Metabolic Profiling of Jasminum grandiflorum L. Flowers and Protective Role against Cisplatin-Induced Nephrotoxicity: Network Pharmacology and In Vivo Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. JGF Extraction
2.2. JGF Analysis
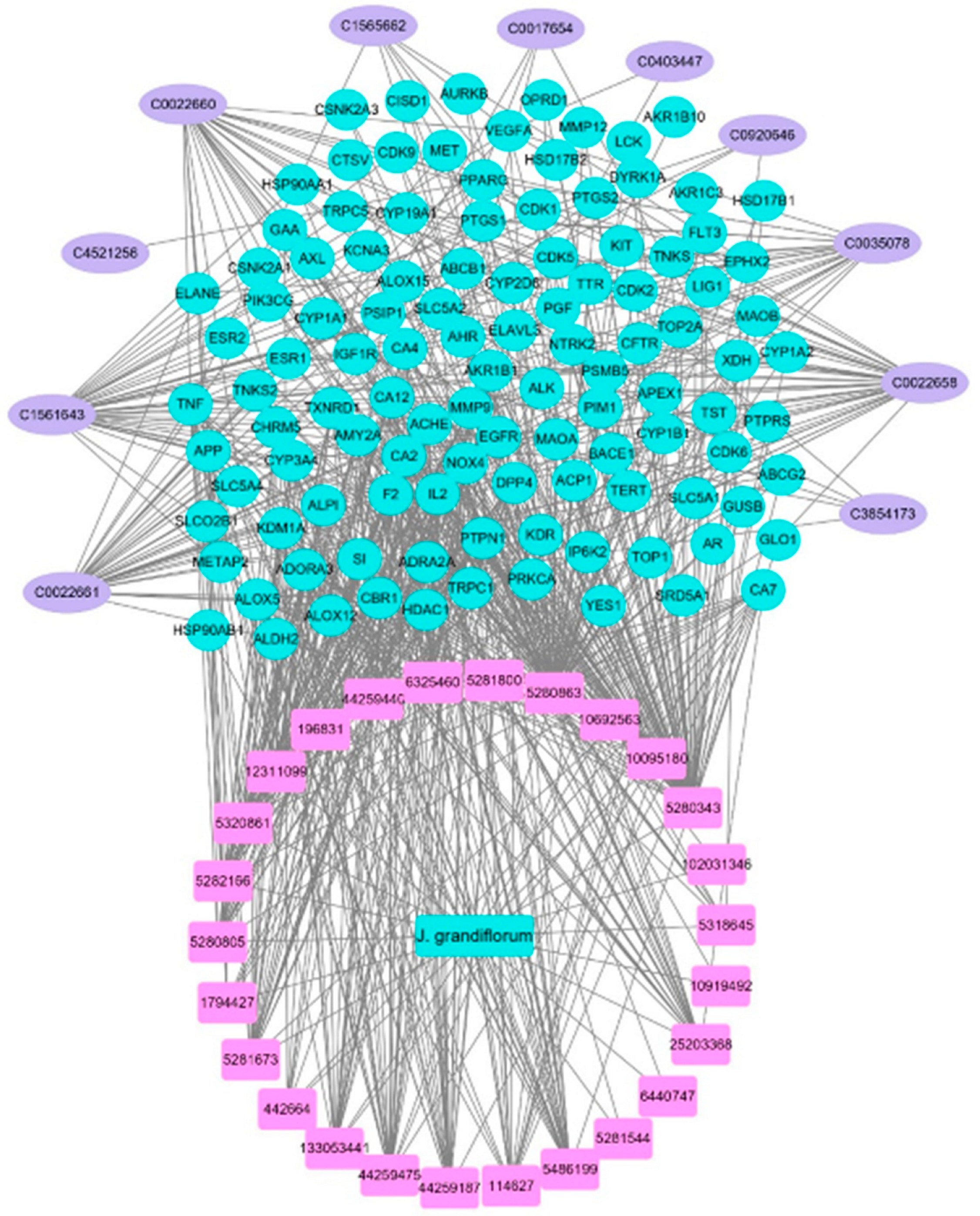
2.3. Prediction of Bioactive Ingredients of JGF
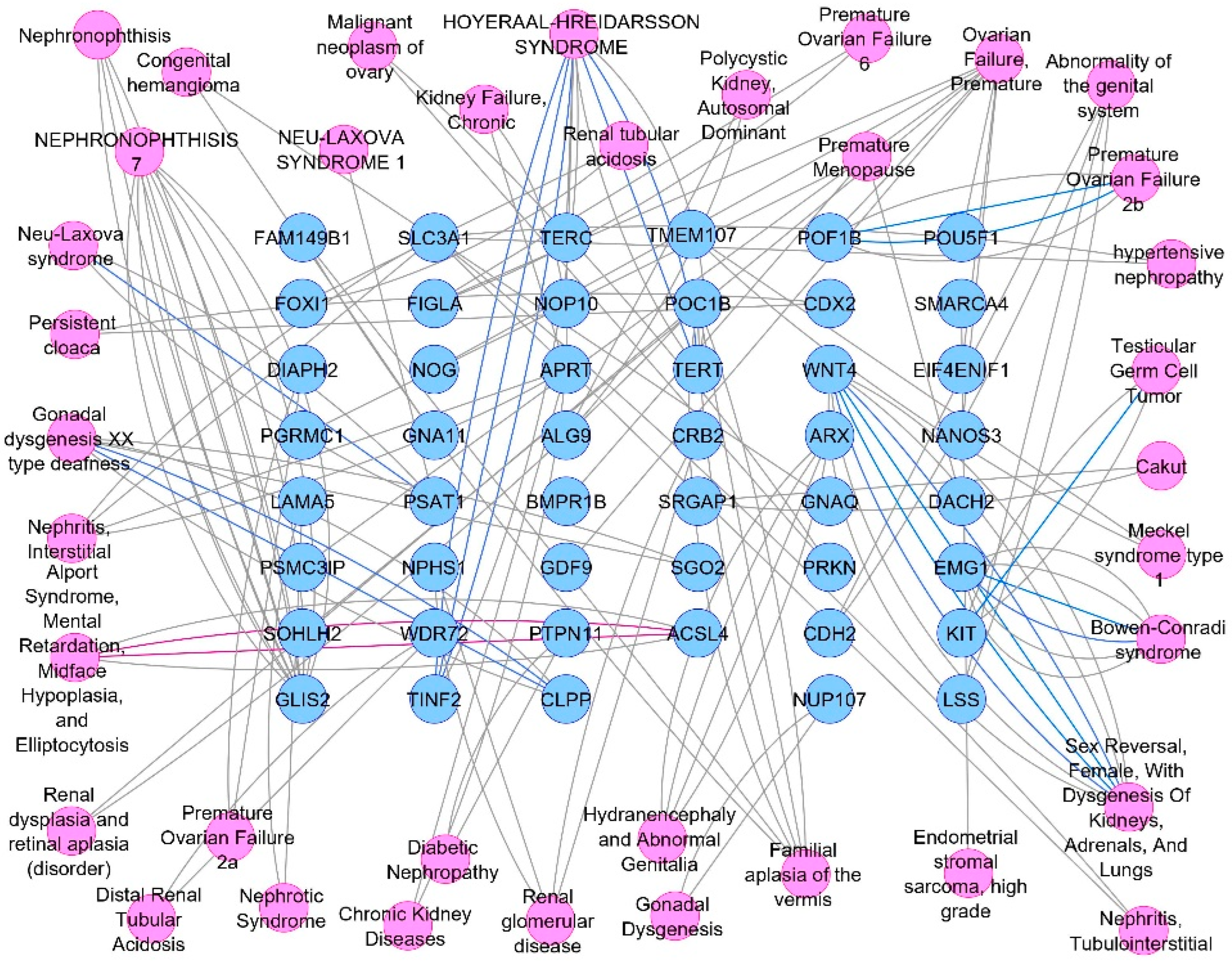
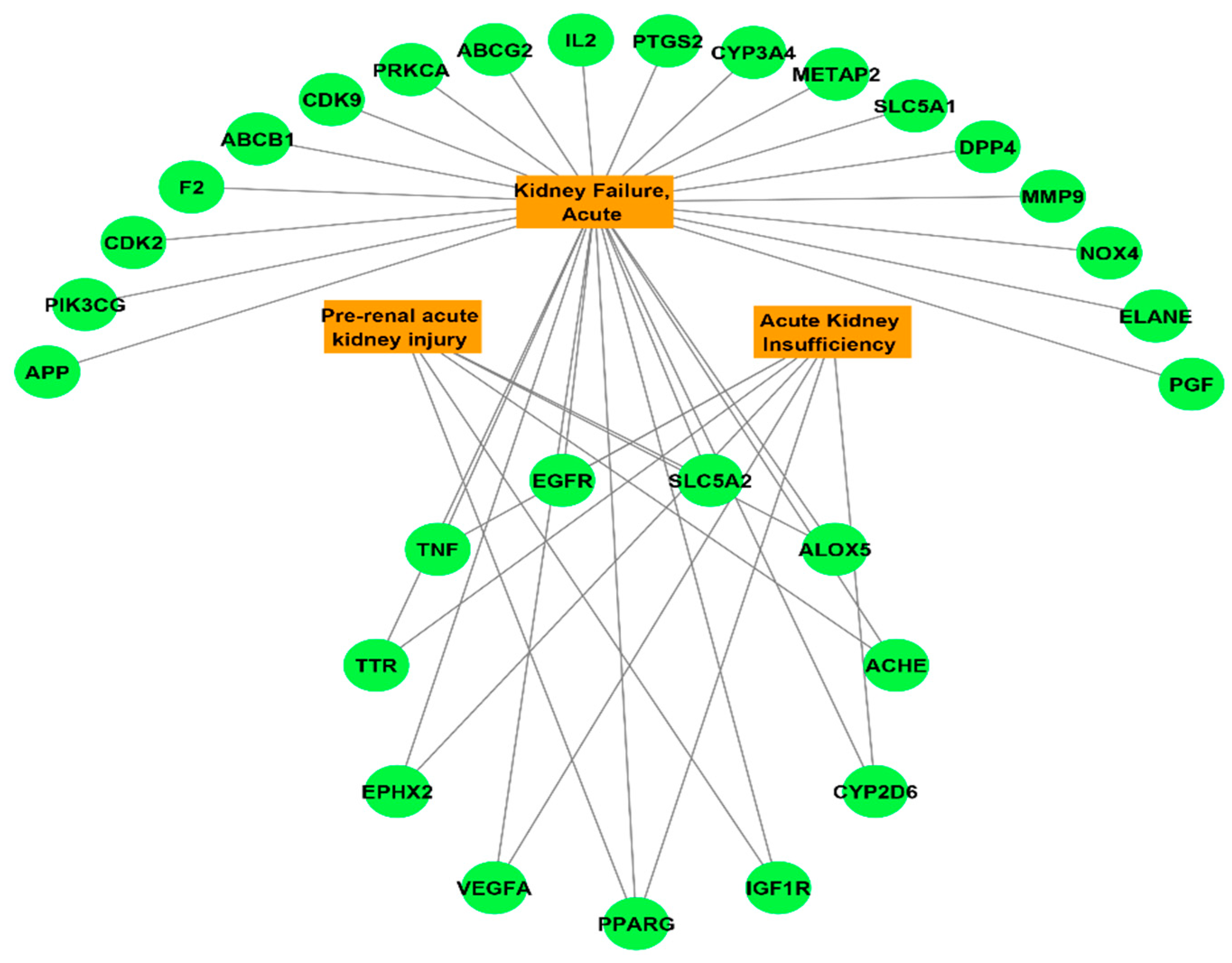
2.4. Potential Targets Intersection of JGF with Disease
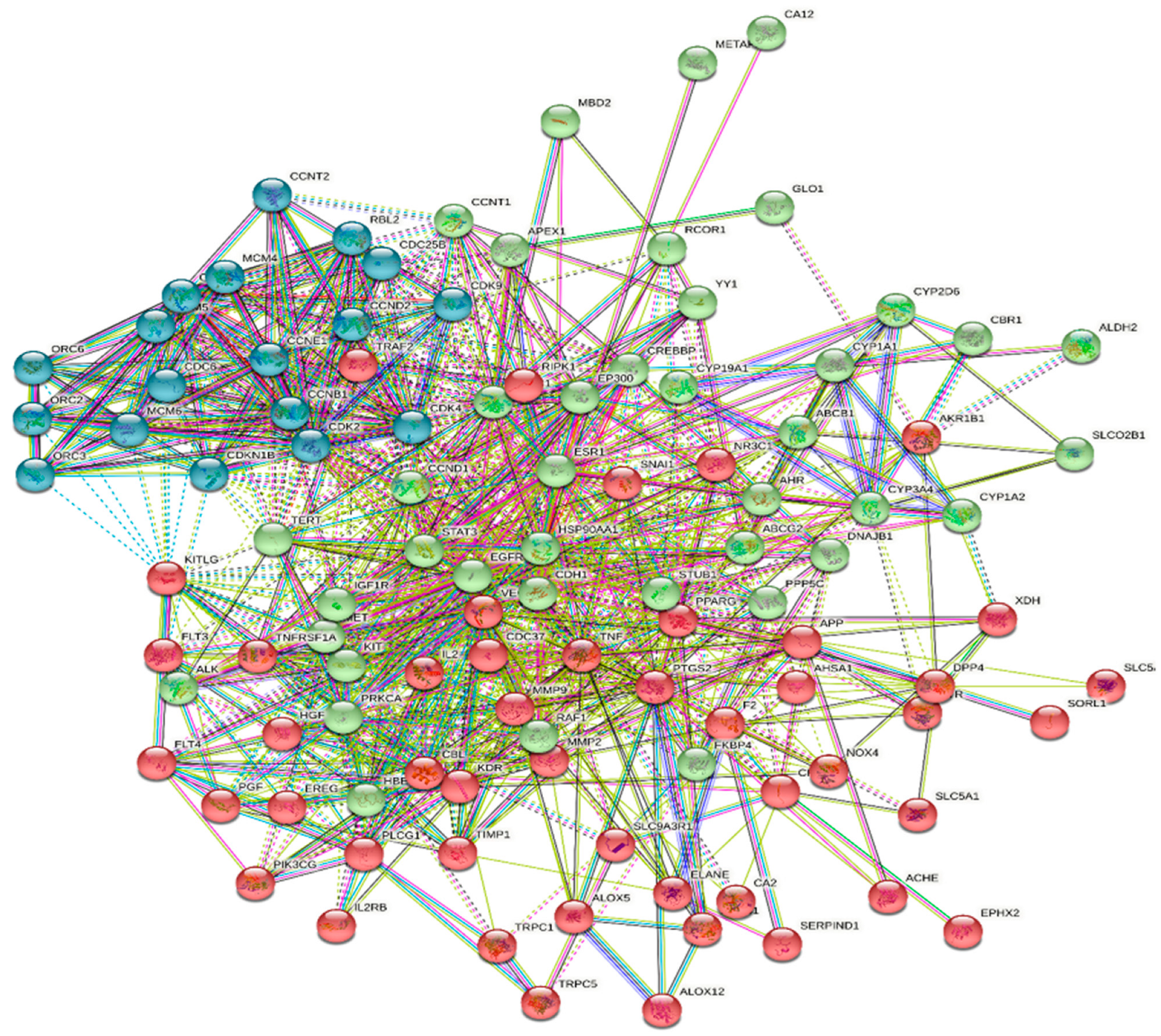
2.5. Protein–Protein Interaction (PPI) Data
2.6. Network Construction and Visualization
2.7. Pathway and Functional Enrichment Analysis
2.8. Animals
2.9. In Vivo Experimental Design
2.10. Estimation of Blood Urea Nitrogen and Serum Creatinine
2.11. Determination of Malondialdehyde (MDA) of Lipid Peroxidation
2.12. Determination of Glutathione Levels (GSH) in Kidney Tissues
2.13. RNA Extraction and Gene Expression Studies
2.14. Histopathology Assessment
2.15. Statistical Analysis
3. Results
3.1. JGF Phytochemical Profile
3.2. Compound–Target Network Construction
3.3. Potential Targets Intersection of JGF with Disease
3.4. Compounds–Common Targets–Renal Failure Pharmacology Network
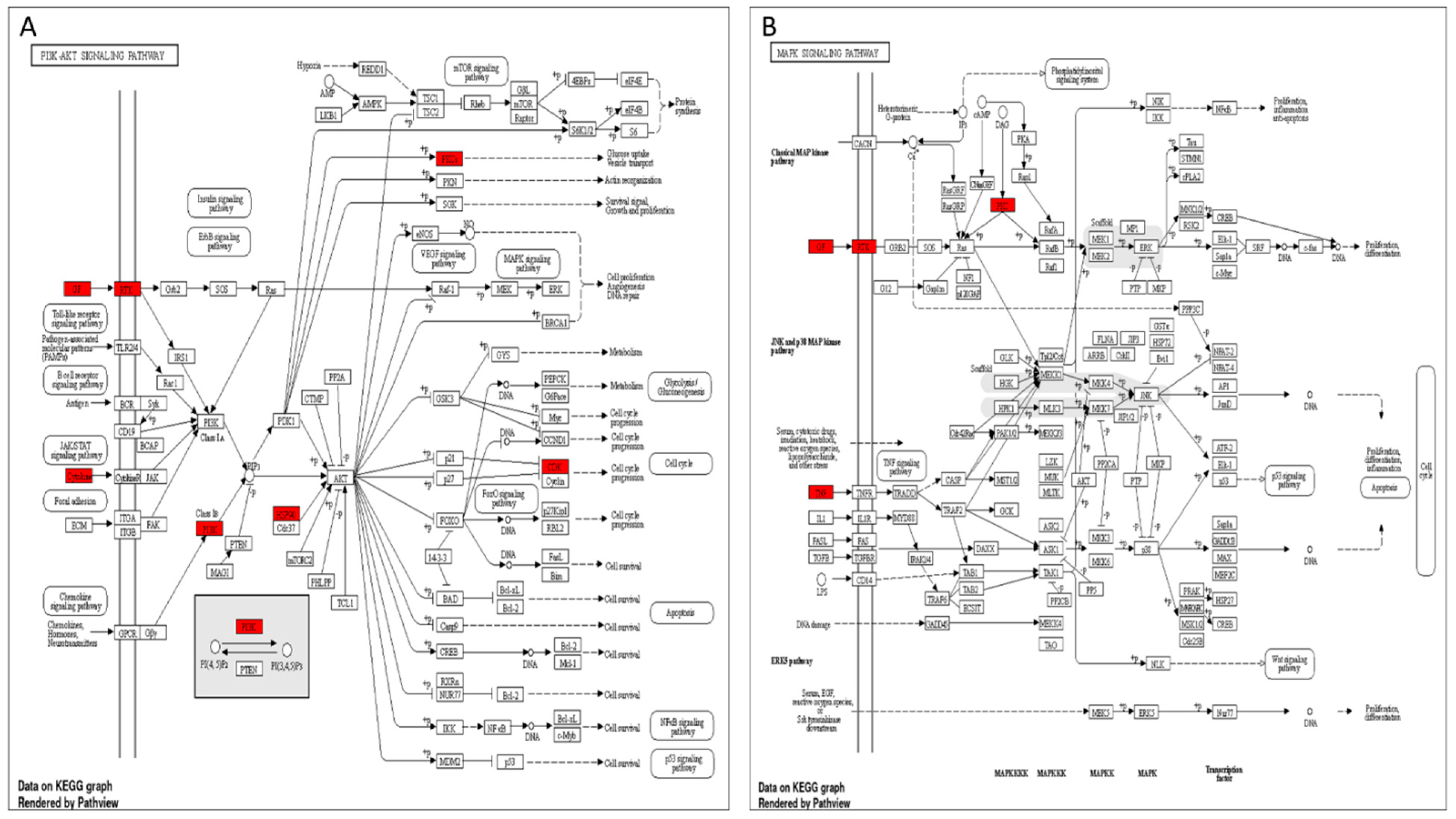
3.5. Target Genes–Pathways Network
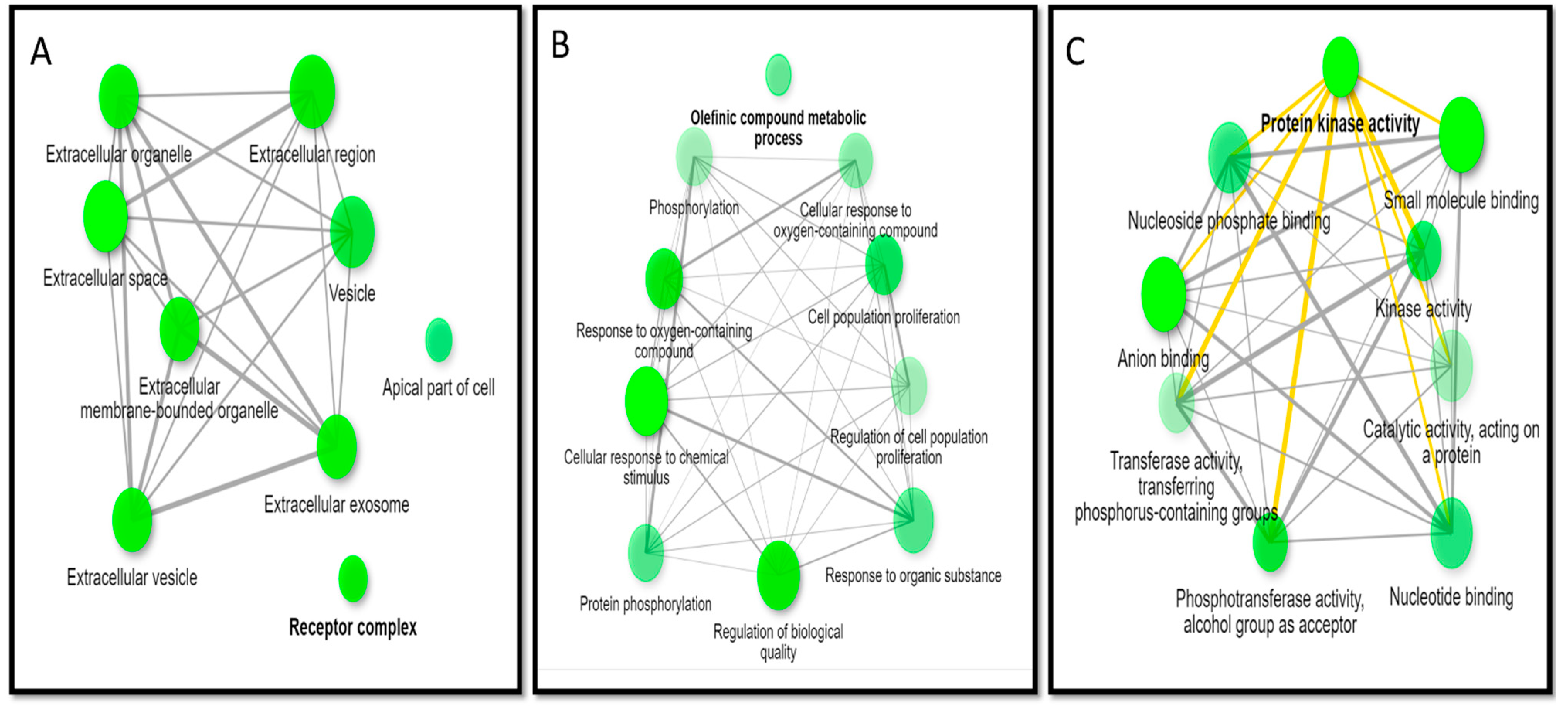
3.6. GO Enrichment Analysis
3.7. In Vivo Studies
3.7.1. Effects of JGF on Bodyweight, BUN, and Serum Creatinine
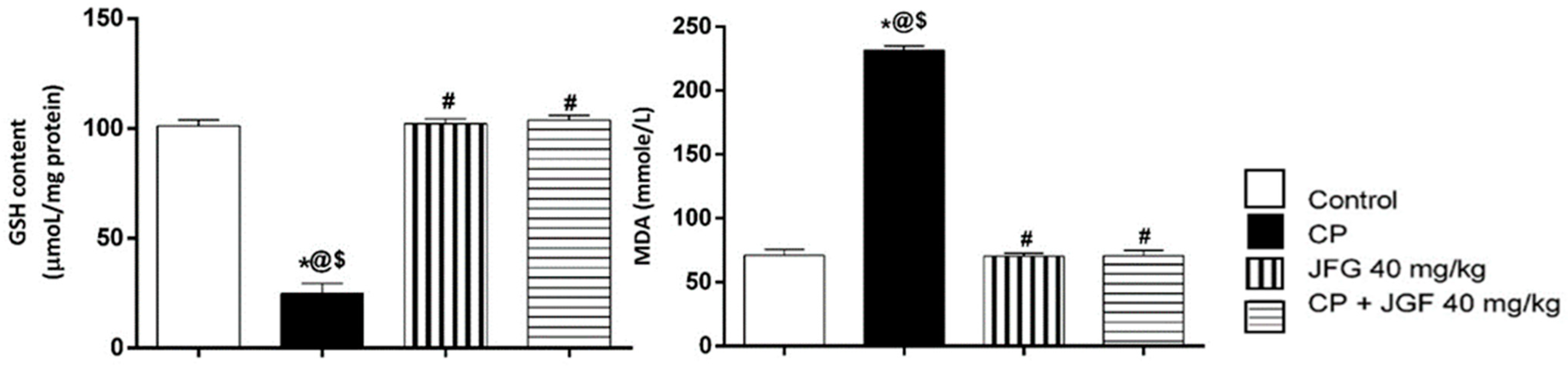
3.7.2. Effects of JGF on Renal Oxidative Stress
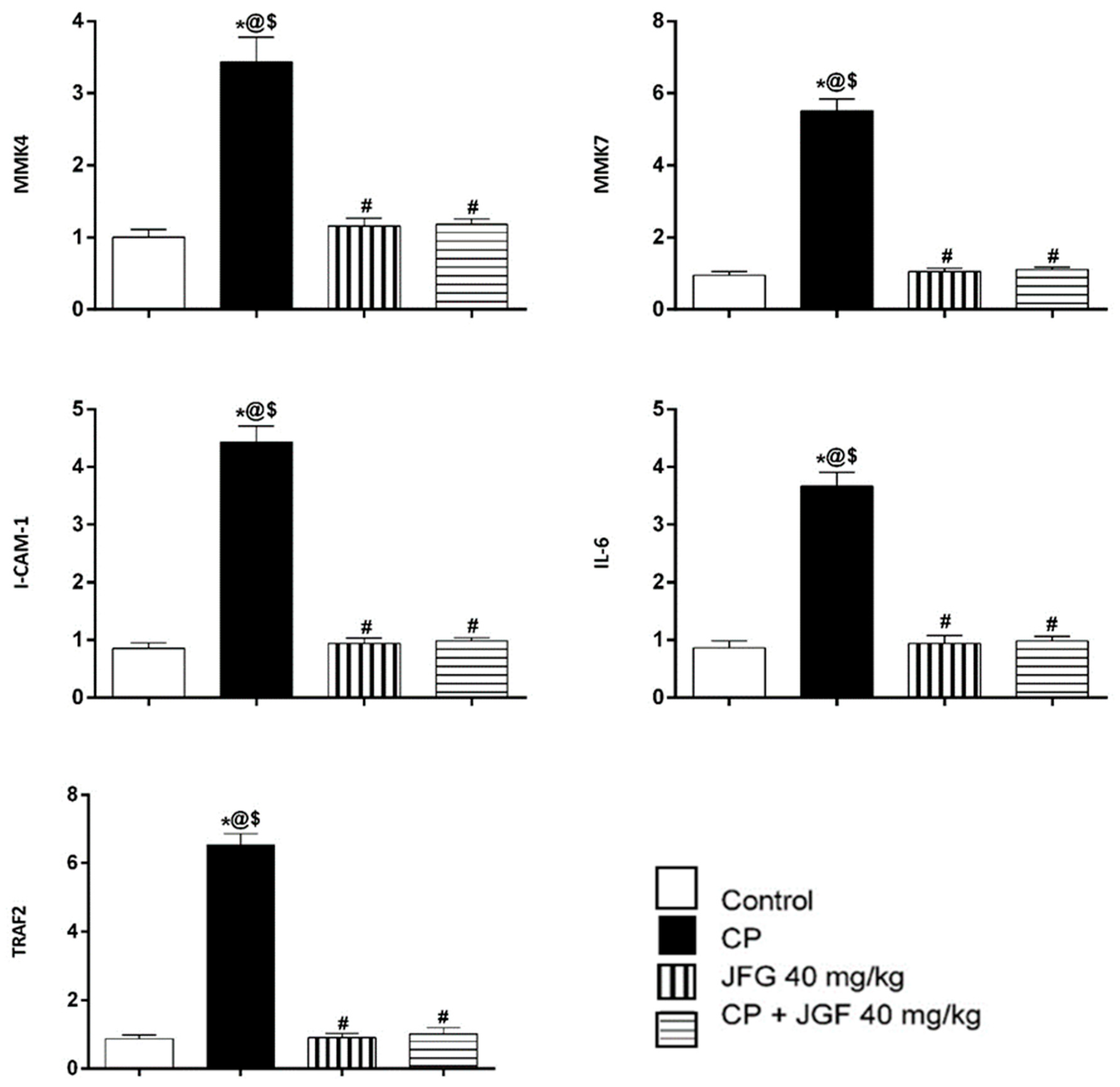
3.7.3. Effects of JGF on the Levels of Gene Expression
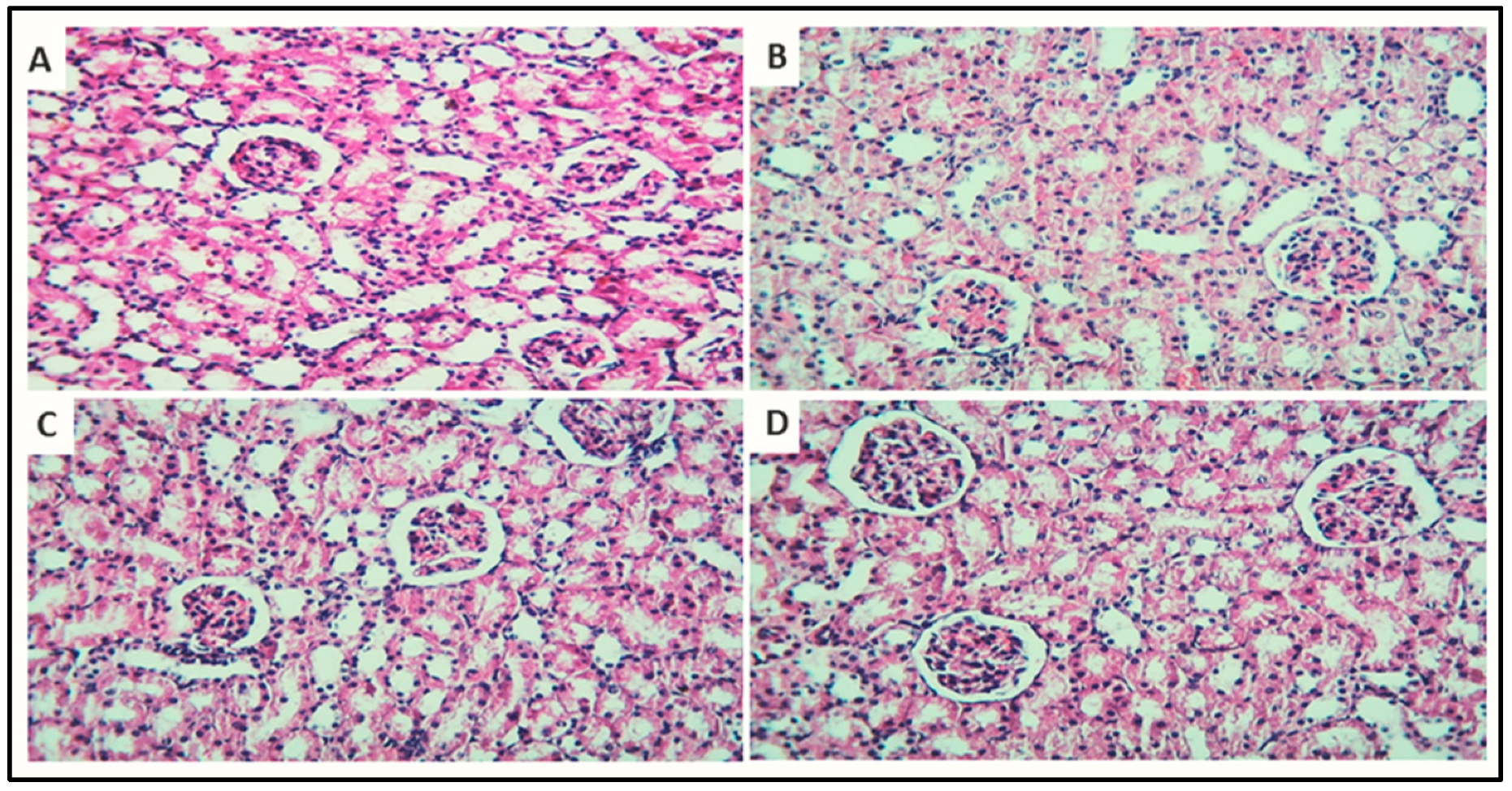
3.7.4. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTPN1 | Receptor-interacting serine/threonine-protein kinase 1 |
| NOX4 | NADPH oxidase 4 |
| TNF | Tumor necrosis factor |
| p38 MAPK | Mitogen-activated protein kinase signaling pathway |
| HSP90AA1 | Heat shock protein HSP 90-alpha |
| ESR1 | Estrogen receptor |
| VEGFA | Vascular endothelial growth factor A |
| EGFR | Epidermal growth factor receptor |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PTGS2 | Cyclooxygenase |
| HSP90AB1 | Heat shock protein HSP 90-beta |
| CA4 | Carbonic anhydrase 4 |
| MMP9 | Matrix metalloproteinase 9 |
| AR | Androgen Receptor |
| CYP3A4 | Cytochrome P450 3A4 |
| HDAC1 | Histone deacetylase |
| APP | beta-Glucuronidase (β-glucuronidase) |
| CDK1 | Cyclin-dependent kinase 1 |
| CDK2 | Cyclin-dependent kinase 2 |
| KDR | Vascular endothelial growth factor receptor 2 |
| ERK | Extracellular signal-regulated kinases |
| JNK | c-Jun N-terminal protein kinases (JNK), also known as stress-activated protein kinases |
| STRING | The STRING database is one of several online resources dedicated to organism-wide protein association networks |
| DisGeNET | A discovery platform containing one of the largest publicly available collections of genes and variants associated with human diseases |
| HDAC1 | Histone deacetylase |
| GO | Gene ontology |
| JGF | Jasminum grandiflorum flowers |
| HPLC-PDA/MS | High-performance liquid chromatographic coupled with diode array detector/mass spectrometry |
References
- Najafi, M.; Tavakol, S.; Zarrabi, A.; Ashrafizadeh, M. Dual role of quercetin in enhancing the efficacy of cisplatin in chemotherapy and protection against its side effects: A review. Arch. Physiol. Biochem. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; El-Masry, T.A.; Elekhnawy, E.; El-Kadem, A.H.; Saleh, A.; Negm, W.A.; Abdelkader, D.H. Aqueous core epigallocatechin gallate PLGA nanocapsules: Characterization, antibacterial activity against uropathogens, and in vivo reno-protective effect in cisplatin induced nephrotoxicity. Drug Deliv. 2022, 29, 1848–1862. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, X.; Li, A.; Zhao, Z.; Li, S. S-Allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation. Nutrients 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sulakhiya, K.; Barua, C.C.; Mundhe, N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol. Cell. Biochem. 2017, 431, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.J.; Ha, C.-M.; Choi, Y.-K.; Park, S.; Choe, M.S.; Jeoung, N.H.; Huh, Y.H.; Kim, H.-J.; Kweon, H.-S.; Lee, J.-m. Pyruvate dehydrogenase kinase 4 deficiency attenuates cisplatin-induced acute kidney injury. Kidney Int. 2017, 91, 880–895. [Google Scholar] [CrossRef]
- Divya, M.K.; Lincy, L.; Raghavamenon, A.C.; Babu, T.D. Ameliorative effect of Apodytes dimidiata on cisplatin-induced nephrotoxicity in Wistar rats. Pharm. Biol. 2016, 54, 2149–2157. [Google Scholar] [CrossRef]
- Romero, F.; Pérez, M.; Chávez, M.; Parra, G.; Durante, P. Effect of uric acid on gentamicin-induced nephrotoxicity in rats–role of matrix metalloproteinases 2 and 9. Basic Clin. Pharmacol. Toxicol. 2009, 105, 416–424. [Google Scholar] [CrossRef]
- Pedraza-Chaverrí, J.; Barrera, D.; Maldonado, P.D.; Chirino, Y.I.; Macías-Ruvalcaba, N.A.; Medina-Campos, O.N.; Castro, L.; Salcedo, M.I.; Hernández-Pando, R. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin. Pharmacol. 2004, 4, 5. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Bhatia, J.; Khan, S.I.; Vasisth, S.; Tomar, A.; Goyal, S.; Kumar, R.; Arya, D.S.; Ojha, S.K. Therapeutic potential and molecular mechanisms of Emblica officinalis Gaertn in countering Nephrotoxicity in rats induced by the chemotherapeutic agent Cisplatin. Front. Pharmacol. 2016, 7, 350. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; El-Sayed, Y.S.; Eldaim, M.A.; Ibrahim, A. Nephroprotective efficacy of ceftriaxone against cisplatin-induced subchronic renal fibrosis in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2017, 390, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dang, C.; Kang, H.; Dai, Z.; Lin, S.; Guan, H.; Liu, X.; Wang, X.; Hui, W. Saikosaponin-D reduces cisplatin-induced nephrotoxicity by repressing ROS-mediated activation of MAPK and NF-κB signalling pathways. Int. Immunopharmacol. 2015, 28, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Megyesi, J.K.; Kaneto, H.; Price, P.M.; Safirstein, R.L. Cisplatin-induced cell death is EGFR/src/ERK signaling dependent in mouse proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2004, 287, F543–F549. [Google Scholar] [CrossRef]
- Negm, W.A.; El-Kadem, A.H.; Hussein, I.A.; Alqahtani, M.J. The Mechanistic Perspective of Bilobetin Protective Effects against Cisplatin-Induced Testicular Toxicity: Role of Nrf-2/Keap-1 Signaling, Inflammation and Apoptosis. Biomedicines 2022, 10, 1134. [Google Scholar] [CrossRef]
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Gamad, N.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Telmisartan ameliorates cisplatin-induced nephrotoxicity by inhibiting MAPK mediated inflammation and apoptosis. Eur. J. Pharmacol. 2015, 748, 54–60. [Google Scholar] [CrossRef]
- Bragado, P.; Armesilla, A.; Silva, A.; Porras, A. Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis 2007, 12, 1733–1742. [Google Scholar] [CrossRef]
- Tsuchida, M.; Yokosawa, T.; Noguchi, T.; Shimada, T.; Yamada, M.; Sekiguchi, Y.; Hirata, Y.; Matsuzawa, A. Pro-apoptotic functions of TRAF2 in p53-mediated apoptosis induced by cisplatin. J. Toxicol. Sci. 2020, 45, 219–226. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Lin, Y.-C.; Chen, H.-M.; Lin, J.-T.; Kao, S.-H. Adenine Combined with Cisplatin Promotes Anticancer Activity against Hepatocellular Cancer Cells through AMPK-Mediated p53/p21 and p38 MAPK Cascades. Pharmaceuticals 2022, 15, 795. [Google Scholar] [CrossRef]
- Negm, W.A.; Abo El-Seoud, K.A.; Kabbash, A.; Kassab, A.A.; El-Aasr, M. Hepatoprotective, cytotoxic, antimicrobial and antioxidant activities of Dioon spinulosum leaves Dyer Ex Eichler and its isolated secondary metabolites. Nat. Prod. Res. 2020, 35, 5166–5176. [Google Scholar] [CrossRef]
- Arun, M.; Satish, S.; Anima, P. Phytopharmacological profile of Jasminum grandiflorum Linn. (Oleaceae). Chin. J. Integr. Med. 2016, 22, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, L.; Lai, X.; Peng, X.; Wen, S.; Zhang, Z.; Xie, Y.; Li, Q.; Chen, R.; Zheng, X. Gastroprotective effects of extract of Jasminum grandiflorum L. flower in HCl/EtOH-induced gastric mucosal ulceration mice. Biomed. Pharmacother. 2021, 144, 112268. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.T. Phytochemical and pharmacological properties of five different species of Jasminum. Plant Arch. 2021, 21, 126–136. [Google Scholar]
- Elhawary, S.; Hala, E.-H.; Mokhtar, F.A.; Mansour Sobeh, E.M.; Osman, S.; El-Raey, M. Green synthesis of silver nanoparticles using extract of Jasminum officinal L. leaves and evaluation of cytotoxic activity towards bladder (5637) and breast cancer (MCF-7) cell lines. Int. J. Nanomed. 2020, 15, 9771. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-Hefnawy, H.M.; Elemeery, M.N.; Osman, S.M.; El-Raey, M.A.; Mokhtar, F.A.; Pan, C.H.; Ibrahim, H.A. The role of active metabolites isolated from Jasminum multiflorum flowers against hepatitis C virus infection and related hepatocellular carcinoma. Nat. Prod. Res. 2022, 36, 2625–2629. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-Hefnawy, H.M.; El-Raey, M.A.; Mokhtar, F.A.; Osman, S.M. Jasminum azoricum L. leaves: HPLC-PDA/MS/MS profiling and in-vitro cytotoxicity supported by molecular docking. Nat. Prod. Res. 2021, 35, 5518–5520. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Attallah, N.G.; Altwaijry, N.; Batiha, G.E.-S.; El-Sherbeni, S.A. Antidiarrheal and Antibacterial Activities of Monterey Cypress Phytochemicals: In Vivo and In Vitro Approach. Molecules 2022, 27, 346. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 2018, 18, 623–632. [Google Scholar] [CrossRef]
- Brown, G.R.; Hem, V.; Katz, K.S.; Ovetsky, M.; Wallin, C.; Ermolaeva, O.; Tolstoy, I.; Tatusova, T.; Pruitt, K.D.; Maglott, D.R. Gene: A gene-centered information resource at NCBI. Nucleic Acids Res. 2015, 43, D36–D42. [Google Scholar] [CrossRef]
- Liu, T.; Lin, Y.; Wen, X.; Jorissen, R.N.; Gilson, M.K. BindingDB: A web-accessible database of experimentally determined protein–ligand binding affinities. Nucleic Acids Res. 2007, 35 (Suppl. S1), D198–D201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Al-Sanea, M.M.; Mostafa, E.M.; Qasim, S.; Abelyan, N.; Mokhtar, F.A. A Network Pharmacology Analysis of Cytotoxic Triterpenes Isolated from Euphorbia abyssinica Latex Supported by Drug-likeness and ADMET Studies. ACS Omega 2022, 7, 17713–17722. [Google Scholar] [CrossRef]
- Franz, M.; Lopes, C.T.; Huck, G.; Dong, Y.; Sumer, O.; Bader, G.D. Cytoscape. js: A graph theory library for visualisation and analysis. Bioinformatics 2016, 32, 309–311. [Google Scholar]
- Du, J.; Li, M.; Yuan, Z.; Guo, M.; Song, J.; Xie, X.; Chen, Y. A decision analysis model for KEGG pathway analysis. BMC Bioinform. 2016, 17, 407. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Tabacco, A.; Meiattini, F.; Moda, E.; Tarli, P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 1979, 25, 336–337. [Google Scholar] [CrossRef]
- Fabiny, D.L.; Ertingshausen, G. Automated reaction-rate method for determination of serum creatinine with the CentrifiChem. Clin. Chem. 1971, 17, 696–700. [Google Scholar] [CrossRef]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sastry, J.; Kellie, S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatric Hematol. Oncol. 2005, 22, 441–445. [Google Scholar] [CrossRef]
- Yang, S.-k.; Han, Y.-c.; He, J.-r.; Yang, M.; Zhang, W.; Zhan, M.; Li, A.-m.; Li, L.; Liu, Y.-t.; Wu, X.-q. Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed. Pharmacother. 2020, 130, 110521. [Google Scholar] [CrossRef]
- Money, M.E.; Hamroun, A.; Shu, Y.; Matthews, C.; Ahmed Eltayeb, S.; Ciarimboli, G.; Metz, C.N. Case Report and Supporting Documentation: Acute Kidney Injury Manifested as Oliguria Is Reduced by Intravenous Magnesium Before Cisplatin. Front. Oncol. 2021, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; Trombatore, G.; Triarico, S.; Capozza, M.A.; Coccia, P.; Attina, G.; Mastrangelo, S.; Maurizi, P. Cisplatin Toxicity in Children with Malignancy. Biomed. Pharmacol. J. 2019, 12, 1603–1612. [Google Scholar] [CrossRef]
- Farooqui, Z.; Ahmed, F.; Rizwan, S.; Shahid, F.; Khan, A.A.; Khan, F. Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed. Pharmacother. 2017, 85, 7–15. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, A.; Hemalatha, S. Protective effect of Withania coagulans fruit extract on cisplatin-induced nephrotoxicity in rats. Pharmacogn. Res. 2017, 9, 354. [Google Scholar]
- Rashed, L.A.; Hashem, R.M.; Soliman, H.M. Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomed. Pharmacother. 2011, 65, 474–480. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Kumari, S.; Ojha, S.; Arya, D.S. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: Role of AGE-RAGE/MAPK pathways. Sci. Rep. 2017, 7, 42027. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Sinha, K.; Banerjee, S.; Sil, P.C. Taurine protects cisplatin induced cardiotoxicity by modulating inflammatory and endoplasmic reticulum stress responses. Biofactors 2016, 42, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Bharara, A.; Grossman, C.; Grinnan, D.; Syed, A.; Fisher, B.; DeWilde, C.; Natarajan, R. Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Rep. Crit. Care 2016, 2016, 8560871. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, B.; Mokhtar, F.A.; El-Masry, T.A.; Elekhnawy, E.; Mostafa, S.A.; Abdelkader, D.H.; Elharty, M.E.; Saleh, A.; Negm, W.A. Antimicrobial Activity of Brassica rapa L. Flowers Extract on Gastrointestinal Tract Infections and Antiulcer Potential Against Indomethacin-Induced Gastric Ulcer in Rats Supported by Metabolomics Profiling. J. Inflamm. Res. 2021, 14, 7411. [Google Scholar] [CrossRef] [PubMed]
- El Hawary, S.; El Sayed, A.; Helmy, M.W.; El Naggar, E.M.b.; Marzouk, H.S.; Bassam, S.M. DNA fingerprinting, biological and chemical investigation of certain Yucca species. Nat. Prod. Res. 2018, 32, 2617–2620. [Google Scholar] [CrossRef] [PubMed]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- Ridzuan, N.R.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-y.; Lou, D.-y.; Zhou, L.-q.; Wang, J.-c.; Yang, B.; He, Q.-j.; Wang, J.-j.; Weng, Q.-j. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef]
- Negm, W.A.; Ibrahim, A.E.-R.S.; El-Seoud, K.A.; Attia, G.I.; Ragab, A.E. A new cytotoxic and antioxidant Amentoflavone Monoglucoside from Cycas revoluta Thunb growing in Egypt. J. Pharm. Sci. Res. 2016, 8, 343. [Google Scholar]
- Oladejo, A.O.; Li, Y.; Imam, B.H.; Ma, X.; Shen, W.; Wu, X.; Jiang, W.; Yang, J.; Lv, Y.; Ding, X. MicroRNA miR-24-3p Mediates the Negative Regulation of Lipopolysaccharide-Induced Endometrial Inflammatory Response by Targeting TNF Receptor-Associated Factor 6 (TRAF6). J. Inflamm. Res. 2022, 15, 807. [Google Scholar] [CrossRef]
- Yeh, W.-C.; Shahinian, A.; Speiser, D.; Kraunus, J.; Billia, F.; Wakeham, A.; De La Pompa, J.L.; Ferrick, D.; Hum, B.; Iscove, N. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 1997, 7, 715–725. [Google Scholar] [CrossRef]
- Rothe, M.; Wong, S.C.; Henzel, W.J.; Goeddel, D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 1994, 78, 681–692. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.-f.; Han, X.-y.; Sun, Y.-s.; Zhang, L.-x.; Liu, W.; Liu, X.-x.; Li, W.; Liu, Y.-y. Kidney protection effect of ginsenoside re and its underlying mechanisms on cisplatin-induced kidney injury. Cell. Physiol. Biochem. 2018, 48, 2219–2229. [Google Scholar] [CrossRef]
- Wang, W.; Sun, H.; Che, Y.; Jiang, X. Rasfonin promotes autophagy and apoptosis via upregulation of reactive oxygen species (ROS)/JNK pathway. Mycology 2016, 7, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Park, J.G.; Aziz, N.; Cho, J.Y. MKK7, the essential regulator of JNK signaling involved in cancer cell survival: A newly emerging anticancer therapeutic target. Ther. Adv. Med. Oncol. 2019, 11, 1758835919875574. [Google Scholar] [CrossRef] [PubMed]
- Mathien, S.; Tesnière, C.; Meloche, S. Regulation of Mitogen-Activated Protein Kinase Signaling Pathways by the Ubiquitin-Proteasome System and Its Pharmacological Potential. Pharmacol. Rev. 2021, 73, 1434–1467. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I.; Lu, Y.; Wang, X.; Wu, D. Synergistic toxic interactions between CYP2E1, LPS/TNFα, and JNK/p38 MAP kinase and their implications in alcohol-induced liver injury. Biol. Basis Alcohol-Induc. Cancer 2015, 815, 145–172. [Google Scholar]


| No | Compound | RT | m/z | Fragments | Ontology |
|---|---|---|---|---|---|
| 1 | 1-Caffeoylquinic acid | 17.04 | 353.16 | 191, 179 | Phenolic acid |
| 2 | Verbascoside | 25.02 | 623.59 | 461, 161 | Phenylpropanoid |
| 3 | Elenolic acid glucopyranoside | 27.38 | 403.95 | 241, 179 | Secoiridoids |
| 4 | Quercetin-3-O-pentosyl (1–2) acetylpentoside | 27.99 | 607.11 | 433, 301 | Flavonoid glycosides |
| 5 | Quercetin 3-rutinoside | 30.09 | 609.22 | 463, 301 | Flavonol glycosides |
| 6 | Quercetin-3-O-sophoroside | 33.14 | 624.94 | 463, 301 | Flavonol glycosides |
| 7 | Myricetin-3-O- glucopyranoside | 34.10 | 479.08 | 317, 195 | Flavonoid-3-glycosides |
| 8 | Eriodictyol-7-O-neohesperidoside | 34.14 | 595.23 | 289, 163 | Flavonoid-7-glycosides |
| 9 | Kaempferol 3,7 Di-glucoside | 35.13 | 609.32 | 285, 179 | Flavonoid glycosides |
| 10 | 10-hydroxyoleuropein | 36.21 | 555.08 | 539, 249 | Secoiridoids |
| 11 | Vicenin-2 | 37.04 | 593.28 | 503, 353 | Flavonoid 8-C-glycosides |
| 12 | Oleuropein glucoside | 38.75 | 701.31 | 529, 223 | Secoiridoids |
| 13 | Kaempferol-7-O-glucoside | 40.46 | 447.34 | 285, 177 | Flavonol glycosides |
| 14 | Isorhamnetin-3-O- glucoside | 41.84 | 477.03 | 315, 193 | Flavonoid 8-C-glycosides |
| 15 | Isoquercitrin | 43.41 | 463.08 | 301, 179 | Flavonoid 8-C-glycosides |
| 16 | Multifloroside | 44.83 | 677.31 | 661, 539 | Secoiridoids |
| 17 | Oleuropein | 46.46 | 539.17 | 377, 233 | Secoiridoids |
| 18 | Kaempferitrin | 47.44 | 577.31 | 285, 179 | Flavonoid 8-C-glycosides |
| 19 | Reynoutrin | 49.18 | 433.10 | 301 | Flavonoid glycosides |
| 20 | Quercetin 3-(6″-acetylglucoside) | 51.08 | 505.02 | 301, 271 | Flavonol glycosides |
| 21 | Myricetin 3-xyloside | 56.41 | 449.05 | 317, 179 | Flavonoid-3-glycosides |
| 22 | Laricitrin 3-O-glucoside | 58.48 | 493.10 | 331, 151 | Flavonoid glycosides |
| 23 | Myricitrin | 59.39 | 463.28 | 317, 179 | Flavonoid-3-O-glycosides |
| 24 | Kaempferol | 61.25 | 285.30 | 269, 241 | Flavonoid glycosides |
| 25 | Quercetin | 61.62 | 301.17 | 245, 179 | Flavonoid |
| 26 | 5-O-Methyllicoricidin | 66.82 | 483.61 | 203, 177 | Flavan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.J.; Mostafa, S.A.; Hussein, I.A.; Elhawary, S.; Mokhtar, F.A.; Albogami, S.; Tomczyk, M.; Batiha, G.E.-S.; Negm, W.A. Metabolic Profiling of Jasminum grandiflorum L. Flowers and Protective Role against Cisplatin-Induced Nephrotoxicity: Network Pharmacology and In Vivo Validation. Metabolites 2022, 12, 792. https://doi.org/10.3390/metabo12090792
Alqahtani MJ, Mostafa SA, Hussein IA, Elhawary S, Mokhtar FA, Albogami S, Tomczyk M, Batiha GE-S, Negm WA. Metabolic Profiling of Jasminum grandiflorum L. Flowers and Protective Role against Cisplatin-Induced Nephrotoxicity: Network Pharmacology and In Vivo Validation. Metabolites. 2022; 12(9):792. https://doi.org/10.3390/metabo12090792
Chicago/Turabian StyleAlqahtani, Moneerah J., Sally A. Mostafa, Ismail A. Hussein, Seham Elhawary, Fatma A. Mokhtar, Sarah Albogami, Michał Tomczyk, Gaber El-Saber Batiha, and Walaa A. Negm. 2022. "Metabolic Profiling of Jasminum grandiflorum L. Flowers and Protective Role against Cisplatin-Induced Nephrotoxicity: Network Pharmacology and In Vivo Validation" Metabolites 12, no. 9: 792. https://doi.org/10.3390/metabo12090792
APA StyleAlqahtani, M. J., Mostafa, S. A., Hussein, I. A., Elhawary, S., Mokhtar, F. A., Albogami, S., Tomczyk, M., Batiha, G. E.-S., & Negm, W. A. (2022). Metabolic Profiling of Jasminum grandiflorum L. Flowers and Protective Role against Cisplatin-Induced Nephrotoxicity: Network Pharmacology and In Vivo Validation. Metabolites, 12(9), 792. https://doi.org/10.3390/metabo12090792










