Effects of Storage Temperature at the Early Postharvest Stage on the Firmness, Bioactive Substances, and Amino Acid Compositions of Chili Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Collection and Temperature Treatment
2.2. Morphological and Firmness Determination
2.3. Water Loss Rate and Relative Electrolyte Leakage
2.4. HPLC Analysis of Capsaicinoids and Ascorbic Acid
2.5. Soluble Protein Assay
2.6. Total Carotenoids Assay
2.7. Amino Acid Detection
2.8. Statistical Analysis
3. Results
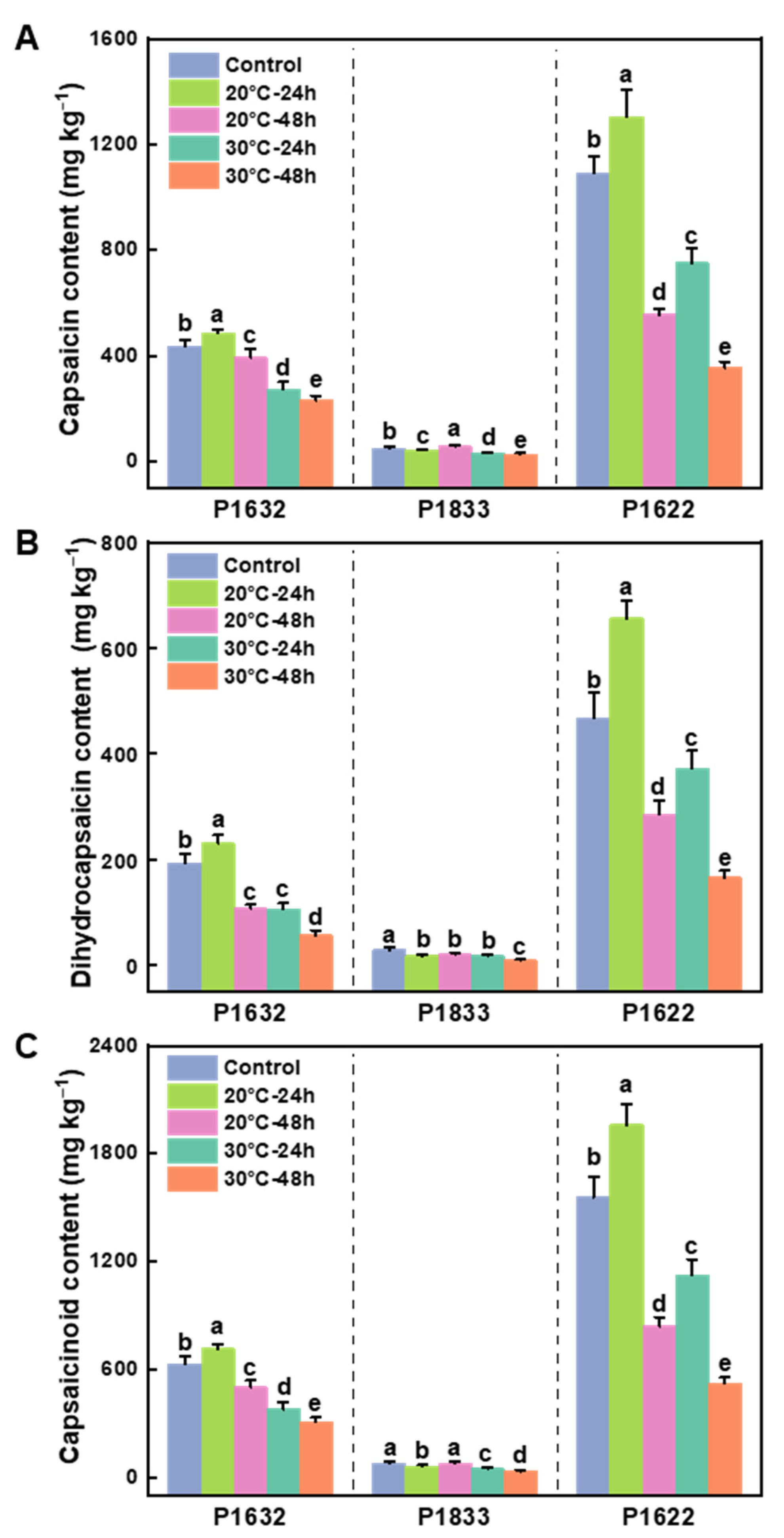
3.1. Effects of Storage Temperature on the Contents of Capsaicinoids
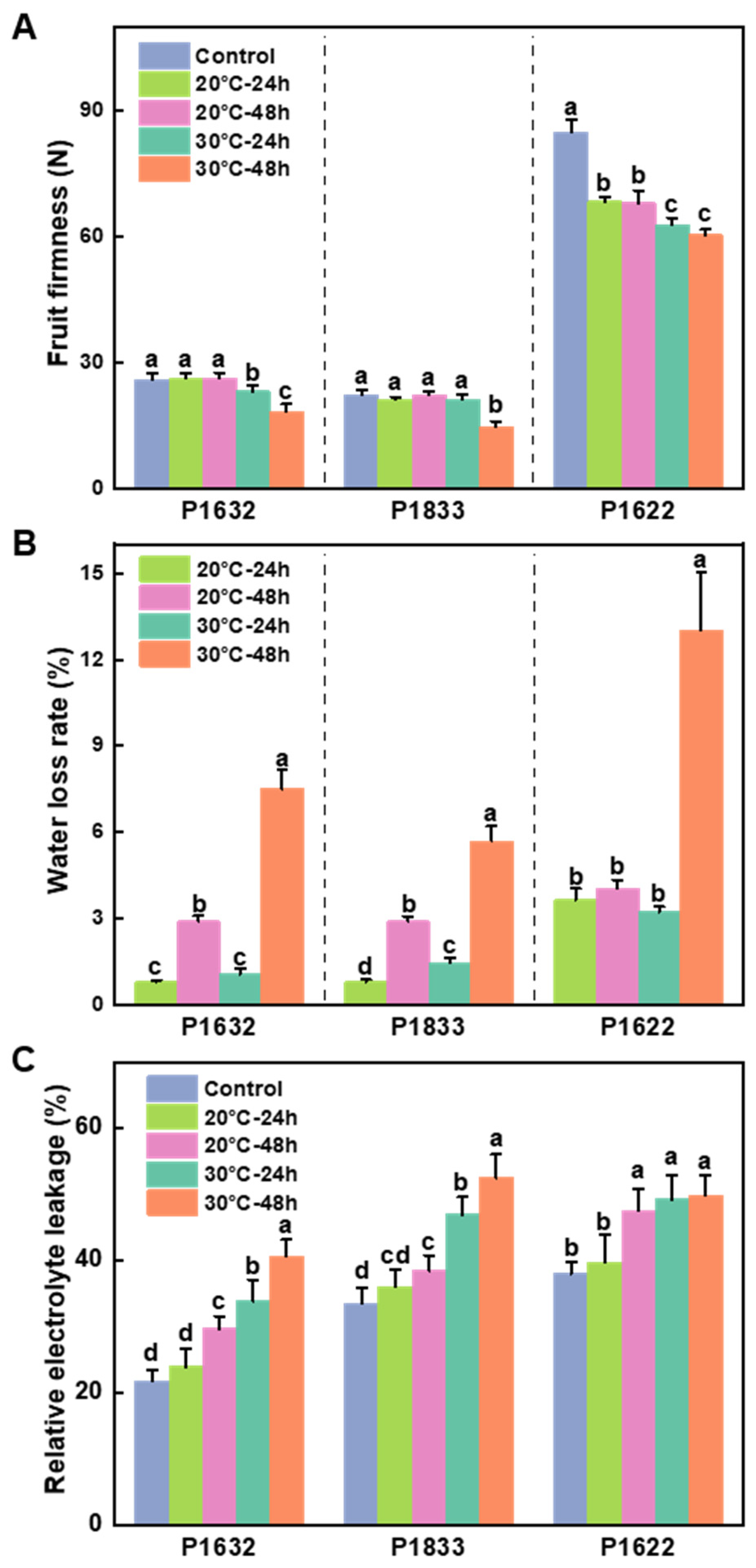
3.2. Effects of Storage Temperature on Fruit Firmness, Water Loss Rate, and Relative Electrolyte Leakage
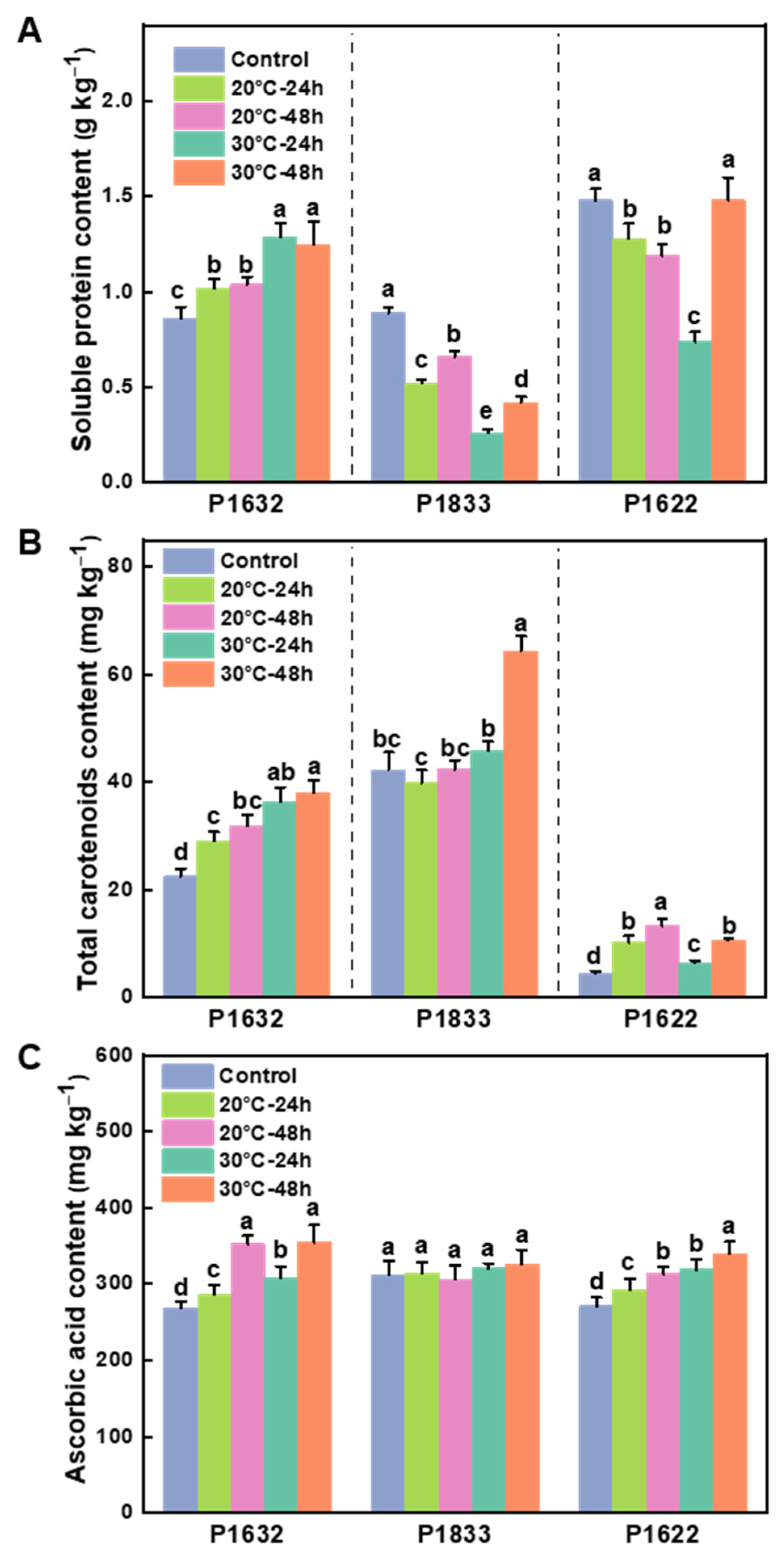
3.3. Effects of Storage Temperature on the Nutrient Quality of Fruits
3.4. Effects of Storage Temperature on the Amino Acid Composition and Contents
3.5. Effects of Storage Temperature on the Essential Amino Acid Percentages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arce-Rodriguez, M.L.; Ochoa-Alejo, N. Biochemistry and molecular biology of capsaicinoid biosynthesis: Recent advances and perspectives. Plant Cell Rep. 2019, 38, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.R.; Rohman, M.M.; Islam, M.R.; Hasanuzzaman, M.; Hassan, L. Screening and evaluation of chilli (Capsicum annuum L.) genotypes for waterlogging tolerance at seedling stage. Biocell 2022, 46, 1613–1627. [Google Scholar] [CrossRef]
- Mendes, N.D.; Goncalves, E.C.B.D. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243. [Google Scholar] [CrossRef]
- Liu, C.C.; Wan, H.J.; Yang, Y.X.; Ye, Q.J.; Zhou, G.Z.; Wang, X.R.; Ahammed, G.J.; Cheng, Y. Post-Harvest LED Light Irradiation Affects Firmness, Bioactive Substances, and Amino Acid Compositions in Chili Pepper (Capsicum annum L.). Foods 2022, 11, 2712. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular Health Benefits of Specific Vegetable Types: A Narrative Review. Nutrients 2018, 10, 595. [Google Scholar] [CrossRef]
- Naves, E.R.; Silva, L.D.; Sulpice, R.; Araujo, W.L.; Nunes-Nesi, A.; Peres, L.E.P.; Zsogon, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.; Sharangi, A.B.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Siddiqui, S.; Saeed, M.; Lee, H.-J.; Yadav, D.K. Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward. Molecules 2022, 27, 6380. [Google Scholar] [CrossRef]
- Lu, M.W.; Chen, C.Y.; Lan, Y.Q.; Xiao, J.; Li, R.; Huang, J.Q.; Huang, Q.R.; Cao, Y.; Ho, C.T. Capsaicin-the major bioactive ingredient of chili peppers: Bio-efficacy and delivery systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Perez, T.; Gomez-Garcia, M.D.; Valverde, M.E.; Paredes-Lopez, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization technical report series; World Health Organization: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Mouritsen, O.G.; Duelund, L.; Petersen, M.A.; Hartmann, A.L.; Frøst, M.B. Umami taste, free amino acid composition, and volatile compounds of brown seaweeds. J. Appl. Phycol. 2019, 31, 1213–1232. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2016, 7, 806s–822s. [Google Scholar] [CrossRef]
- Sun, B.; Tian, Y.X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Li, T.; Shi, Q.; Zhu, W.; Wang, X. Cold shock precooling improves the firmness of chili pepper during postharvest storage and the molecular mechanisms related to pectin. Food Chem. 2023, 419, 136052. [Google Scholar] [CrossRef]
- Pola, W.; Sugaya, S.; Photchanachai, S. Influence of Postharvest Temperatures on Carotenoid Biosynthesis and Phytochemicals in Mature Green Chili (Capsicum annuum L.). Antioxidants 2020, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.E.L.; Sargent, S.A.; Cantliffe, D.J.; Berry, A.D.; Shaw, N.L. Harvest Maturity and Storage Temperature Affect Postharvest Quality of ‘Wanda’ Datil Hot Pepper Grown under Protected Culture. Horttechnology 2019, 29, 402–407. [Google Scholar] [CrossRef]
- Miranda-Molina, F.D.; Valle-Guadarram, S.; Guerra-Ramirez, D.; Arevalo-Galarza, M.D.L.; Perez-Grajales, M.; Artes-Hernandez, F. Quality attributes and antioxidant properties of Serrano chili peppers (Capsicum annuum L.) affected by thermal conditions postharvest. Int. Food Res. J. 2019, 26, 1889–1898. [Google Scholar]
- Shil, S.; Mandal, J. Evaluation of postharvest quality of four local chilli (Capsicum frutescens) genotypes of Tripura under zero energy cool chamber. J. Pharmacogn. Phytochem. 2018, 7, 3698–3702. [Google Scholar]
- Wei, F.; Fu, M.; Li, J.; Yang, X.; Chen, Q.; Tian, S. Chlorine dioxide delays the reddening of postharvest green peppers by affecting the chlorophyll degradation and carotenoid synthesis pathways. Postharvest Biol. Technol. 2019, 156, 110939. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Benard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poessel, J.L.; Caris-Veyrat, C.; Genard, M. How does tomato quality (sugar, acid, and nutritional quality) vary with ripening stage, temperature, and irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Nakajima, N.; Hasegawa, Y. Effect of Postharvest Temperature and Ethylene on Carotenoid Accumulation in the Flavedo and Juice Sacs of Satsuma Mandarin (Citrus unshiu Marc.) Fruit. J. Agric. Food Chem. 2009, 57, 4724–4732. [Google Scholar] [CrossRef]
- Thanaraj, T.; Terry, L.A. Temporal change in taste- and health-related compounds during postharvest ripening of Sri Lankan mango fruit (Mangifera indica L.). Acta Hortic. 2010, 877, 1183–1189. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Nguyen, T.H.; Dang, T.M.Q.; Do, T.V.T.; Reungsang, A.; Chaiwong, N.; Rachtanapun, P. Effect of Pectin/Nanochitosan-Based Coatings and Storage Temperature on Shelf-Life Extension of “Elephant” Mango (Mangifera indica L.) Fruit. Polymers 2021, 13, 3430. [Google Scholar] [CrossRef]
- Ishikawa, T.; Maruta, T.; Yoshimura, K.; Smirnoff, N. Biosynthesis and Regulation of Ascorbic Acid in Plants. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–179. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Significance of Genetic, Environmental, and Pre- and Postharvest Factors Affecting Carotenoid Contents in Crops: A Review. J. Agric. Food Chem. 2018, 66, 5310–5324. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Hameed, R.; Malik, A.U.; Khan, A.S.; Imran, M.; Umar, M.; Riaz, R. Evaluating the Effect of Different Storage Conditions on Quality of Green Chillies (Capsicum annuum L.). Trop. Agric. Res. 2015, 24, 391–399. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.B.; Fawole, O.A.; Verboven, P.; Zhang, X.R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K.S. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Finger, F.L.; Pereira, G.M. Physiology and Postharvest of Pepper Fruits. In Production and Breeding of Chilli Peppers (Capsicum spp.); Ramalho do Rêgo, E., Monteiro do Rêgo, M., Finger, F.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 27–40. [Google Scholar] [CrossRef]
- Arce-Rodriguez, M.L.; Ochoa-Alejo, N. Silencing AT3 gene reduces the expression of pAmt, BCAT, Kas, and Acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Biol. Plant. 2015, 59, 477–484. [Google Scholar] [CrossRef]
- Liu, H.R.; Meng, F.L.; Miao, H.Y.; Chen, S.S.; Yin, T.T.; Hu, S.S.; Shao, Z.Y.; Liu, Y.Y.; Gao, L.X.; Zhu, C.Q.; et al. Effects of postharvest methyl jasmonate treatment on main health-promoting components and volatile organic compounds in cherry tomato fruits. Food Chem. 2018, 263, 194–200. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Yue, Z.C.; Zhong, X.M.; Lei, J.L.; Tao, P.; Li, B.Y. Distribution of primary and secondary metabolites among the leaf layers of headed cabbage (Brassica oleracea var. capitata). Food Chem. 2020, 312, 126028. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Guang, Y.L.; Yang, Y.X.; Chen, J.Y. Mechanisms of elevated CO2-induced thermotolerance in plants: The role of phytohormones. Plant Cell Rep. 2021, 40, 2273–2286. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X. Elevated carbon dioxide-induced regulation of ethylene in plants. Environ. Exp. Bot. 2022, 202, 105025. [Google Scholar] [CrossRef]
- Liu, C.C.; Luo, S.D.; Zhao, Y.; Miao, Y.N.; Wang, Q.; Ye, L.; Gao, L.X.; Ahammed, G.J.; Cheng, Y. Multiomics analyses reveal high temperature-induced molecular regulation of ascorbic acid and capsaicin biosynthesis in pepper fruits. Environ. Exp. Bot. 2022, 201, 104941. [Google Scholar] [CrossRef]
- Gonzalez-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Perez-Morales, R.; Ortiz, J.C.R.; Garcia-Hernandez, J.L. Characterization of Different Capsicum Varieties by Evaluation of Their Capsaicinoids Content by High Performance Liquid Chromatography, Determination of Pungency and Effect of High Temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef] [PubMed]
- Zamljen, T.; Medic, A.; Hudina, M.; Veberic, R.; Slatnar, A. Biostimulatory Effects of Amino Acids on Phenylalanine Ammonia Lyase, Capsaicin Synthase, and Peroxidase Activities in Capsicum baccatum L. Biology 2022, 11, 674. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Muy-Rangel, M.D.; Mascorro, A.G. Fruit size and stage of ripeness affect postharvest water loss in bell pepper fruit (Capsicum annuum L.). J. Sci. Food Agric. 2007, 87, 68–73. [Google Scholar] [CrossRef]
- Barzegar, T.; Fateh, M.; Razavi, F. Enhancement of postharvest sensory quality and antioxidant capacity of sweet pepper fruits by foliar applying calcium lactate and ascorbic acid. Sci. Hortic. 2018, 241, 293–303. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic Acid Foliar Application Increases Crop Yield and Quality Parameters of Green Pepper Fruit during Postharvest Storage. Agronomy 2021, 11, 2263. [Google Scholar] [CrossRef]
- Olatunde, A.; Tijjani, H.; Ishola, A.A.; Egbuna, C.; Hassan, S.; Akram, M. Carotenoids as Functional Bioactive Compounds. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 415–444. [Google Scholar] [CrossRef]
- Heinemann, B.; Hildebrandt, T.M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections Between Amino Acid Metabolisms in Plants: Lysine as an Example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Multiple Amino Acids Inhibit Postharvest Senescence of Broccoli. Horticulturae 2021, 7, 71. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef] [PubMed]
| Cultivar | Plant Height (cm) | Canopy Width (cm) | Fruit Length (mm) | Fruit Diameter (mm) | Single Fruit Weight (g) | Fruit Number Plant−1 |
|---|---|---|---|---|---|---|
| P1632 | 49.25 ± 2.50 b | 66.75 ± 3.59 b | 135.13 ± 7.70 a | 26.17 ± 1.55 a | 29.65 ± 1.54 a | 60.25 ± 11.87 b |
| P1833 | 75.50 ± 2.89 a | 69.50 ± 6.03 ab | 103.87 ± 1.36 b | 24.74 ± 1.25 a | 20.61 ± 1.65 b | 22.50 ± 3.32 c |
| P1622 | 44.75 ± 2.75 c | 71.75 ± 1.71 a | 88.85 ± 4.37 c | 11.42 ± 0.18 b | 5.18 ± 0.55 c | 106.50 ± 8.27 a |
| P1632 | P1833 | P1622 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 20 °C-48 h | 30 °C-48 h | Control | 20 °C-48 h | 30 °C-48 h | Control | 20 °C-48 h | 30 °C-48 h | |
| Total amino acids (T) | 48.91 ± 2.82 a | 38.07 ± 2.25 c | 43.05 ± 2.42 b | 44.39 ± 2.81 a | 42.23 ± 2.51 b | 31.97 ± 1.90 b | 47.97 ± 2.55 a | 43.85 ± 2.28 b | 43.71 ± 2.15 b |
| Essential amino acids (A) | 20.35 ± 1.27 a | 14.99 ± 0.98 c | 17.32 ± 1.11 b | 18.29 ± 1.18 a | 16.93 ± 1.06 b | 14.00 ± 0.86 c | 20.09 ± 0.97 a | 17.91 ± 0.85 b | 18.11 ± 0.81 b |
| Non-essential amino acids (B) | 28.55 ± 1.55 a | 23.08 ± 1.27 c | 25.73 ± 1.31 b | 26.10 ± 1.63 a | 25.29 ± 1.45 a | 17.97 ± 1.04 b | 27.88 ± 1.58 a | 25.94 ± 1.43 b | 25.60 ± 1.34 b |
| Children’s essential amino acids (C) | 4.09 ± 0.16 a | 3.28 ± 0.14 c | 3.48 ± 0.17 b | 3.76 ± 0.21 a | 3.61 ± 0.22 a | 2.77 ± 0.14 b | 4.19 ± 0.20 a | 3.87 ± 0.24 b | 3.88 ± 0.17 b |
| Tasty amino acids (D) | 9.01 ± 0.58 a | 7.18 ± 0.49 c | 8.44 ± 0.51 b | 8.11 ± 0.59 a | 8.02 ± 0.55 a | 5.18 ± 0.33 b | 7.90 ± 0.53 a | 8.06 ± 0.52 a | 7.46 ± 0.46 b |
| Sweet amino acids (E) | 12.45 ± 0.73 a | 10.05 ± 0.52 c | 11.17 ± 0.50 b | 11.47 ± 0.68 a | 10.44 ± 0.54 b | 8.60 ± 0.51 c | 12.63 ± 0.66 a | 11.17 ± 0.52 b | 11.34 ± 0.54 b |
| Aromatic amino acids (F) | 5.39 ± 0.26 a | 3.23 ± 0.17 c | 4.49 ± 0.25 b | 4.77 ± 0.26 a | 4.71 ± 0.23 a | 3.02 ± 0.15 b | 5.47 ± 0.26 a | 4.81 ± 0.28 b | 4.86 ± 0.24 b |
| A/T % | 41.62 ± 2.60 a | 39.37 ± 2.57 b | 40.23 ± 2.58 b | 41.20 ± 2.66 b | 40.10 ± 2.51 c | 43.80 ± 2.69 a | 41.87 ± 2.02 a | 40.85 ± 1.94 c | 41.44 ± 1.85 b |
| B/T % | 58.38 ± 3.17 b | 60.63 ± 3.34 a | 59.77 ± 3.04 a | 58.80 ± 3.67 a | 59.90 ± 3.43 a | 56.20 ± 3.25 b | 58.13 ± 3.29 a | 59.15 ± 3.24 a | 58.56 ± 3.07 a |
| C/T % | 8.36 ± 0.33 ab | 8.60 ± 0.37 a | 8.08 ± 0.39 b | 8.46 ± 0.47 a | 8.56 ± 0.52 a | 8.66 ± 0.44 a | 8.73 ± 0.42 a | 8.82 ± 0.55 a | 8.87 ± 0.39 a |
| D/T % | 18.41 ± 1.19 b | 18.85 ± 1.29 b | 19.60 ± 1.18 a | 18.26 ± 1.33 a | 18.99 ± 1.30 a | 16.20 ± 1.03 b | 16.47 ± 1.10 b | 18.39 ± 1.19 a | 17.06 ± 1.05 b |
| E/T % | 25.46 ± 1.49 b | 26.40 ± 1.37 a | 25.94 ± 1.16 ab | 25.84 ± 1.53 b | 24.73 ± 1.28 c | 26.89 ± 1.60 a | 26.33 ± 1.38 a | 25.46 ± 1.19 a | 25.93 ± 1.24 a |
| F/T % | 11.01 ± 0.53 a | 8.49 ± 0.45 c | 10.43 ± 0.58 b | 10.74 ± 0.59 b | 11.17 ± 0.54 a | 9.44 ± 0.47 c | 11.41 ± 0.54 a | 10.98 ± 0.64 b | 11.12 ± 0.55 b |
| Essential | Amino Acid Pattern Spectrum | P1632 | P1833 | P1622 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid | Control | 20 °C-48 h | 30 °C-48 h | Control | 20 °C-48 h | 30 °C-48 h | Control | 20 °C-48 h | 30 °C-48 h | |
| Thr | 2.30 | 5.59 ± 0.31 a | 5.70 ± 0.37 a | 5.40 ± 0.28 b | 5.57 ± 0.38 a | 4.65 ± 0.28 b | 5.83 ± 0.38 a | 5.77 ± 38 a | 5.52 ± 0.30 a | 5.55 ± 0.25 a |
| Val | 3.90 | 5.42 ± 0.27 a | 5.44 ± 0.32 a | 4.98 ± 0.28 b | 5.25 ± 0.20 b | 5.25 ± 0.24 b | 6.02 ± 0.28 a | 5.25 ± 0.23 a | 5.20 ± 0.21 a | 5.36 ± 0.27 a |
| Ile | 3.00 | 4.49 ± 0.31 a | 4.48 ± 0.29 a | 4.16 ± 0.23 b | 4.32 ± 0.25 a | 4.27 ± 0.26 a | 4.49 ± 0.25 a | 4.25 ± 0.17 a | 4.10 ± 0.23 a | 4.28 ± 0.21 a |
| Leu | 5.90 | 11.00 ± 0.76 a | 11.16 ± 0.87 a | 10.70 ± 0.88 b | 11.00 ± 0.79 a | 10.82 ± 0.88 a | 10.98 ± 0.72 a | 11.14 ± 0.54 a | 10.80 ± 0.50 b | 11.09 ± 0.43 a |
| Lys | 4.50 | 7.30 ± 0.51 a | 7.53 ± 0.47 a | 7.27 ± 0.49 a | 7.21 ± 0.54 b | 7.31 ± 0.50 b | 8.11 ± 0.63 a | 7.44 ± 0.40 a | 7.28 ± 0.30 a | 7.19 ± 0.30 a |
| Met + Cys | 2.20 | 2.00 ± 0.12 a | 2.07 ± 0.13 a | 1.96 ± 0.14 a | 2.11 ± 0.11 b | 2.06 ± 0.09 b | 2.59 ± 0.13 a | 2.08 ± 0.08 b | 2.22 ± 0.11 a | 2.25 ± 0.11 a |
| Phe + Tyr | 3.80 | 11.01 ± 0.53 a | 8.49 ± 0.45 c | 10.43 ± 0.58 b | 10.74 ± 0.59 b | 11.17 ± 0.54 a | 9.44 ± 0.47 c | 11.41 ± 0.54 a | 10.98 ± 0.64 b | 11.12 ± 0.55 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Gao, C.; Luo, S.; Yao, Z.; Ye, Q.; Wan, H.; Zhou, G.; Liu, C. Effects of Storage Temperature at the Early Postharvest Stage on the Firmness, Bioactive Substances, and Amino Acid Compositions of Chili Pepper (Capsicum annuum L.). Metabolites 2023, 13, 820. https://doi.org/10.3390/metabo13070820
Cheng Y, Gao C, Luo S, Yao Z, Ye Q, Wan H, Zhou G, Liu C. Effects of Storage Temperature at the Early Postharvest Stage on the Firmness, Bioactive Substances, and Amino Acid Compositions of Chili Pepper (Capsicum annuum L.). Metabolites. 2023; 13(7):820. https://doi.org/10.3390/metabo13070820
Chicago/Turabian StyleCheng, Yuan, Chengan Gao, Shaodan Luo, Zhuping Yao, Qingjing Ye, Hongjian Wan, Guozhi Zhou, and Chaochao Liu. 2023. "Effects of Storage Temperature at the Early Postharvest Stage on the Firmness, Bioactive Substances, and Amino Acid Compositions of Chili Pepper (Capsicum annuum L.)" Metabolites 13, no. 7: 820. https://doi.org/10.3390/metabo13070820
APA StyleCheng, Y., Gao, C., Luo, S., Yao, Z., Ye, Q., Wan, H., Zhou, G., & Liu, C. (2023). Effects of Storage Temperature at the Early Postharvest Stage on the Firmness, Bioactive Substances, and Amino Acid Compositions of Chili Pepper (Capsicum annuum L.). Metabolites, 13(7), 820. https://doi.org/10.3390/metabo13070820






