When Is the Right Moment to Pick Blueberries? Variation in Agronomic and Chemical Properties of Blueberry (Vaccinium corymbosum) Cultivars at Different Harvest Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pomological Traits
2.3. Reagents and Standards
2.4. Sample Preparation
2.5. Total Phenolic Content (TPC)
2.6. Radical Scavenging Activity (RSA)
2.7. Total Anthocyanin Content (TAC)
2.8. UHPLC–DAD MS/MS Analysis of Polyphenolic Compounds
2.9. HPAEC/PAD Analysis of Sugars and Sugar Alcohols (Ion Chromatography with Amperometric Detection)
2.10. Statistical Analysis
3. Results
3.1. Climatic Conditions
3.2. Yield and Pomological Traits
3.3. Determination of TPC, RSA, and TAC
3.4. Phenolic Composition of Blueberry Extracts
3.5. Sugar and Sugar Alcohol Profiles
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalska, A.; Lysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors influencing strik the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- FAO Stat. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 3 July 2022).
- Kurtović, M.; Maličević, A. Vodič za Proizvodnju Jagodastog Voća; Poljoprivredno-Prehrambeni Fakultet: Sarajevo, Bosnia and Herzegovina; Heko d.o.o.: Bugojno, Bosnia and Herzegovina, 2013; pp. 1–215. [Google Scholar]
- Zorenc, Z.; Veberic, R.; Stampar, F.; Koron, D.; Petkovsek-Mikulic, M. Changes in berry quality of northern highbush blueberry (Vaccinium corymbosum L.) during the harvest season. Turk. J. Agric. 2016, 40, 855–864. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.-Y.; Li, J.; Zhang, H.; Li, Y.; Farooq, S.; Acha, S.A.S.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar]
- Betz, M.; Kulozik, U. Whey protein gels for the entrapment of bioactive anthocyanins from bilberry extract. Inter. Dairy J. 2011, 21, 703–710. [Google Scholar]
- Ordóñez-Díaz, J.; Pereira-Caro, G.; Cardeñosa, V.; Muriel, J.L.; Moreno-Rojas, J.M. Study of the Quality Attributes of Selected Blueberry (Vaccinium corymbosum L.) Varieties Grown under Different Irrigation Regimes and Cultivation Systems. Appl. Sci. 2020, 10, 8459. [Google Scholar] [CrossRef]
- Bryla, D.R.; Strik, C.B. Effect of Cultivar and Plant of Spacing on the Seasonal Water Requirements of Highbush Blueberry. J. Am. Soc. Hortic. Sci. 2007, 132, 270–277. [Google Scholar]
- Brun, A.C.; De Vetter, L.; Benedict, K. Growing Small Fruits in the Home Garden; WSU Extension Bulletin EM103E 2016; Washington State University Extension, WSU: Pullman, WA, USA, 2016; pp. 1–64. [Google Scholar]
- Mengist, M.F.; Grace, M.H.; Xiong, J.; Kay, C.D.; Bassil, N.; Hummer, K.; Ferruzzi, M.G.; Lila, M.A.; Iorizzo, M. Diversity in Metabolites and Fruit Quality Traits in Blueberry Enables Ploidy and Species Differentiation and Establishes a Strategy for Future Genetic Studies. Front. Plant Sci. 2020, 11, 370. [Google Scholar] [CrossRef]
- Milivojević, J.; Miletić, N. The Blueberry; University of Belgrade, Faculty of Agriculture and AgroNET, Center for Education and Research: Belgrade, Serbia, 2022. [Google Scholar]
- Hancock, J.; Draper, A. Blueberry culture in North America. Hort. Sci. 1989, 24, 551–556. [Google Scholar]
- Sousa, M.B.; Curado, T.; Lavadinho, C.; Moldão-Martins, M. A Survey of Quality Factors in Highbush and Rabbiteye Blueberry Cultivars in Portugal. Acta Hortic. 2006, 715, 567–572. [Google Scholar] [CrossRef]
- Ścibisz, I.; Mitek, M. Antioxidant propreties of highbush blueberry fruit cultivars. Electron. J. Pol. Agric. Univ. 2007, 10, 34. [Google Scholar]
- Castrejon, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar]
- Balkhoven, J.; Peppelman, G. Chandler obtains the largest fruits, Brigitta Blue the largest production/Chandler geeft grootste vruchten, Brigitta Blue grootste productie. Fruitteelt 2002, 92, 12–13. [Google Scholar]
- Ehlenfeldt, M.K.; Martin, R.B., Jr. Seed set, berry weight, and yield interactions in the highbush blueberry cultivars (Vaccinium corymbosum L.) ‘Bluecrop’ and ‘Duke’. J. Am. Pomol. Soc. 2010, 64, 162–172. [Google Scholar]
- Sargent, A.S.; Takeda, F.; Williamson, G.J.; Berry, D.A. Harvest of Southern Highbush Blueberry with a Modified, Over-the-Row Mechanical Harvester: Use of Soft-Catch Surfaces to Minimize Impact Bruising. Agronomy 2021, 11, 1412. [Google Scholar] [CrossRef]
- Vance, A.J. The Impact of Organic Blueberry Production Systems on Performance of Cultivars and Yield Components (Final Report 2013–2014). 2014. Available online: http://oregonstate.edu/dept/NWREC/sites (accessed on 6 June 2022).
- Haffner, K.; Vestrheim, S.; Grønnerød, K. Qualitätseigenschaften von Kulturheidelbeeren sorten Vaccinium corymbosum L. Erwerbs-Obstbau 1998, 40, 112–116. [Google Scholar]
- Skupien, K. Chemical composition of selected cultivars of high bus blueberry fruit (Vaccinium corymbosum L.). Folia Hortic. 2006, 18, 47–56. [Google Scholar]
- Iglesias, I.; Alegre, S. The effect of anti-hail nets on fruit protection, radiation, temperature, quality and profitability of ‘Mondial Gala’ apples. J. Appl. Hortic. 2006, 8, 91–100. [Google Scholar]
- Ciucu, M.; Hoza, D. Research on the Biochemical Quality of Fruits on Some Highbush Blueberry Cultivars. In Proceedings of the Online International Conference “Young People and Multidisciplinary Research in Applied Life Sciences”, Online, 23 October 2020; University of Agricultural Sciences and Veterinary Medicine: Bucharest, Romania, 2020. [Google Scholar]
- Sun, Y.; Li, M.; Mitra, S.; Muhammad, R.H.; Debnath, B.; Lu, X.; Qiu, D. Comparative phytochemical profiles and antioxidant enzyme activity analyses of the southern highbush blueberry (Vaccinium corymbosum) at different developmental stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Rescic, J.; Schmitzer, V.; Stampar, F.; Slatnar, A.; Koron, D.; Veberic, R. Changes in fruit quality parameters of four Ribes species during ripening. Food Chem. 2015, 173, 363–374. [Google Scholar] [CrossRef]
- Foney, C.F. Postharvest issues in blueberry and cranberry and methods to improve market-life. Acta Hortic. 2009, 810, 785–798. [Google Scholar]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef]
- Calò, R.; Marabini, L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B 2014, 132, 27–35. [Google Scholar]
- Milivojevic, J.; Maksimovic, V.; Maksimovic, J.D.; Radivojevic, D.; Poledica, M.; Ercişli, S. A comparison of major taste- and health-related compounds of Vaccinium berries. Turk. J. Biol. 2012, 36, 738–745. [Google Scholar]
- Ştefănescu, B.E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crişan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef]
- Kalt, W.; Howell, A.; Duy, J.C.; Forney, C.F.; McDonald, J.E. Horticultural factors affecting antioxidant capacity of blueberries and other small fruits. HortTechnology 2001, 11, 523–528. [Google Scholar]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar]
- Brambilla, A.; Scalzo, R.L.; Bertolo, G.; Torreggiani, D. Steam-blanched highbush blueberry (Vaccinium corymbosum L.) juice: Phenolic profile and antioxidant capacity in relation to cultivar selection. J. Agric. Food Chem. 2008, 56, 2643–2648. [Google Scholar]
- Kim, S.-M.; Shang, Y.F.; Um, B.-H. Preparative separation of chlorogenic acid by centrifugal partition chromatography from highbush blueberry leaves (Vaccinium corymbosum L.). Phytochem. Anal. 2010, 21, 457–462. [Google Scholar]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.L.; Arnason, J.T. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar]
- Pantelić, M.; Dabić Zagorac, D.; Davidović, S.; Todić, S.; Bešlić, Z.; Gašić, U.; Tešić, Ž.; Natić, M. Identification and quantification of phenolic compounds in berry skin. pulp. and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolyb-dic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Natić, M.; Dabić, D.; Papetti, A.; Fotirić Akšić, M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž. Analysis and characterization of phytochemicals in Mulberry (Morus alba L.) fruits grown in Vojvodina. North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar]
- Pavlović, A.; Dabić, D.; Momirović, M.; Dojčinović, B.; Milojković-Opsenica, D.; Tešić, Ž.; Natić, M. Chemical composition of two different extracts of berries harvested in Serbia. J. Agric. Food Chem. 2013, 61, 4188–4194. [Google Scholar]
- Gašić, U.; Natić, M.; Mišić, D.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Lušić, D. Chemical markers for the authentication of unifloral Salvia officinalis L. honey. J. Food Compos. Anal. 2015, 44, 128–138. [Google Scholar]
- Fotirić Akšić, M.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of Sugar Profile between Leaves and Fruits of Blueberry and Strawberry Cultivars Grown in Organic and Integrated Production System. Plants 2019, 8, 205. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar]
- Okan, O.T.; Denìz, I.; Yayli, N.; Şat, I.G.; Öz, M.; Hatìpoğlu Serdar, G. Antioxidant Activity, Sugar Content and Phenolic Profiling of Blueberries Cultivars: A Comprehensive Comparison. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 639–652. [Google Scholar]
- Dawidowicz, A.L.; Typek, R. Transformation of 5-O-caffeoylquinic acid in blueberries during high-temperature processing. J. Agric. Food Chem. 2014, 62, 10889–10895. [Google Scholar]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Sredojević, M.; Milivojević, J.; Gašić, U.; Meland, M.; Natić, M. Chemometric characterization of strawberries and blueberries according to their phenolic profile: Combined effect of cultivar and cultivation system. Molecules 2019, 24, 4310. [Google Scholar]
- Milivojević, J.; Radivojević, D.; Nikolić, M.; Dragišić-Maksimović, J. Changes in fruit quality of highbush blueberries (Vaccinium corymbosum) during the ripening season. Acta Hortic. 2016, 1139, 657–664. [Google Scholar]
- Heiberg, N.; Stubhaug, E. First results from cultivar trials with highbush blueberry in NORWAY. Acta Hortic. 2006, 715, 307–312. [Google Scholar] [CrossRef]
- Kumudini, S. Effect of radiation, temperature on cranberry photosynthesis and characterization of diurnal change in photosynthesis. J. Am. Soc. Hort. Sci. 2004, 129, 106–111. [Google Scholar]
- Milošević, T.; Milošević, N.; Simović, S. Ocena pomoloških osobina novijih sorti borovnice (Vaccinium corymbosum L.) pomoću multivariacione analize. In Proceedings of the XX Symposium of Biotechnology with International Participation, Čačak, Serbia, 13–14 March 2015; Volume 20, pp. 175–183. [Google Scholar]
- Tanny, J.; Cohen, S.; Grava, A.; Naor, A.; Lukyanov, V. The effect of shading screens on microclimate of apple orchards. Acta Hortic. 2009, 807, 103–108. [Google Scholar]
- Strik, B.C.; Bryla, D.R.; Sullivan, D.M. Organic Production Systems in Northern Highbush Blueberry: I. Impact of Planting Method, Cultivar, Fertilizer, and Mulch on Yield and Fruit Quality from Planting through Maturity. Hortscience 2017, 52, 1201–1213. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Viskelis, P.; Jasutiene, I.; Duchovskis, P.; Bobinas, C. Changes in biologically active constituents during ripening in black currants. J. Fruit Ornam. Plant Res. 2006, 14, 237–246. [Google Scholar]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest date affects aronia juice polyphenols, sugars, and antioxidant activity, but not anthocyanin stability. Food Chem. 2015, 187, 189–196. [Google Scholar]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 2015, 95, 776–785. [Google Scholar]
- Ketata, M.; Desjardins, Y.; Ratti, C. Effect of liquid nitrogen pretreatments on osmotic dehydration of blueberries. J. Food Eng. 2013, 116, 202–212. [Google Scholar]
- Helena, D.; Guimaraes, P.E.; Alves, G.L.; Querido, A.F. Influence of gelling substance on sensory quality blueberry (climax) jelly. Int. J. Biotech. Food Sci. 2014, 2, 116–120. [Google Scholar]
- Garcia-Alonso, M.; De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar]
- Gündoğdu, M.; Ercisli, S.; Canan, I.; Orman, E.; Sameeullah, M.; Naeem, M.; Ayed, R.B. Diversity in phenolic compounds, biochemical and pomological characteristics of Arbutus unedo fruits. Folia Hortic. 2018, 30, 139–146. [Google Scholar] [CrossRef]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci. Rep. 2019, 9, 5894. [Google Scholar]
- Kim, J.G.; Kim, H.G.; Kim, S.J.; Park, K.-S. Fruit quality, anthocyanin and total phenolic contents, and antioxidant activities of 45 blueberry cultivars grown in Suwon, Korea. J. Zhejiang Univ. Sci. B 2013, 14, 793–799. [Google Scholar]
- İsbilir, S.S.; Orak, H.H.; Yagar, H.; Ekinci, N. Determination of antioxidant activities of strawberry tree (Arbutus unedo L.) Flowers and fruits at different ripening stages. Acta Sci. Pol. Hortorum Cultus 2012, 11, 223–237. [Google Scholar]
- Elmastaş, M.; Demir, A.; Genc, N.; Dolek, U.; Guneş, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef]
- Pedisic, S.; Levaj, B.; Dragovic-Uzelac, V.; Kos, K. Physicochemical composition, Phenolic Content and Antioxidant Activity of Sour Cherry Cv. Marasca during Ripening. Agric. Conspec. Sci. 2007, 72, 295–300. [Google Scholar]
- Liu, H.; Jiang, W.; Cao, J.; Li, Y. Changes in extractable and non-extractable polyphenols and their antioxidant properties during fruit on-tree ripening in five peach cultivars. Hortic. Plant J. 2019, 5, 137–144. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Veberič, R.; Štampar, F. Quality changes during ripening of plums (Prunus domestica L.). Food Chem. 2008, 111, 830–836. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Bertoft, E.; Acar, C.; Turna, I. Effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium arctostaphylos and V. myrtillus) native to Turkey. N. Z. J. Crop Hortic. Sci. 2001, 29, 137–141. [Google Scholar]
- Šne, E.; Kampuse, S.; Berņa, E. The composition of sugars and sugar-acid ratio of highbush blueberry varieties grown in Latvia. In Proceedings of the Annual 17th International Scientific Conference Proceedings, “Research for Rural Development 2011”, Jelgava, Latvia, 18–20 May 2011; Volume 1, pp. 140–144. [Google Scholar]
- Kader, F.; Rovel, B.; Metche, M. Role of invertase in sugar content in highbush blueberries (Vaccinium corymbosum L.). LWT Food Sci. Technol. 1993, 26, 593–595. [Google Scholar]
- Nindo, C.I.; Tang, J.; Powers, J.R.; Singh, P. Viscosity of blueberry and raspberry juices for processing applications. J. Food Eng. 2005, 69, 335–343. [Google Scholar]
- Forney, C.F.; Kalt, W.; Jordan, J.A.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E. Compositional changes in blueberry and cranberry fruit during ripening. Acta Hortic. 2012, 926, 331–337. [Google Scholar] [CrossRef]
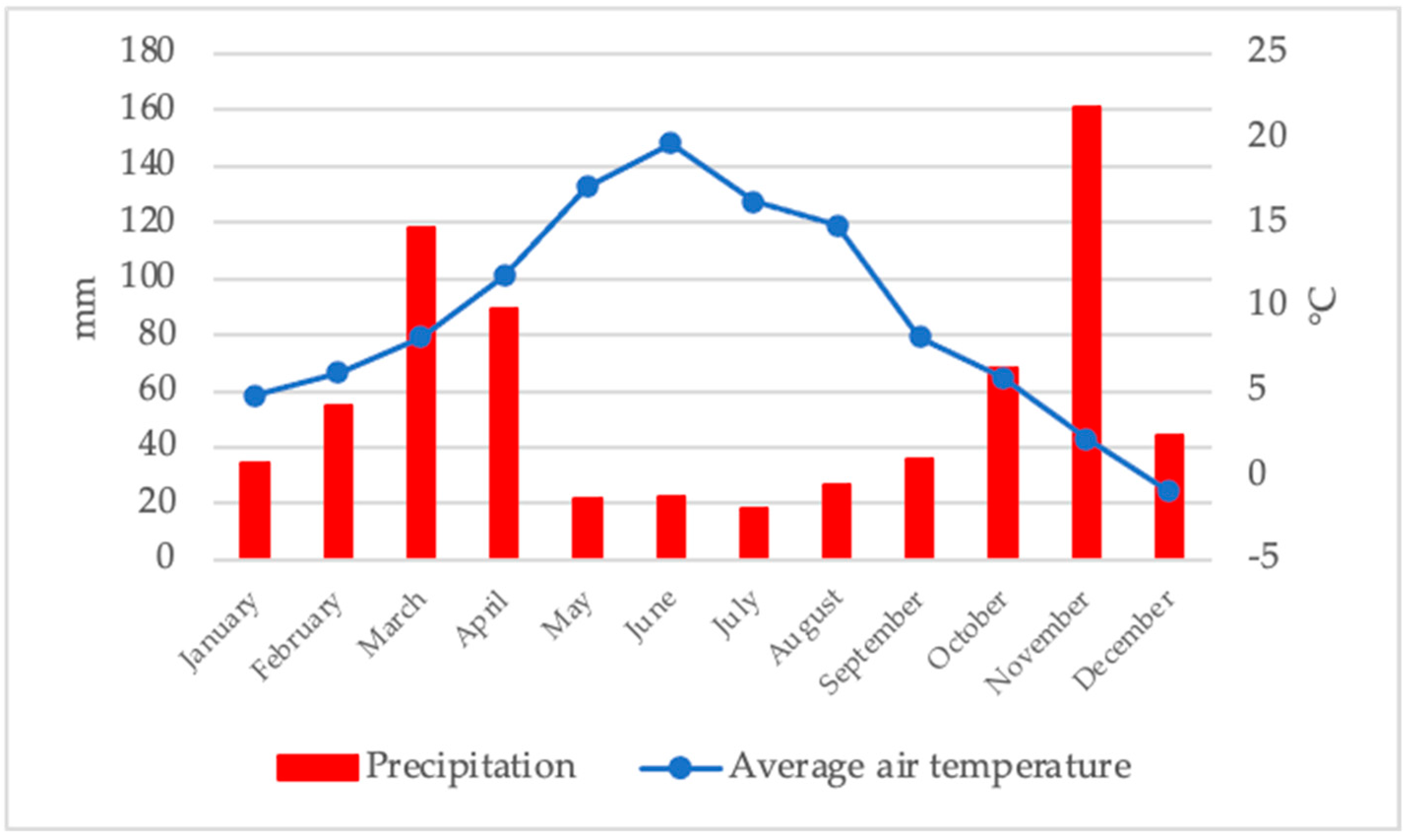
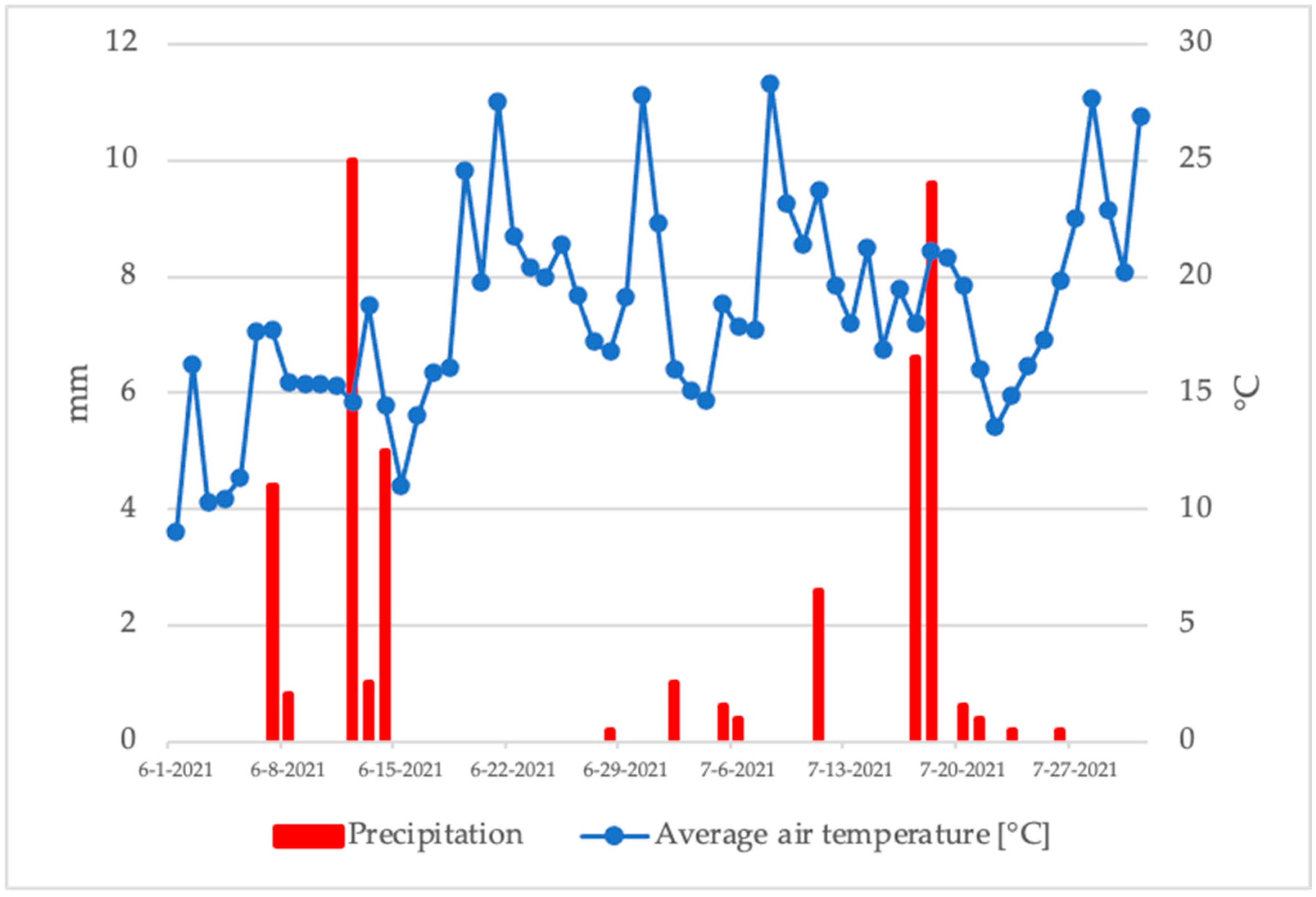

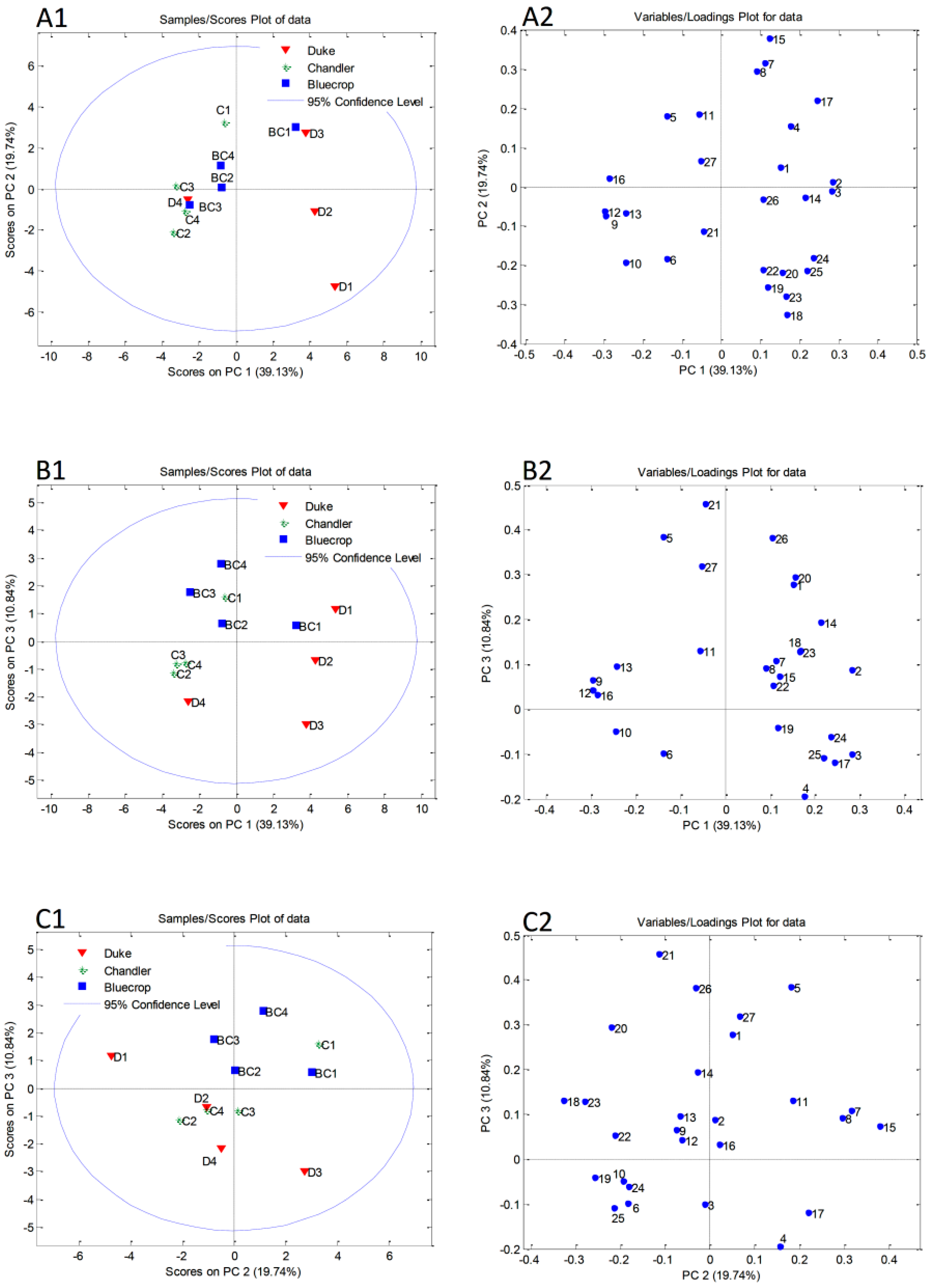
| Cultivar × Harvest Date | Abbrevation | Harvest Dates | % of Mature Fruits |
|---|---|---|---|
| ‘Duke’–1st harvest | D1 | 14 June 2021 | 10 |
| ‘Duke’–2nd harvest | D2 | 21 June 2021 | 20 |
| ‘Duke’–3rd harvest | D3 | 28 June 2021 | 50 |
| ‘Duke’–4th harvest | D4 | 05 July 2021 | 20 |
| ‘Bluecrop’–1st harvest | BC1 | 21 June 2021 | 10 |
| ‘Bluecrop’–2nd harvest | BC2 | 28 June 2021 | 15 |
| ‘Bluecrop’–3rd harvest | BC3 | 05 July 2021 | 35 |
| ‘Bluecrop’–4th harvest | BC4 | 12 July 2021 | 40 |
| ‘Chandler’–1st harvest | C1 | 05 July 2021 | 10 |
| ‘Chandler’–2nd harvest | C2 | 12 July 2021 | 20 |
| ‘Chandler’–3rd harvest | C3 | 19 July 2021 | 40 |
| ‘Chandler’–4th harvest | C4 | 26 July 2021 | 30 |
| Cultivar | Bluecrop | Duke | Chandler |
|---|---|---|---|
| 2301.80 | 5331.40 | 6085.20 | |
| S | 109.28 | 232.11 | 191.37 |
| Cv | 10.61 | 9.73 | 7.03 |
| Variety | Yield (kg/bush) | Yield (t/ha) |
|---|---|---|
| Bluecrop | 3.66 | 8.13 |
| Duke | 7.52 | 17.39 |
| Chandler | 7.59 | 16.87 |
| Cultivar × Harvest Date | Fruit Weight (g) | Fruit Shape Index | Total Soluble Solids (%) | Total Acids (%) | Total Soluble Solids/Total Acids Ratio | Fruit Juice pH Value |
|---|---|---|---|---|---|---|
| D1 | 1.73 ± 0.05 de* | 0.68 ± 0.0060 a | 11.03 ± 0.22 ab | 0.71 ± 0.13 ab | 16.57 ± 3.13 ab | 3.17 ± 0.07 |
| D2 | 1.69 ± 0.05 d | 0.73 ± 0.0035 bc | 11.74 ± 0.87 bc | 0.85 ± 0.17 ab | 15.10 ± 3.31 ab | 3.03 ± 0.07 |
| D3 | 1.78 ± 0.01 de | 0.72 ± 0.0052 b | 12.56 ± 1.78 cd | 0.71 ± 0.08 ab | 17.83 ± 2.19 ab | 3.03 ± 0.07 |
| D4 | 1.16 ± 0.03 ab | 0.69 ± 0.0054 a | 13.82 ± 0.33 d | 0.55 ± 0.13 a | 28.93 ± 8.24 bc | 3.07 ± 0.09 |
| C1 | 1.16 ± 0.005 ab | 0.78 ± 0.0060 d | 11.62 ± 0.37 ab | 0.78 ± 0.07 ab | 15.03 ± 1.13 ab | 3.03 ± 0.07 |
| C2 | 1.24 ± 0.04 b | 0.77 ± 0.0103 cd | 12.20 ± 0.18 cd | 1.45 ± 0.05 c | 8.43 ± 0.31 a | 2.90 ± 0.06 |
| C3 | 1.50 ± 0.01 c | 0.77 ± 0.0082 cd | 12.98 ± 0.36 cd | 0.84 ± 0.16 ab | 16.57 ± 3.09 ab | 3.03 ± 0.03 |
| C4 | 1.09 ± 0.03 a | 0.82 ± 0.0161 e | 10.55 ± 0.41 a | 1.07 ± 0.16 b | 10.47 ± 2.17 a | 3.00 ± 0.06 |
| BC1 | 1.81 ± 0.04 e | 0.70 ± 0.0180 ab | 11.54 ± 1.05 ab | 0.96 ± 0.18 b | 13.28 ± 3.18 ab | 3.00 ± 0.06 |
| BC2 | 1.70 ± 0.05 d | 0.71 ± 0.0033 ab | 11.98 ± 0.71 bc | 0.44 ± 0.18 a | 38.63 ± 15.28 c | 3.33 ± 0.26 |
| BC3 | 1.12 ± 0.03 a | 0.71 ± 0.0037 ab | 15.04 ± 0.92 e | 0.90 ± 0.21 b | 18.47 ± 4.28 ab | 3.17 ± 0.03 |
| BC4 | 1.24 ± 0.02 b | 0.75 ± 0.0108 c | 12.46 ± 0.28 cd | 1.12 ± 0.06 bc | 11.18 ± 0.60 a | 2.87 ± 0.03 |
| Samples | TPC (g GAE/kg) | RSA (mmol TE/kg) | TAC (g cy-3-glu/kg) |
|---|---|---|---|
| D1 | 2.52 ± 0.02 a* | 27.64 ± 1.46 bcde | 1.65 ± 0.01 b |
| D2 | 2.52 ± 0.02 a | 29.66 ± 0.00 a | 1.75 ± 0.01 a |
| D3 | 2.35 ± 0.01 b | 27.78 ± 0.51 cd | 1.61 ± 0.02 c |
| D4 | 1.45 ± 0.02 i | 24.83 ± 0.06 f | 0.49 ± 0.00 k |
| C1 | 1.78 ± 0.01 f | 25.91 ± 1.11 ef | 0.97 ± 0.01 e |
| C2 | 1.64 ± 0.02 h | 24.71 ± 1.48 ef | 0.78 ± 0.00 g |
| C3 | 1.74 ± 0.01 g | 26.02 ± 1.33 def | 0.96 ± 0.01 ef |
| C4 | 1.61 ± 0.01 h | 26.16 ± 0.60 e | 0.73 ± 0.00 h |
| BC1 | 2.51 ± 0.00 a | 29.07 ± 1.39 abc | 1.34 ± 0.01 d |
| BC2 | 2.21 ± 0.01 c | 29.05 ± 1.66 abc | 0.95 ± 0.00 f |
| BC3 | 1.86 ± 0.01 e | 29.98 ± 1.43 abc | 0.56 ± 0.00 j |
| BC4 | 1.98 ± 0.04 d | 29.02 ± 1.34 abc | 0.71 ± 0.00 i |
| Samples | Aesculin | p-Hydroxybenzoic Acid | 5-O-Caffeoylquinic Acid | Caffeic Acid | Aesculetin | Rutin | Quercetin 3-O-glucoside | Ellagic acid | Kaempferol 3-O-glucoside | Quercetin 3-O-rhamnoside | Phloridzin | Quercetin | Kaempferol | Isorhamnetin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | 0.467 c* | 0.000 | 6.405 d | 0.344 c | 0.378 b | 6.956 a | 17.239 b | 0.000 | 0.410 a | 0.000 | 0.309 d | 62.995 b | 1.032 a | 2.857 c |
| D2 | 0.661 d | 0.000 | 2.789 ab | 0.283 b | 0.451 c | 6.043 a | 17.432 b | 0.000 | 0.471 a | 0.000 | 0.236 c | 85.858 cd | 1.306 a | 5.023 e |
| D3 | 1.276 f | 0.000 | 2.191 a | 0.566 e | 0.630 e | 4.780 a | 13.985 a | 0.000 | 0.327 a | 0.000 | 0.179 b | 119.136 e | 1.191 a | 6.999 f |
| D4 | 0.302 ab | 0.000 | 8.737 f | 0.285 b | 0.555 d | 25.642 d | 21.462 c | 0.000 | 5.127 d | 0.000 | 0.091 a | 69.045 b | 2.409 c | 0.752 a |
| C1 | 0.455 c | 11.048 f | 3.746 c | 0.709 f | 0.833 f | 16.367 b | 18.261 b | 0.708 b | 3.392 b | 7.318 a | 0.143 ab | 130.78 f1 | 2.109 bc | 3.927 d |
| C2 | 0.277 a | 0.970 a | 34.793 h | 0.318 bc | 0.230 a | 24.612 cd | 22.717 c | 0.000 | 5.767 d | 15.452 c | 0.159 b | 47.120 a | 2.042 b | 1.127 b |
| C3 | 0.297 a | 4.693 c | 3.241 bc | 0.284 b | 0.252 a | 24.277 cd | 24.354 d | 0.298 a | 7.151 f | 12.307 b | 0.167 b | 90.864 d | 2.397 c | 0.956 a |
| C4 | 0.348 b | 6.058 d | 7.584 e | 0.187 a | 0.356 b | 22.736 c | 22.449 c | 0.000 | 6.575 e | 13.912 b | 0.137 ab | 57.267 ab | 1.913 b | 0.863 a |
| BC1 | 0.463 c | 3.407 b | 3.202 bc | 0.552 e | 0.549 d | 5.644 a | 12.012 a | 0.000 | 0.256 a | 0.000 | 0.338 d | 136.391 f | 1.109 a | 6.905 f |
| BC2 | 0.774 e | 4.080 c | 6.493 d | 0.279 b | 0.330 b | 21.309 c | 22.406 c | 0.000 | 5.229 d | 12.174 b | 0.288 cd | 81.966 c | 1.996 b | 1.471 b |
| BC3 | 0.398 bc | 8.238 e | 9.982 g | 0.271 b | 0.419 c | 26.254 d | 23.189 cd | 0.000 | 6.041 e | 12.395 b | 0.140 ab | 72.343 b | 2.081 b | 0.700 a |
| BC4 | 0.440 c | 6.702 d | 3.589 c | 0.449 d | 0.569 d | 21.425 c | 16.110 b | 0.000 | 4.816 c | 5.929 a | 0.207 bc | 92.895 d | 2.054 b | 1.257 b |
| Samples | Sorbitol | Trehalose | Glucose | Fructose | Sucrose | Isomaltose | Turanose | Gentiobiose | Maltose | Maltotriose |
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | 3.781 e* | 0.019 c | 44.471 c | 32.659 d | 2.267 e | 0.077 b | 0.088 d | 0.002 | 0.144 d | 0.036 ab |
| D2 | 1.872 d | 0.001 a | 39.859 bc | 28.124 c | 1.564 c | 0.003 a | 0.125 e | 0.002 | 0.061 b | 0.025 a |
| D3 | 0.608 ab | 0.004 b | 24.708 a | 15.159 a | 1.209 bc | 0.002 a | 0.038 c | 0.001 | 0.026 a | 0.018 a |
| D4 | 0.842 b | 0.009 a | 27.749 a | 19.197 a | 1.097 b | 0.001 a | 0.002 a | 0.000 | 0.028 a | 0.031 a |
| C1 | 0.730 b | 0.003 ab | 26.864 a | 33.680 d | 1.844 d | 0.002 a | 0.002 a | 0.000 | 0.071 b | 0.073 c |
| C2 | 0.712 b | 0.002 a | 26.516 a | 33.978 d | 1.731 d | 0.001 a | 0.002 a | 0.000 | 0.027 a | 0.038 ab |
| C3 | 0.485 a | 0.002 a | 22.424 a | 27.080 c | 0.922 ab | 0.001 a | 0.003 a | 0.000 | 0.017 a | 0.037 ab |
| C4 | 1.044 c | 0.004 b | 24.586 a | 24.908 b | 1.015 b | 0.001 a | 0.003 a | 0.001 | 0.049 ab | 0.090 d |
| BC1 | 0.412 a | 0.001 a | 28.632 a | 29.396 c | 0.682 a | 0.002 a | 0.005 a | 0.000 | 0.123 c | 0.028 a |
| BC2 | 0.734 b | 0.003 ab | 24.809 a | 24.993 b | 0.850 a | 0.003 a | 0.008 b | 0.000 | 0.196 e | 0.057 b |
| BC3 | 1.256 c | 0.000 | 38.287 b | 37.820 e | 1.023 b | 0.002 a | 0.003 a | 0.000 | 0.073 b | 0.020 a |
| BC4 | 0.825 b | 0.001 a | 36.775 b | 31.960 d | 1.071 b | 0.001 a | 0.004 a | 0.000 | 0.126 c | 0.329 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvetković, M.; Kočić, M.; Dabić Zagorac, D.; Ćirić, I.; Natić, M.; Hajder, Đ.; Životić, A.; Fotirić Akšić, M. When Is the Right Moment to Pick Blueberries? Variation in Agronomic and Chemical Properties of Blueberry (Vaccinium corymbosum) Cultivars at Different Harvest Times. Metabolites 2022, 12, 798. https://doi.org/10.3390/metabo12090798
Cvetković M, Kočić M, Dabić Zagorac D, Ćirić I, Natić M, Hajder Đ, Životić A, Fotirić Akšić M. When Is the Right Moment to Pick Blueberries? Variation in Agronomic and Chemical Properties of Blueberry (Vaccinium corymbosum) Cultivars at Different Harvest Times. Metabolites. 2022; 12(9):798. https://doi.org/10.3390/metabo12090798
Chicago/Turabian StyleCvetković, Miljan, Milana Kočić, Dragana Dabić Zagorac, Ivanka Ćirić, Maja Natić, Đurađ Hajder, Aleksandar Životić, and Milica Fotirić Akšić. 2022. "When Is the Right Moment to Pick Blueberries? Variation in Agronomic and Chemical Properties of Blueberry (Vaccinium corymbosum) Cultivars at Different Harvest Times" Metabolites 12, no. 9: 798. https://doi.org/10.3390/metabo12090798
APA StyleCvetković, M., Kočić, M., Dabić Zagorac, D., Ćirić, I., Natić, M., Hajder, Đ., Životić, A., & Fotirić Akšić, M. (2022). When Is the Right Moment to Pick Blueberries? Variation in Agronomic and Chemical Properties of Blueberry (Vaccinium corymbosum) Cultivars at Different Harvest Times. Metabolites, 12(9), 798. https://doi.org/10.3390/metabo12090798









