Abstract
The link between nutrition and autism spectrum disorder (ASD) as a neurodevelopmental condition, which is clinically presented as significant delays or deviations in interaction and communication, has provided a fresh point of view and signals that nutrition may play a role in the etiology of ASD, as well as playing an effective role in treatment by improving symptoms. In this study, 36 male albino rat pups were used. They were randomly divided into five groups. The control group was fed only a standard diet and water for the 30 days of the experiment. The second group, which served as a propionic acid (PPA)-induced rodent model of ASD, received orally administered PPA (250 mg/kg body weight (BW)) for 3 days, followed by feeding with a standard diet until the end of the experiment. The three other groups were given PPA (250 mg/kg body weight (BW)) for 3 days and then fed a standard diet and orally administered yogurt (3 mL/kg BW/day), artichokes (400 mL/kg BW/day), and a combination of Lacticaseibacillus rhamnosus GG at 0.2 mL daily (1 × 109 CFU; as the probiotic of yogurt) and luteolin (50 mg/kg BW/day; as the major antioxidant and anti-inflammatory ingredient of artichokes) for 27 days. Biochemical markers, including gamma-aminobutyric acid (GABA), reduced glutathione (GSH), glutathione peroxidase (GPx1), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-10 (IL-10), were measured in brain homogenates in all groups. The data showed that while PPA demonstrated oxidative stress and neuroinflammation in the treated rats, yogurt, Lacticaseibacillus rhamnosus GG as a probiotic, and luteolin as a prebiotic ingredient in artichokes were effective in alleviating the biochemical features of ASD. In conclusion, nutritional supplementation seems to be a promising intervention strategy for ASD. A combined dietary approach using pro- and prebiotics resulted in significant amelioration of most of the measured variables, suggesting that multiple interventions might be more relevant for the improvement of biochemical autistic features, as well as psychological traits. Prospective controlled trials are needed before recommendations can be made regarding the ideal ASD diet.
1. Introduction
Autism spectrum disorder (ASD) is a prevalent neurodevelopmental disorder with substantial clinical heterogeneity. The role of neuroinflammation in ASD has become increasingly evident, and previous studies have demonstrated neuroinflammation in the cerebral cortex, cerebellum, and white matter of patients with ASD. Furthermore, the cerebrospinal fluid (CSF) and serum of living patients with ASD show significantly higher proinflammatory cytokine profiles.
The gut–brain axis, which refers to the bidirectional route between gut bacteria and the brain, has a significant impact on numerous brain processes. These mechanisms include oxidative stress; neuroinflammation; glutamate excitotoxicity; blood–brain barrier construction; neurogenesis; microglia maturation, GABA, noradrenaline, and dopamine synthesis; and behavioral variance, which is a key component of ASD [1,2,3,4].
Because there is no cure for autism, treatments often focus on speech and behavioral interventions to address the disorder’s hallmark social, behavioral, and communication difficulties [5]. Gastrointestinal (GI) disturbances are prevalent comorbidities that are thought to be both a sign of ASD and an etiological cause [6]. The gut microbiota is altered in ASD, with diverse alterations described at different taxonomic levels, highlighting the necessity of examining the gut–brain axis in the treatment of these disorders [7].
In numerous investigations involving human and animal models of autism, dysbiosis of the gut microbiota has been shown to exist. These investigations have revealed that in ASD, aberrant bacterial species prefer the environment of the gut. Biopsy samples from children with ASD have been shown to include abnormal Firmicutes-to-Bacteroidetes ratios [8,9,10]. The imbalance between these two bacterial families varied throughout the various compartments of the gut, which was linked to compositional dysbiosis [11]. Exhibiting higher amounts of proteobacteria [12], lactobacillus [13], bacteroides [14], desulfovibrio [15], and clostridium [16], patients with ASD consistently demonstrate a dysfunctional imbalance. Reduced abundances of bifidobacterium [17], dialister [18], prevotella [19], veillonella, and turicibacter [18] are frequently observed in conjunction with this. Consequently, nutritional interventions are used by the majority of patients with ASD, both with and without clinical management, to relieve GI and behavioral symptoms. Despite considerable interest in dietary interventions, no agreement has been reached regarding optimal nutritional intervention strategies [19].
Food choices and dietary patterns are also suggested to play a role in the development of ASD. Recent evaluations have emphasized the importance of nutrition in regulating or lowering ASD symptoms. It is generally known that consuming prebiotics and probiotics provides various health benefits by positively modifying gut flora. People with autism spectrum disorder (ASD) have an imbalanced gut microbiota. The use of probiotics, prebiotics, and synbiotics is a promising technique for regulating the gut flora and lowering ASD symptoms [20]. Despite the infrequency of studies related to the supplementation of probiotics and prebiotics in individuals with ASD, a promising improvement has been noted in the severity of social interactions associated with an increase in beneficial bacteria and a decrease in pathogens in the GI tract, leading to an improvement in recurrent GI problems, suggesting both pre- and probiotics as promising alternative complementary medicine [19,20].
There is increasing evidence regarding the use of inulin as a prebiotic for the selective growth of bifidobacteria and lactobacilli as beneficial gut bacteria linked to several health benefits. Costabile et al. [21] reported that the daily consumption of inulin extracted from globe artichokes exerts a pronounced prebiotic effect on the composition of human fecal microbiota. Although a pronounced variation in chemical composition and nutritional value was observed in different artichoke genotypes, all have high nutritional value and are significantly recommended as part of a healthy and balanced diet [22].
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is well known as a common component in plants. Luteolin-rich plants have been used ethnopharmacologically for the treatment of inflammation. Both luteolin supplements and extracts from luteolin-rich plants, such as artichokes, have been studied using several models and demonstrated anti-inflammatory activity [23].
Yogurt, fermented milk, and fermented vegetables are all excellent probiotic sources [24]. Consuming probiotics may be advantageous for the improvement of neurological and neurodevelopmental diseases, such as ASD, because the gut microbiota has been found to have a bidirectional link with the brain [25].
The formation of short-chain fatty acids, such as PPA, by intestinal clostridia and desulfovibrio is thought to play a role in the development of ASD symptoms [26]. The PPA model shows several characteristics that are typical of children with autism. In the PPA model, increasing oxidative stress and free radicals cause mitochondrial malfunction, which releases potent cytokines that irritate and change several neurotransmitters. Additionally, the PPA model and patients with ASD are found to share pathophysiological similarities and gastrointestinal problems. Using appropriate behavior testing and modeling criteria, multiple studies have demonstrated that PPA can fulfil more than three aspects. The PPA model offers the most difficult situation and affects a specific brain area to make it the closest one to autism and distinguish it. It is also regarded as a low-cost and simple method of testing novel treatments [26,27,28]. Most recently, Ali et al. [29] proved the validity of the PPA model of ASD.
This information sparked our interest in examining the ability of luteolin and lactobacillus, either as supplements or in food-rich sources, such as artichoke or yogurt, to ameliorate specific biochemical variables related to oxidative stress, neurochemistry, and neuroinflammation, which are the three major etiological mechanisms of ASD, as well as the biomarkers of PPA-induced neurotoxicity in rodent models of ASD [30]. Also investigated was a combined pre- and probiotic intervention (L. rhamnosus GG plus luteolin). It is crucial to emphasize that the same nutritional interventions significantly improved the gut microbiomes of ASD animal models that had been induced by PPA (unpublished work under review).
2. Results
2.1. Effect of the Nutritional Interventions on GPX1 and GSH as Oxidative Stress Markers and the Impaired GABA Neurotransmitter
Data are presented as means ± S.D., together with the percentage changes in all the measured variables (Table 1 and Table 2 and Figure 1 and Figure 2). Table 1 and Figure 1 demonstrate a significant decrease in GPX1 (−32.78%), GSH (−52.17%), and GABA (−28.83%) in the PPA-treated group as a rodent model of ASD, together with the remarkable ameliorative effects of the four nutritional interventions used in treatments. Although artichokes remarkably increased GPX1 and GSH in PPA-treated rats, the PPA-treated rats still demonstrated significantly lower GSH levels compared to controls (−25.95%). In contrast, the yogurt-treated group recorded more or less similar GSH and GPX1 levels to those of the controls and significantly higher levels compared to the PPA-treated group.

Table 1.
Effect of nutrition with yogurt, artichokes, and combined L. rhamnosus GG + luteolin on levels of GPX1 (U/mg protein), GSH (µg/mg protein), and GABA (Pg/mg protein) in the brain homogenates of the PPA-induced rodent model of autism.

Table 2.
Effect of nutrition with yogurt, artichokes, and combined L. rhamnosus GG + luteolin on levels of TNF-α (pg/mg protein), IL10 (pg/mg protein), and IL-6 (pg/mg protein) in the brain homogenates of the PPA-induced rodent model of autism.
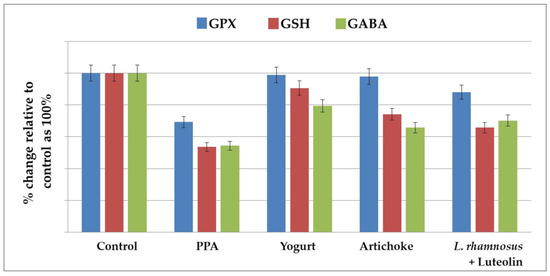
Figure 1.
The percentage change in GPX, GSH, and GABA levels in the brain homogenates of an untreated PPA-induced autism model and nutritionally treated groups of yogurt, artichokes, and combined L. rhamnosus + luteolin relative to the control, presented as 100%.
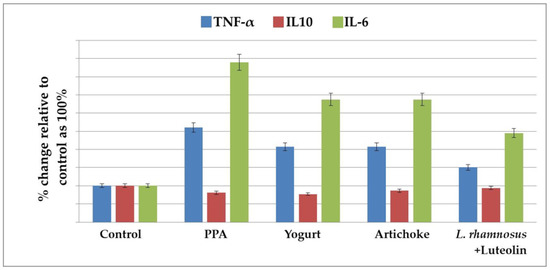
Figure 2.
The percentage change in TNF-α, IL10, and IL-6 levels in the brain homogenates of an untreated PPA-induced autism model and nutritionally treated groups of yogurt, artichokes, and combined L. rhamnosus GG + luteolin relative to the control, presented as 100%.
2.2. Effect of the Nutritional Interventions on the Levels of TNF-α, IL-10, and IL-6 as Neuroinflammatory Markers
Table 2 and Figure 2 demonstrate the levels of TNF-α, IL-10, and IL-6 in the five studied groups. PPA treatment induced a highly significant increase in TNF-α and IL-6 as pro-inflammatory cytokines, recording percentage increases of 196.81% and 482.8%, respectively, compared to the control group of rats. IL-10, as an anti-inflammatory cytokine, was significantly lower in the PPA-treated group and did not increase after nutritional interventions. Yogurt, artichoke, and L. rhamnosus GG + luteolin treatments demonstrated significantly lower levels of TNF-α and IL-6 compared to the PPA-treated group, but these levels were still remarkably higher than in the controls. The L. rhamnosus GG + luteolin-treated group recorded the lowest values of both pro-inflammatory cytokines.
2.3. Receiver Operating Characteristic Analysis for Evaluating Predictive Values of the Measured Variables in the PPA-Induced Autism Model and Different Nutritionally Treated Groups
Table 3 and Table 4 demonstrate the area under the curves (AUCs) of the receiver operating characteristics (ROC) curves, cut-off values, specificity, and sensitivity of the six measured variables in all the groups. It can be noted that most of the parameters recorded high AUCs, together with satisfactory specificity and sensitivity.

Table 3.
ROC results of GPX, GSH, and GABA in the tissue homogenates of the PPA-induced rodent model of autism and yogurt, artichoke, and combined L. rhamnosus GG + luteolin nutritionally treated groups relative to the control group.

Table 4.
ROC results of TNF-α, IL-10, and IL-6 in the tissue homogenates of the PPA-induced rodent model of autism and yogurt, artichoke, and combined L. rhamnosus GG + luteolin nutritionally treated the groups relative to the control group.
3. Discussion
It is well accepted that the etiology of ASD may involve complex interactions between genetic factors and certain environmental toxicants that may act synergistically or in parallel during critical periods of neurodevelopment, increasing the likelihood of developing ASD in at least a subset of children.
The current treatment of psychiatric disorders primarily focuses on the use of psychotropic medicine to treat symptoms, although its efficiency varies between people, and it is typically linked with severe adverse effects. In recent years, nutritional therapies for the prevention and treatment of mental diseases have gained a lot of attention. However, data supporting nutritional interventions in autism spectrum disorder are still limited and of poor quality [31].
The lower recorded GPX1 activity in the combined lactobacillus-and-luteolin-treated group compared to the independently treated yogurt and artichoke groups could be attributed to the fact that luteolin, as a prebiotic, quenches ROS and prevents their damaging effects on brain cells and that lactobacilli, as excellent organic acid producers, convert sugars into lactic acid and other by-products, including H2O2, a substrate of GPX1, which could affect its enzymatic activity [32]. They produce small molecules as well. Regarding GABA, there was a significant decrease in PPA-treated groups, together with a remarkable elevation in the yogurt-treated group, whereas both the artichoke and L. rhamnosus GG + luteolin treatments were ineffective in inducing GABA levels.
Many children with ASD have been observed to suffer from co-morbidities, such as GI distress and abnormal sensory processing, which may restrict their nourishment. To compensate for nutritional deficiencies attributable to the reduction in food selectivity and the abnormal eating habits of patients with ASD, several dietary strategies have been applied by caregivers, such as the supplementation of diets with probiotics, a large amount of fiber, omega-3 fatty acids, antioxidants, and vitamins and minerals, but most of these are still confusing and inconclusive [33].
There is growing interest in the use of combined prebiotics, such as oligosaccharides, and probiotics to support human health. Combining these two to create a successful synbiotic could maximize their therapeutic effects. Simply, prebiotics can improve the composition of the gut microbiome, support the immune system by increasing the number of protective microorganisms, and reduce the number of harmful or pathogenic microorganisms [19,20]. In this study, we investigated selected nutritional intervention strategies using a PPA-induced animal model of ASD [28,30]. Among these strategies are pure probiotics, probiotic-rich food, fiber- and flavonoid-rich food, and luteolin either independently or in combination.
Previous research has found that children with ASD have reduced GSH levels. Nutritional therapies aimed at increasing GSH levels have been demonstrated to improve ASD behaviors [34,35]. GSH and GPX1 play a role in the antioxidant defense against a wide range of environmental pollutants, including PPA [36,37,38]. Table 1 and Figure 1 demonstrate the significant decrease in GPX1 and GSH observed in both PPA-treated groups in the rodent model of ASD. This result can find support in the recent work carried out by Al Suhaibani et al. [39], in which PPA-treated animals demonstrated a significant reduction in GSH compared with controls. Additional support can be also found in the previous studies by Macfabe et al. and El-Ansary et al. [30,40], who both reported reductions in GSH in PPA-treated rats. They hypothesized that increased levels of PPA could induce oxidative stress in the brain, first when orally administrated and second when intraventricularly administrated, along with repetitive, social, and object-directed behaviors [40]. Table 1 also presents the antioxidant effects of yogurt, as shown by the amelioration of GSH and GPX depletion. This can find support in the work of Gjorgievski et al. [41], which proved that yogurt fermented with different microbiological cultures, including symbiotic Lactobacillus spp., shows health-promoting effects and strong antioxidant activity compared with unfermented milk.
Table 1 and Figure 1 also demonstrate the antioxidant effects of artichokes and luteolin as active ingredients of artichokes. Both demonstrated significant potency in amending the oxidative stress induced by PPA as a neurotoxicant. Luteolin demonstrated higher antioxidant effects compared to whole-artichoke extracts. This finding is in good agreement with the previous work in [42], which proved that artichoke leaf extract displays high potential as a natural source of minerals and phytochemical compounds with antioxidant and anti-inflammatory properties. The authors proved that methanol extract from artichokes shows a significant decrease in lipid peroxides as an indicator of oxidative behavior in children given luteolin at 100 mg/capsule per 10 kg (22 lb.) weight per day with food for 26 weeks. They attributed the improvement in behavior to the antioxidant, anti-inflammatory, and neuroprotective effects of luteolin. The remarkable increase in GABA observed in prebiotic- and probiotic-treated groups can find support in multiple previous works that have proven that food-derived Lactobacillus strains, such as Lactobacillus plantarum [43], Lactobacillus paracasei, Lacticaseibacillus rhamnosus GG [44], and Lactobacillus brevis, are effective in alleviating the decreased GABA levels usually associated with depression and anxiety brain disorders [44].
Chronic neuroinflammation has been identified in ASD [45]. This includes chronic glia activation and changed inflammatory function, which could be somewhat responsible for the abnormal behavior observed in ASD. It is well accepted that chronic peripheral inflammation and abnormal inflammatory responses in the brain may lead to cognitive dysfunction [46]. Table 2 and Figure 2 demonstrate significant increases in the proinflammatory cytokines TN-α and IL-6, together with a non-significant decrease in IL-10 as an anti-inflammatory cytokine, in PPA-treated rats. This result was supported by the most recent work of Abdelli et al. [47] and Abuaish et al. [28], who demonstrated a remarkable increase in gliosis and neuron-inflammatory biomarkers in a PPA-rodent model of ASD. Table 2 and Figure 2 also show the significant therapeutic effects of yogurt, artichokes, luteolin, L rhamnosus, and combined L rhamnosus + luteolin. This finding is supported by multiple previous studies that have proved that probiotic yogurt intake is associated with significant anti-inflammatory effects that parallel the increase in the peripheral pool of T (reg) cells in patients with IBD [48]. Furthermore, yoghurt and its associated probiotics may improve intestinal barrier function by maintaining tight-junction protein expression and aiding in the prevention of gut inflammation and tissue injury [49]. Yogurt containing Lactobacillus bulgaricus strains and Streptococcus thermophilus strains reduced mortality and prevented chemically induced intestinal inflammation in mice [50]. Furthermore, yoghurt without additional probiotic strains inhibited induced colitis in mice by increasing IgA-producing cells and decreasing CD8+ T-cells 2 weeks after chemical toxin treatment [51]. Based on the fact that leaky gut and tight-junction protein impairment are well-documented features in patients with ASD, this study could help suggest yogurt consumption as a therapeutic strategy working through the gut–brain axis in patients with ASD [52]. The significant therapeutic effects of artichokes shown in Table 2 and Figure 2 are in good agreement with multiple studies that have demonstrated remarkable decreases in pro-inframammary markers in artichoke-treated mice with colitis induced by dextran sulfate sodium [53]. Additionally, Wauquier et al. [54] demonstrated that plant-derived nutrients and especially polyphenols from artichokes may represent a relevant alternative for nutritional strategies addressing multiple inflammatory chronic diseases. The anti-inflammatory effects of luteolin can be easily observed in Table 2 and Figure 2 as significant decreases in TNF-α and IL-6 induced in PPA-treated groups. This is in good agreement with a previous study by Aziz et al. [23], which proved that luteolin, as a flavonoid commonly found in medicinal plants, such as artichokes, has strong anti-inflammatory activity in vitro and in vivo. The anti-inflammatory effects of luteolin occur mostly through the inhibition of the nuclear factor (NF)-κB pathway, mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription 3 (STAT3). Additionally, a clinical trial with a formulation containing luteolin revealed therapeutic effects against multiple inflammation-associated diseases. Luteolin, as a component of artichokes, demonstrates remarkably higher anti-inflammatory effects than whole-plant extract. Probiotic microorganisms are thought to benefit human health primarily through three basic modes of action [23,55]. First, some probiotics have the ability to remove or suppress pathogens, either directly or through their effects on commensal microbiota [56,57]. A second mechanism is the capacity of specific probiotics to increase epithelial barrier function by regulating signaling pathways, such as NF-kB, Akt, and MAPK, which results in the induction of mucus [58] or improved tight-junction function. Third, most probiotics have the ability to regulate host immunological responses [59]. Many interactions between probiotic bacteria and intestinal epithelial and immune cells are hypothesized to be mediated by molecular structures known as microbe-associated molecular patterns (MAMPs), which can be identified by pattern recognition receptors (PRRs), such as TLRs [60]. L rhamnosus is one of the most commonly used probiotics, demonstrating both antioxidant and anti-inflammatory effects in this study (Table 1 and Table 2 and Figure 1 and Figure 2). These findings are supported by the work of Ayyanna et al. [61], who observed L. rhamnosus GG-induced downregulation of pro-inflammatory cytokines, including IL-6, and significant decreases in lipid peroxides and ROS as markers of oxidative stress [62].
The therapeutic effects of pre- and probiotics observed in this study can find support in multiple recent studies that have demonstrated the effectiveness of prebiotics and prebiotics used as psychobiotics in treating the symptoms of schizophrenia and its comorbidities, attention deficit hyperactivity disorder (ADHD), bipolar disorder, and other neuropsychiatric disorders affecting children and adolescents [63,64,65,66]. Although still in its early stages, the use of prebiotics and probiotics to treat the symptoms of neurological disorders is quite promising.
Table 3 demonstrates that ROC curve analysis is an appropriate statistical tool for evaluating both the sensitivity and specificity of a biochemical variable or biomarker. It helps ascertain optimal cut-off points for a measured variable for potential follow-ups for future clinical applications [67]. The absence of false positives and false negatives for any measured variable means that this method demonstrates perfect performance. ROC analysis produces an AUC, which is a measure of how well a parameter can discriminate between two studied groups (i.e., PPA-intoxicated or prebiotic- and probiotic-treated groups relative to controls in this study). The AUC usually ranges from 0.5 (no discriminant capacity) to 1.0 (perfect discriminant capacity) [67].
4. Materials and Methods
4.1. Materials
In March 2019, fresh Cynara scolymus L. (artichokes) exported from the Netherlands was purchased from local supermarkets in Riyadh, Saudi Arabia (SA). Yogurt was purchased from local supermarkets in Riyadh, Saudi Arabia. Following collection, the samples were stored aseptically in a refrigerator at a low temperature (4 °C) to preserve them from contamination and deterioration [53]. Probiotic Lacticaseibacillus rhamnosus GG and prebiotic luteolin supplements were purchased from Swanson Health Products (Fargo, ND, USA).
4.2. Preparation of Cynara Scolymus L. (Artichoke) Extract
Cynara scolymus L. heads were divided into petal, choke, and heart sections. Each component was cleaned, cut, shade-dried at room temperature, and then processed into powder in a coffee grinder. The petal, choke, and heart total dry powder weights were 3.16 kg, 1.21 kg, and 0.371 kg, respectively. The powder of the Cynara scolymus L. (artichoke) head petal, choke, and heart was extracted separately with methanol/water (80/20, V/V) over 72 h using an orbital shaker at 150 rpm. Next, it was filtered through Whatman paper and re-extracted four times using a new solvent (methanol/water). The artichoke extract in the flask was immersed in a water bath during the evaporation process. The extract was placed in the hood for 24 h to ensure complete methanol evaporation, and then, a few drops of chloroform were added to prevent fungal contamination. The final dry extract was stored at 4 °C until further use.
4.3. Animals
Thirty-six male Sprague–Dawley albino rat pups were used, with an average weight of 70 g ± 20 g (approximately 3 weeks old). They were divided into 5 groups randomly. The control group was fed only a standard diet and water for the 30 days of the experiment. The second group served as a PPA-induced rodent model of ASD, orally administered PPA (250 mg/kg body weight (BW)) for 3 days, followed by feeding with a standard diet until the end of the experiment. The three other groups were given PPA(250 mg/kg body weight (BW)) for 3 days and then fed a standard diet and orally administered yogurt (3 mL/kg BW/day) [68], artichokes (400 mL/kg BW/day) [69], and a combination of L. rhamnosus GG 0.2 mL daily (1 × 109 CFU) [56] and luteolin (50 mg/kg BW/day) [70,71,72] for 27 days. The biochemical markers glutamate, gamma-aminobutyric acid (GABA), glutathione (GSH), glutathione peroxidase 1(GPX1), tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL6), and interleukin 10 (IL10) were measured in brain homogenates in all the groups. The Graduate Studies and Scientific Research Ethical Committee of Bioethics of King Saud University (KSU; reference no. SE-19-142) accepted the protocol for this study, and it was carried out in accordance with its rules. Our investigation was conducted in accordance with the ARRIVE recommendations. The 5 study groups are shown in Table 5 including the control group, the PPA-induced rodent model, and the three nutritionally treated groups.

Table 5.
Number of animals and the pro/prebiotic dosages fed to animals post orally administered PPA (250 mg /kg BW for 3 day).
4.4. Preparation of Brain Tissue Homogenates
Deeply anaesthetized (with ketamine/xylazine + D.W. (91, respectively 9 mg/kg BW, I.P.) animals were beheaded at the end of the feeding sessions. Brain tissues were extracted from the five groups of rats and dissected into minute pieces before being homogenized in bi-distilled water (1:10, w/v) and kept at −30 °C until further use.
4.5. Biochemical Analyses
4.5.1. Determination of GSH
GSH was measured in brain homogenates using a competitive ELISA kit (GPX1; Cat.No: CEA294Ge; Cloud Clone Corp., 23603 W. Fernhurst Dr., Unit 2201, Katy, TX 77494, USA). The assay was performed according to the manufacturer’s protocols. Its sensitivity is typically less than 0.52 μg/mL.
4.5.2. Determination of GPX1
GPX1 was measured in brain homogenates using a competitive ELISA kit (GPX1; Cat.No: SEA295Ra; Cloud Clone Corp., 23603 W. Fernhurst Dr., Unit 2201, Katy, TX 77494, USA). The assay was performed according to the manufacturer’s protocols. Its sensitivity is typically less than 0.61 ng/mL.
4.5.3. Determination of GABA
GABA was measured in brain homogenates using a competitive ELISA kit (GPX1; Cat.No: CEA900Ge; Cloud Clone Corp., 23603 W. Fernhurst Dr., Unit 2201, Katy, TX 77494, USA). The assay was performed according to the manufacturer’s protocols. Its sensitivity is typically less than 2.17 pg/mL. Samples were measured at a wavelength of 450 nm ± 10 nm.
4.5.4. Determination of IL-6
A competitive ELISA kit (GPX1; Cat. No. SEA079Ra; Cloud Clone Corp., 23603 W. Fernhurst Dr., Unit 2201, Katy, TX 77494, USA) was used to quantify IL-6 in brain homogenates. The test was conducted in accordance with the manufacturer’s instructions. Typically, its sensitivity is lower than 3.3 pg/mL.
4.5.5. Determination of IL-10
IL-10 was measured in brain homogenates using a competitive ELISA kit (GPX1; Cat.No: SEA056Ra; Cloud Clone Corp., 23603 W. Fernhurst Dr.; Unit 2201; Katy; TX 77494; USA). The assay was performed according to the manufacturer’s protocols. Its sensitivity is typically less than 5.8 pg/mL.
4.6. Statistical Analyses
The data are presented as means ± standard deviations. All statistical comparisons between the control group and the PPA- and probiotic-treated rat groups were made using SPSS Statistics version 16.0, with one-way analysis of variance (ANOVA) tests, together with Dunnett’s test for multiple comparisons. The threshold for significance was set at p < 0.05. Analysis of the ROC curve was also carried out. Calculations were carried out to determine the AUCs, levels of sensitivity and specificity, and cut-off values.
5. Conclusions
Taken together, these results support the potential effectiveness of probiotic (Lacticaseibacillus rhamnosus GG) and prebiotic (artichoke and Luteolin) treatments, either independently or in combination, as nutritional intervention strategies to amend oxidative stress and neuroinflammation as neurotoxic effects of an orally administered PPA rodent model of ASD.
6. Future Perspectives
In the light of the effectiveness of probiotics and prebiotics that have been used as supplements or food-rich diets to improve induced biochemical autistic features, we expect that future studies will be able to assess the whole physiological effects of these diets. Such information can direct the development of interventions that are more informed, less constrictive, free from the negative effects of limiting certain nutrients, and still keep the components that promote the beneficial behavioral amendment of ASD.
Author Contributions
S.R.M.A. acquired the data; H.A.A. co-drafted the manuscript; S.R.M.A., A.Y.A., N.A.A. and A.B.B. carried out the experimental work; and A.E.-A. suggested the topic and drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded through the Researchers Supporting Project (no. RSP-2021/341), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
This work was approved by the Ethics Committee, College of Science, King Saud University (IRB no. KSU-SE-19-142).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets and analyses generated during this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (no. RSP-2021/341), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial Activation and Neuroinflammation in the Brain of Patients With Autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the Microbiota, Immune and Nervous Systems in Health and Disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2017, 17, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.T.; Chana, G.; Pardo, C.A.; Achim, C.; Semendeferi, K.; Buckwalter, J.; Courchesne, E.; Everall, I.P. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol. Psychiatry 2010, 68, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Vismara, L.A.; Rogers, S.J. Behavioral Treatments in Autism Spectrum Disorder: What Do We Know? Annu. Rev. Clin. Psychol. 2016, 6, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Louis, P. Does the Human Gut Microbiota Contribute to the Etiology of Autism Spectrum Disorders? Dig. Dis. Sci. 2012, 57, 1987–1989. [Google Scholar] [CrossRef]
- Abdellatif, B.; McVeigh, C.; Bendriss, G.; Chaari, A. The Promising Role of Probiotics in Managing the Altered Gut in Autism Spectrum Disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; Mcdonough-means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and Metabolic Status of Children with Autism vs. Neurotypical Children, and the Association with Autism Severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Finegold, S.M.; Downes, J.; Summanen, P.H. Microbiology of Regressive Autism. Anaerobe 2012, 18, 260–262. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Cho Paik, M.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Liu, Y.; Rhoads, J.M. Can Probiotics Benefit Children with Autism Spectrum Disorders? World J. Gastroenterol. 2016, 22, 10093–10102. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Roberfroid, M.B. Critical Review Dietary Modulation of the Human Colonie Microbiota: Introducing the Concept of Prebiotics. Am. Inst. Nutr. 1995, 125, 1401–1412. [Google Scholar]
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A. Autism Spectrum Disorders and Intestinal Microbiota. Gut Microbes 2015, 6, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered Composition and Function of Intestinal Microbiota in Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2019, 9, 13. [Google Scholar] [CrossRef]
- Kang, D.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; Mcdonough-means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Kang, D.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Daniel, P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-brown, R. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Brandão, T.L.S.; Silva, J.C.L.; Campos, S.É.D.; Francelino, J.O. Supplementation of Prebiotics and Probiotics in Autistic Children: Integrative Review. Res. Soc. Dev. 2022, 2022, e12811124061. [Google Scholar] [CrossRef]
- Pranckutė, R.; Kaunietis, A.; Kuisiene, N.; Čitavičius, D.J. Combining Prebiotics with Probiotic Bacteria Can Enhance Bacterial Growth and Secretion of Bacteriocins. Int. J. Biol. Macromol. 2016, 89, 669–679. [Google Scholar] [CrossRef]
- Costabile, A.; Frohberg, C.; Kolida, S.; Klinder, A.; Gietl, E.; Ba, M.; Gibson, G.R. Bifidogenic Effect of a Very-Long-Chain Inulin Extracted from Globe Artichoke (Cynara Scolymus) in Healthy Human Subjects. Br. J. Nutr. 2010, 104, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value and Chemical Composition of Greek Artichoke Genotypes. Food Chem. 2017, 267, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and Other Fermented Foods as Sources of Health-Promoting Bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A.; Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Shultz, S.R.; Macfabe, D.F. Propionic Acid Animal Model of Autism Propionic Acid Animal Model of Autism. Compr. Guide Autism 2014, 1755–1778. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.; Kim, J.; Lee, S.; Hong, Y. Pathophysiological and Neurobehavioral Characteristics of a Propionic Acid-Mediated Autism-like Rat Model. PLoS ONE 2018, 13, e0192925. [Google Scholar]
- Abuaish, S.; Al-Otaibi, N.M.; Aabed, K.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; Bhat, R.S.; Arzoo, S.; Algahtani, N.; Moubayed, N.M.; et al. The Efficacy of Fecal Transplantation and Bifidobacterium Supplementation in Ameliorating Propionic Acid-Induced Behavioral and Biochemical Autistic Features in Juvenile Male Rats. J. Mol. Neurosci. 2022, 72, 372–381. [Google Scholar] [CrossRef]
- Ali, E.; Elmalahy, H.; Abbas, O.; Abu Almaaty, A. Is propionic acid a suitable model for autism? Alfarama J. Basic Appl. Sci. 2022, 3, 45–63. [Google Scholar] [CrossRef]
- El-Ansary, A.K.; Bacha, A.B.; Kotb, M. Etiology of Autistic Features: The Persisting Neurotoxic Effects of Propionic Acid. J. Neuroinflamm. 2012, 9, 74. [Google Scholar] [CrossRef]
- Müller-stierlin, A.S.; Teasdale, S.; Sabrina, M. Brain, Behavior & Immunity—Health Nutritional Psychiatry in the Treatment of Psychotic Disorders: Current Hypotheses and Research Challenges. Brain Behav. Immun. Health 2020, 19, 100070. [Google Scholar] [CrossRef]
- Kling, D.N.; Marcial, G.E.; Roberson, D.N.; Lorca, G.L.; Gonzalez, C.F. The Synergistic Contribution of Lactobacillus and Dietary Phytophenols in Host Health. In Probiotics and Prebiotics in Human Nutrition and Health; IntechOpen: London, UK, 2016. [Google Scholar]
- Bölte, S. Is Autism Curable? Dev. Med. Child Neurol. 2014, 56, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental Toxicants and Autism Spectrum Disorders: A Systematic Review. Transl. Psychiatry 2014, 4, e360-23. [Google Scholar] [CrossRef] [PubMed]
- Pugsley, K.; Scherer, S.W.; Bellgrove, M.A. Environmental Exposures Associated with Elevated Risk for Autism Spectrum Disorder May Augment the Burden of Deleterious de Novo Mutations among Probands. Mol. Psychiatry 2021, 27, 710–730. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X. Oxidative Stress in Autism Spectrum Disorder—Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef]
- Castejon, A.M.; Spaw, J.A.; Rozenfeld, I.; Sheinberg, N.; Kabot, S.; Shaw, A.; Hardigan, P.; Faillace, R.; Packer, E.E. Improving Antioxidant Capacity in Children With Autism: A Randomized, Double-Blind Controlled Study with Cysteine-Rich Whey Protein. Front. Psychiatry 2021, 12, 669089. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione Metabolism in Brain Metabolic Interaction between Astrocytes and Neurons in the Defense against Reactive Oxygen Species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Al Suhaibani, A.; Ben Bacha, A.; Alonazi, M.; Bhat, R.S.; El-Ansary, A. Testing the Combined Effects of Probiotics and Prebiotics against Neurotoxic Effects of Propionic Acid Orally Administered to Rat Pups. Food Sci. Nutr. 2021, 9, 4440–4451. [Google Scholar] [CrossRef]
- Macfabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.; Cain, D.P. Effects of the Enteric Bacterial Metabolic Product Propionic Acid on Object-Directed Behavior, Social Behavior, Cognition, and Neuroinflammation in Adolescent Rats: Relevance to Autism Spectrum Disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef]
- Gjorgievski, N.; Tomovska, J.; Dimitrovska, G.; Makarijoski, B.; Shariati, M.A. Determination of The Antioxidant Activity in Yogurt. J. Hyg. Eng. Des. 2014, 8, 88–91. [Google Scholar]
- Ben Salem, M.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals Compositions, Antioxidant and Anti-Inflammatory Activity of Cynara Scolymus Leaves Extracts, and Analysis of Major Bioactive Polyphenols by HPLC. Evid.-Based Complement. Altern. Med. 2017, 2017, 4951937. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, J.W.; Lim, S.D. The Probiotic Characteristics and GABA Production of Lactobacillus Plantarum K154 Isolated from Kimchi. Food Sci. Biotechnol. 2014, 23, 1951–1957. [Google Scholar] [CrossRef]
- Kochalska, K.; Oakden, W.; Słowik, T.; Chudzik, A.; Pankowska, A.; Łazorczyk, A.; Kozioł, P.; Andres-Mach, M.; Pietura, R.; Rola, R.; et al. Dietary Supplementation with Lactobacillus Rhamnosus JB-1 Restores Brain Neurochemical Balance and Mitigates the Progression of Mood Disorder in a Rat Model of Chronic Unpredictable Mild Stress. Nutr. Res. 2020, 82, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune Dysfunction and Neuroinflammation in Autism Spectrum Disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, L.; Depino, A.M. Altered Peripheral and Central Inflammatory Responses in a Mouse Model of Autism. Autism Res. 2014, 7, 273–289. [Google Scholar] [CrossRef]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic Acid Induces Gliosis and Neuro-Inflammation through Modulation of PTEN/AKT Pathway in Autism Spectrum Disorder. Sci. Rep. 2019, 9, 8824. [Google Scholar] [CrossRef]
- Baroja, M.L.; Kirjavainen, P.V.; Hekmat, S.; Reid, G. Anti-Inflammatory Effects of Probiotic Yogurt in Inflammatory Bowel Disease Patients. Clin. Exp. Immunol. 2007, 149, 470–479. [Google Scholar] [CrossRef]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt Inhibits Intestinal Barrier Dysfunction in Caco-2 Cells by Increasing Tight Junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef]
- Chaves, S.; Perdigon, G.; De Moreno De Leblanc, A. Yoghurt Consumption Regulates the Immune Cells Implicated in Acute Intestinal Inflammation and Prevents the Recurrence of the Inflammatory Process in a Mouse Model. J. Food Prot. 2011, 74, 801–811. [Google Scholar] [CrossRef]
- Gobbato, N.; Rachid, M.; Perdigón, G. Anti-Inflammatory Effect of Yoghurt in an Experimental Inflammatory Bowel Disease in Mouse. J. Dairy Res. 2008, 75, 497–504. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.; Zayed, N.; Bhat, R.S.; Moubayed, N.M.S.; Al-Muammar, M.N.; El-Ansary, A. The Use of Biomarkers Associated with Leaky Gut as a Diagnostic Tool for Early Intervention in Autism Spectrum Disorder: A Systematic Review. Gut Pathog. 2021, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal Anti-Inflammatory Effects of Artichoke Pectin and Modified Pectin Fractions in the Dextran Sulfate Sodium Model of Mice Colitis. Artificial Neural Network Modelling of Inflammatory Markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.J.J.; Verhoeven, T.L.A.; Vanderleyden, J.; Keersmaecker, S.C.J. De Exopolysaccharides of Lactobacillus Rhamnosus GG Form a Protective Shield against Innate Immune. Microb. Biotechnol. 2010, 4, 368–374. [Google Scholar] [CrossRef]
- Corr, S.C.; Hill, C.; Gahan, C.G.M. Understanding the Mechanisms by Which Probiotics Inhibit Gastrointestinal Pathogens. Adv Food Nutr. Res. 2009, 56, 1–15. [Google Scholar]
- Mack, D.R.; Ahrne, S.; Hyde, L.; Wei, S.; Hollingsworth, M.A. Extracellular MUC3 Mucin Secretion Follows Adherence Of. Gut 2003, 52, 827–834. [Google Scholar] [CrossRef]
- Wells, J.M. Immunomodulatory Mechanisms of Lactobacilli. Microb. Cell Factories 2011, 10, S17. [Google Scholar] [CrossRef]
- Abreu, M.T. Erratum: Toll-like Receptor Signalling in the Intestinal Epithelium: How Bacterial Recognition Shapes Intestinal Function (Nature Reviews Immunology (2010) 10 (131-144)). Nat. Rev. Immunol. 2010, 10, 215. [Google Scholar] [CrossRef]
- Ayyanna, R.; Ankaiah, D.; Arul, V. Anti-Inflammatory and Antioxidant Properties of Probiotic Bacterium Lactobacillus Mucosae AN1 and Lactobacillus Fermentum SNR1 in Wistar Albino Rats. Front. Microbiol. 2018, 14, 3063. [Google Scholar] [CrossRef]
- Oksaharju, A.; Kooistra, T.; Kleemann, R.; Van Duyvenvoorde, W.; Miettinen, M.; Lappalainen, J.; Lindstedt, K.A.; Kovanen, P.T.; Korpela, R.; Kekkonen, R.A. Effects of Probiotic Lactobacillus Rhamnosus GG and Propionibacterium Freudenreichii Ssp. Shermanii JS Supplementation on Intestinal and Systemic Markers of Inflammation in ApoE*3Leiden Mice Consuming a High-Fat Diet. Br. J. Nutr. 2013, 110, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.W.; Gorbovskaya, I.; Hahn, M.K.; Müller, D.J. The Gut Microbiome in Schizophrenia and the Potential Benefits of Prebiotic and Probiotic Treatment. Nutrients 2021, 13, 1152. [Google Scholar] [CrossRef] [PubMed]
- Mcguinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; Hely, M.O.; Simpson, C.A.; Green, J. OPEN A Systematic Review of Gut Microbiota Composition in Observational Studies of Major Depressive Disorder, Bipolar Disorder and Schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Ligezka, A.N.; Sonmez, A.I.; Corral-frias, M.P.; Golebiowski, R.; Lynch, B.; Croarkin, P.E.; Romanowicz, M. A systematic review of microbiome changes and impact of probiotic supplementation in children and adolescents with neuropsychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 8, 110187. [Google Scholar] [CrossRef]
- Shahrbabaki, M.E.; Sabouri, S.; Sabahi, A.; Barfeh, D.; Divsalar, P. The Efficacy of Probiotics for Treatment of Bipolar Disorder-Type 1: A Randomized, Double-Blind, Placebo Controlled Trial. Iran J. Psychiatry 2020, 15, 10–16. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver Operating Characteristic Curve: Overview and Practical Use for Clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Issazadeh, K.; Ali Abadi, M.A.; Kazemi Darsanaki, R.; Alikhani, F.; Dadras, H.; Tajehmiri, A. Isolation, Identification and Analysis of Probiotic Properties of Lactobacillus spp. from Traditional Yoghurts in North of Iran. J. Pure Appl. Microbiol. 2013, 7, 2965–2971. [Google Scholar]
- Kusuma, G.D.; Paseephol, T.; Sherkat, F. Prebiotic and Rheological Effects of Jerusalem Artichoke Inulin in Low-Fat Yogurt. Aust. J. Dairy Technol. 2009, 64, 59–163. [Google Scholar]
- Heidarian, E.; Soofiniya, Y. Hypolipidemic and Hypoglycemic Effects of Aerial Part of Cynara Scolymus in Streptozotocin-Induced Diabetic Rats. J. Med. Plants Res. 2011, 5, 2717–2723. [Google Scholar]
- Alghamdi, M.A.; Al-ayadhi, L.; Hassan, W.M.; Bhat, R.S.; Alonazi, M.A.; El-ansary, A. Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders. Metabolites 2022, 12, 562. [Google Scholar] [CrossRef]
- Abu-Elsaad, N.; El-Karef, A. Protection against Nonalcoholic Steatohepatitis through Targeting IL-18 and IL-1alpha by Luteolin. Pharmacol. Rep. 2019, 71, 688–694. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).