermentation of Pichia kluyveri and Saccharomyces cerevisiae Integrated with Two-Step Sugar-Supplement for Preparing High-Alcohol Kiwifruit Wine. Metabolites 2024, 14, 310. https://doi.org/10.3390/metabo14060310
Wu Q, Yuan Q, Wang X, Chen L, Yi S, Huang X, Wang J, Wang X. Synergistic Fermentation of Pichia kluyveri and Saccharomyces cerevisiae Integrated with Two-Step Sugar-Supplement for Preparing High-Alcohol Kiwifruit Wine. Metabolites. 2024; 14(6):310. https://doi.org/10.3390/metabo14060310
Chicago/Turabian StyleWu, Qiang, Qiaoling Yuan, Xi Wang, Lingying Chen, Senlin Yi, Xiaodan Huang, Jun Wang, and Xutong Wang. 2024. "Synergistic Fermentation of Pichia kluyveri and Saccharomyces cerevisiae Integrated with Two-Step Sugar-Supplement for Preparing High-Alcohol Kiwifruit Wine" Metabolites 14, no. 6: 310. https://doi.org/10.3390/metabo14060310
APA StyleWu, Q., Yuan, Q., Wang, X., Chen, L., Yi, S., Huang, X., Wang, J., & Wang, X. (2024). Synergistic Fermentation of Pichia kluyveri and Saccharomyces cerevisiae Integrated with Two-Step Sugar-Supplement for Preparing High-Alcohol Kiwifruit Wine. Metabolites, 14(6), 310. https://doi.org/10.3390/metabo14060310







