Profile of Selected Secondary Metabolites and Antioxidant Activity of Valerian and Lovage Grown in Organic and Low-Input Conventional System
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Phenolic Compound Extraction and Identification
2.3. Antioxidant Activity
2.4. Statistical Analyses
3. Results and Discussion
3.1. Phenolic Acid, Flavonoid and Antioxidant Activity of Lovage and Valerian Roots
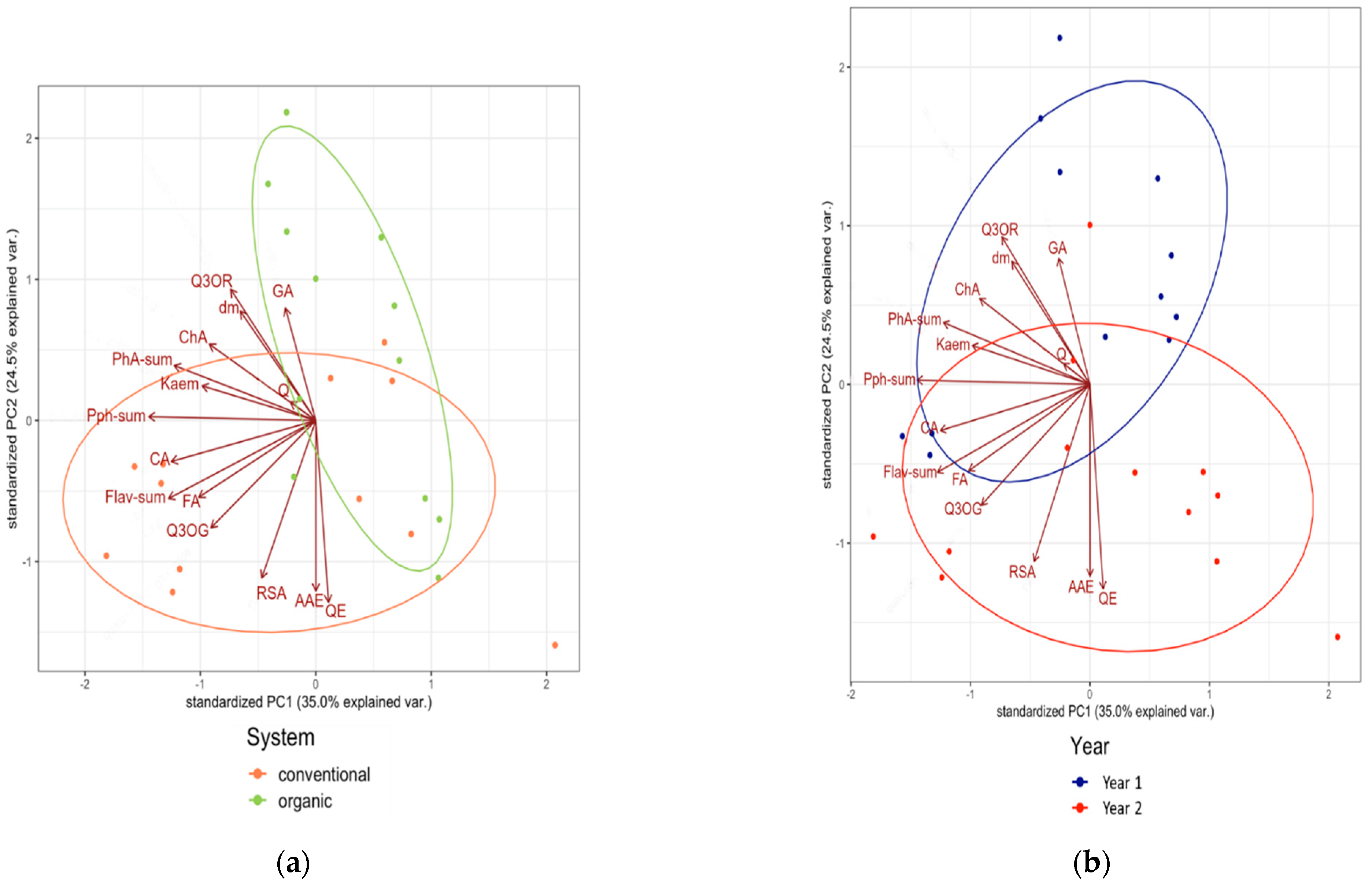
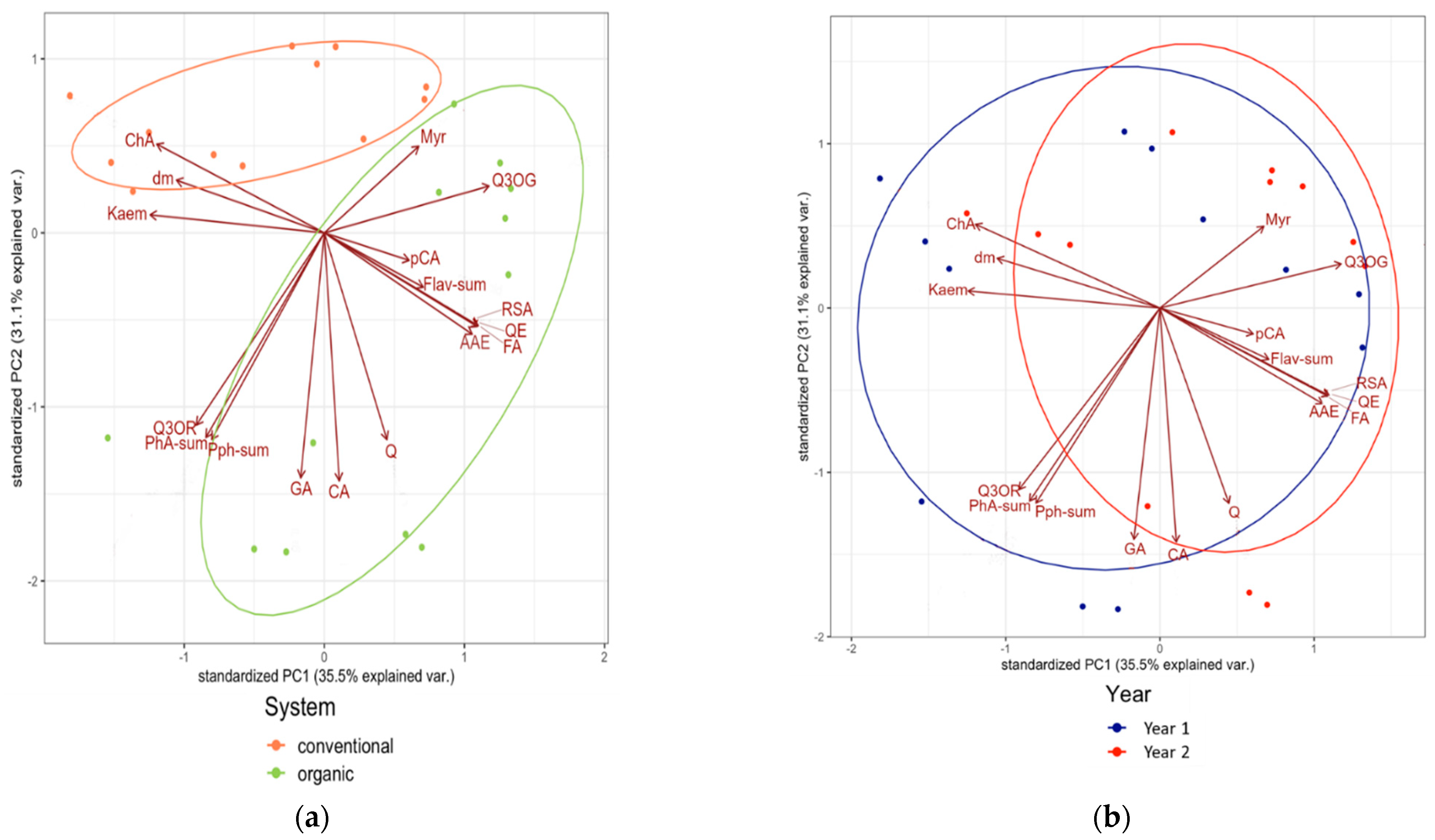
3.2. Associations between Composition Data of the Tested Medicinal Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adel Pilerood, S.; Prakash, J. Nutritional and medicinal properties of valerian (Valeriana Officinalis) herb: A review. Int. J. Food Nutr. Diet. 2013, 1, 25–32. [Google Scholar]
- Braun, L.; Cohen, M. Herbs and Natural Supplements. An Evidence-Based Guide, 2nd ed.; Elsevier: Chatswood, Australia, 2007. [Google Scholar]
- Malva, J.O.; Santos, S.; Macedo, T. Neuroprotective properties of Valeriana officinalis extracts. Neurotox. Res. 2004, 6, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, S.; Narayanan, K.B.; Ilango, K. Valeriana officinalis: A review of its traditional uses, phytochemistry and pharmacology. Asian J. Pharm. Clin. Res. 2018, 11, 36. [Google Scholar] [CrossRef]
- Zizovic, I.; Stamenić, M.; Ivanović, J.; Orlović, A.; Ristić, M.; Djordjević, S.; Petrović, S.D.; Skala, D. Supercritical carbon dioxide extraction of sesquiterpenes from valerian root. J. Supercrit. Fluids 2007, 43, 249–258. [Google Scholar] [CrossRef]
- Letchamo, W.; Ward, W.; Heard, B.; Heard, D. Essential oil of Valeriana officinalis L. cultivars and their antimicrobial activity as influenced by harvesting time under commercial organic cultivation. J. Agric. Food Chem. 2004, 52, 3915–3919. [Google Scholar] [CrossRef]
- Gränicher, F.; Christen, P.; Kapetanidis, I. Essential oils from normal and hairy roots of Valeriana officinalis var. sambucifolia. Phytochemistry 1995, 40, 1421–1424. [Google Scholar] [CrossRef]
- Murray, M.T. 123—Valeriana officinalis (Valerian). In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Churchill Livingstone: St. Louis, MO, USA, 2020; pp. 902–905.e1. ISBN 978-0-323-52342-4. [Google Scholar]
- Mirjalili, M.; Salehi, P.; Sonboli, A.; Hadian, J.; Ebrahimi, S.; Yousefzadi, M. The composition and antibacterial activity of the essential oil of Levisticum officinale Koch flowers and fruits at different developmental stages. J. Serbian Chem. Soc. 2010, 75, 1661–1669. [Google Scholar] [CrossRef]
- Mohamadi, N.; Rajaei, P.; Moradalizadeh, M.; Amiri, M.S. Essential oil composition and antioxidant activity of levisticum officinale Koch. at Various phenological stages. J. Med. Plants 2017, 16, 45–55. [Google Scholar]
- Raal, A.; Arak, E.; Orav, A.; Kailas, T.; Müürisepp, M. Composition of the essential oil of Levisticum officinale W.D.J. Koch from some European Countries. J. Essent. Oil Res. 2008, 20, 318–322. [Google Scholar] [CrossRef]
- Roslon, W.; Osińska, E.; Wajs-Bonikowska, A. Effect of plantation establishment and raw material stabilization on the usefull traits of lovage leaves (Levisticum officinale koch.). Acta Sci. Pol. Hortorum Cultus. 2013, 12, 141–155. [Google Scholar]
- Ravindran, P.N.; Pillai, G.S.; Nirmal Babu, K. 5—Under-utilized herbs and spices. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Peter, K.V., Ed.; Woodhead Publishing: Thorston, UK, 2004; pp. 53–103. ISBN 978-1-85573-721-1. [Google Scholar]
- Najda, A.; Dyduch, W.; Baj, T. Determination of quantitative composition of poliphenolic compounds occur in anatomically different parts of Levisticum officinale Koch. Electron. J. Polish Agric. Univ. Ser. Hortic. 2003, 6, 2. [Google Scholar]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak Aleksandra, A.D.; Tarko, T.; Rus, M. Antioxidant activity of selected herbal plants. Herba Pol. 2009, 55, 66–77. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef]
- European Commission Council Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91, OJ L 189, 20.7.2007; The Official Journal of the European Union, Publications Office of the EU: Luxembourg, 2007; pp. 1–23.
- European Parliament; European Council. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007; The Official Journal of the European Union, Publications Office of the EU: Luxembourg, 2018. [Google Scholar]
- Smith, L.G.; Kirk, G.J.D.; Jones, P.J.; Williams, A.G. The greenhouse gas impacts of converting food production in England and Wales to organic methods. Nat. Commun. 2019, 10, 4641. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F. Sustainability of agro-ecological practices in organic horticulture: Yield, energy-use and carbon footprint. Agroecol. Sustain. Food Syst. 2019, 44, 726–746. [Google Scholar] [CrossRef]
- Rempelos, L.; Baranski, M.; Wang, J.; Adams, T.N.; Adebusuyi, K.; Beckman, J.J.; Brockbank, C.J.; Douglas, B.S.; Feng, T.; Greenway, J.D.; et al. Integrated soil and crop management in organic agriculture: A logical framework to ensure food quality and human health? Agronomy 2021, 11, 2494. [Google Scholar] [CrossRef]
- Vaitkevičienė, N.; Kulaitienė, J.; Jarienė, E.; Levickienė, D.; Danillčenko, H.; Średnicka-Tober, D.; Rembiałkowska, E.; Hallmann, E. Characterization of bioactive compounds in colored potato (Solanum tuberosum L.) cultivars grown with conventional, organic, and biodynamic methods. Sustainability 2020, 12, 2701. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Kazimierczak, R.; Ponder, A.; Kopczyńska, K.; Hallmann, E. Selected antioxidants in organic vs. conventionally grown apple fruits. Appl. Sci. 2020, 10, 2997. [Google Scholar] [CrossRef]
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; et al. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem. 2018, 66, 10369–10379. [Google Scholar] [CrossRef] [PubMed]
- Wegener, C.; Jansen, G.; Jurgens, H.-U. Influence of drought and wounding stress on soluble phenols and proteins in potato tubers. Sustain. Agric. Res. 2014, 3, 3. [Google Scholar] [CrossRef][Green Version]
- Hallmann, E.; Kazimierczak, R.; Marszałek, K.; Drela, N.; Kiernozek, E.; Toomik, P.; Matt, D.; Luik, A.; Rembiałkowska, E. The nutritive value of organic and conventional white cabbage (Brassica oleracea L. var. Capitata) and anti-apoptotic activity in gastric adenocarcinoma cells of sauerkraut juice produced therof. J. Agric. Food Chem. 2017, 65, 8171–8183. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- R Core Team An Introduction to R; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Brandt, K.; Srednicka-Tober, D.; Barański, M.; Sanderson, R.; Leifert, C.; Seal, C. Methods for comparing data across differently designed agronomic studies: Examples of different meta-analysis methods used to compare relative composition of plant foods grown using organic or conventional production methods and a protocol for a systemati. J. Agric. Food Chem. 2013, 61, 7173–7180. [Google Scholar] [CrossRef]
- Rempelos, L.; Almuayrifi, M.S.B.; Baranski, M.; Tetard-Jones, C.; Barkla, B.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; Hall, G.; et al. The effect of agronomic factors on crop health and performance of winter wheat varieties bred for the conventional and the low input farming sector. F. Crop. Res. 2020, 254, 107822. [Google Scholar] [CrossRef]
- Margaritopoulou, T.; Toufexi, E.; Kizis, D.; Balayiannis, G.; Anagnostopoulos, C.; Theocharis, A.; Rempelos, L.; Troyanos, Y.; Leifert, C.; Markellou, E. Reynoutria sachalinensis extract elicits SA-dependent defense responses in courgette genotypes against powdery mildew caused by Podosphaera xanthii. Sci. Rep. 2020, 10, 3354. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhang, J.; Coulter, J.A.; Li, L.; Gan, Y. Diversifying crop rotation improves system robustness. Agron. Sustain. Dev. 2019, 39, 38. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Reilly, K.; Valverde, J.; Finn, L.; Gaffney, M.; Rai, D.K.; Brunton, N. A note on the effectiveness of selenium supplementation of Irish-grown Allium crops. Irish J. Agric. Food Res. 2014, 53, 91–99. [Google Scholar]
- Kazimierczak, R.; Siłakiewicz, A.; Hallmann, E.; Srednicka-Tober, D.; Rembiałkowska, E. Chemical composition of selected beetroot juices in relation to beetroot production system and processing technology. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 491–498. [Google Scholar] [CrossRef]
- Radfar, R.; Hosseini, H.; Farhodi, M.; Ghasemi, I.; Średnicka-Tober, D.; Shamloo, E.; Khaneghah, A.M. Optimization of antibacterial and mechanical properties of an active LDPE/starch/nanoclay nanocomposite film incorporated with date palm seed extract using D-optimal mixture design approach. Int. J. Biol. Macromol. 2020, 158, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Gutiérrez-Del-Río, I.; Entrialgo-Cadierno, R.; Villar, C.J.; Lombó, F. De novo biosynthesis of myricetin, kaempferol and quercetin in Streptomyces albus and Streptomyces coelicolor. PLoS ONE 2018, 13, e0207278. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]

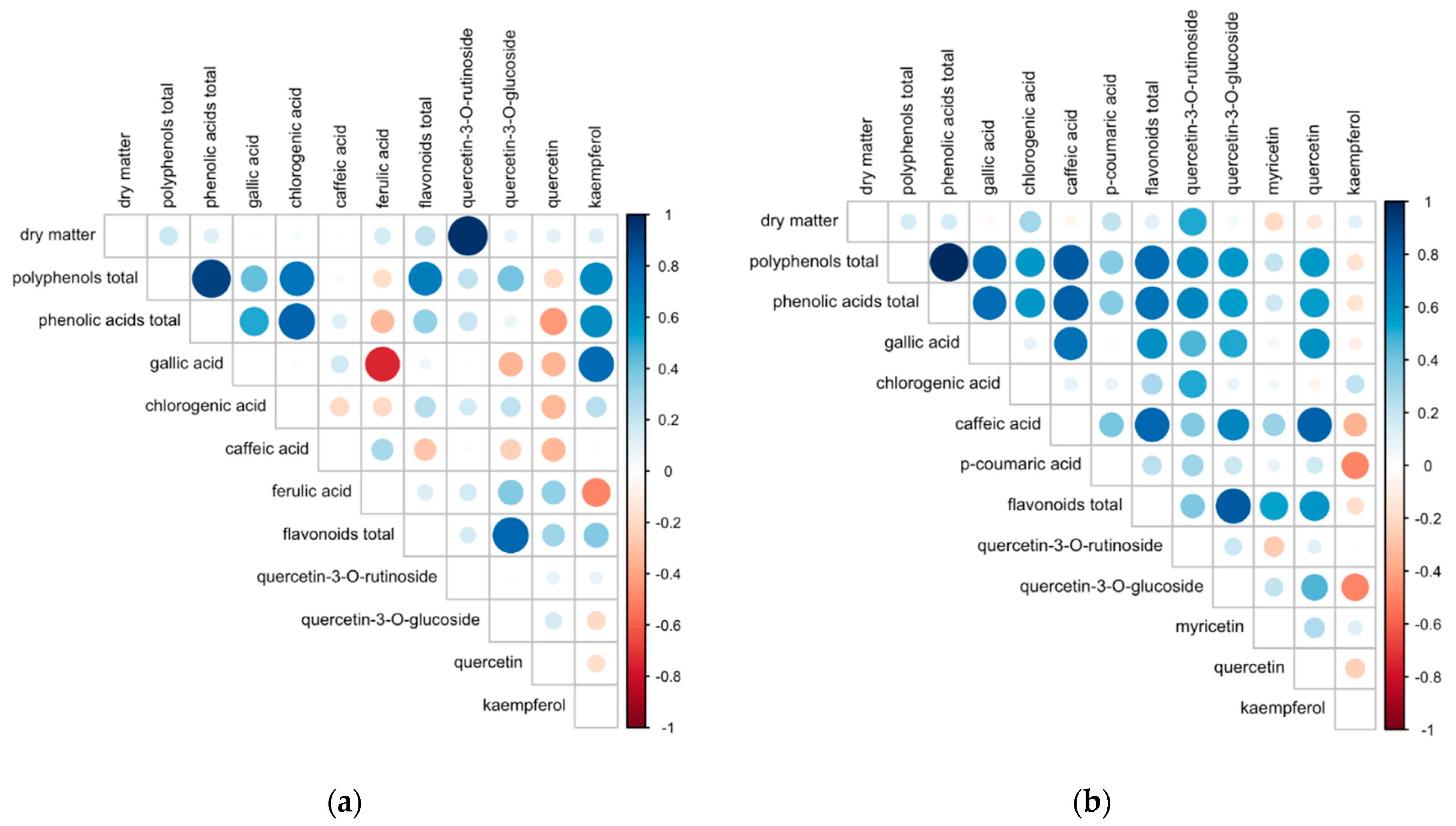
| Phenolics (Sum) | Phenolic Acids (Sum) | Chlorogenic Acid | Ferulic Acid | Gallic Acid | Caffeic Acid | Flavonoids (Sum) | Quercetin-3-O- Rutinoside | Quercetin-3-O- Glucoside | Quercetin | Kaempferol | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/100 g f.w. | |||||||||||
| System (SYS) | |||||||||||
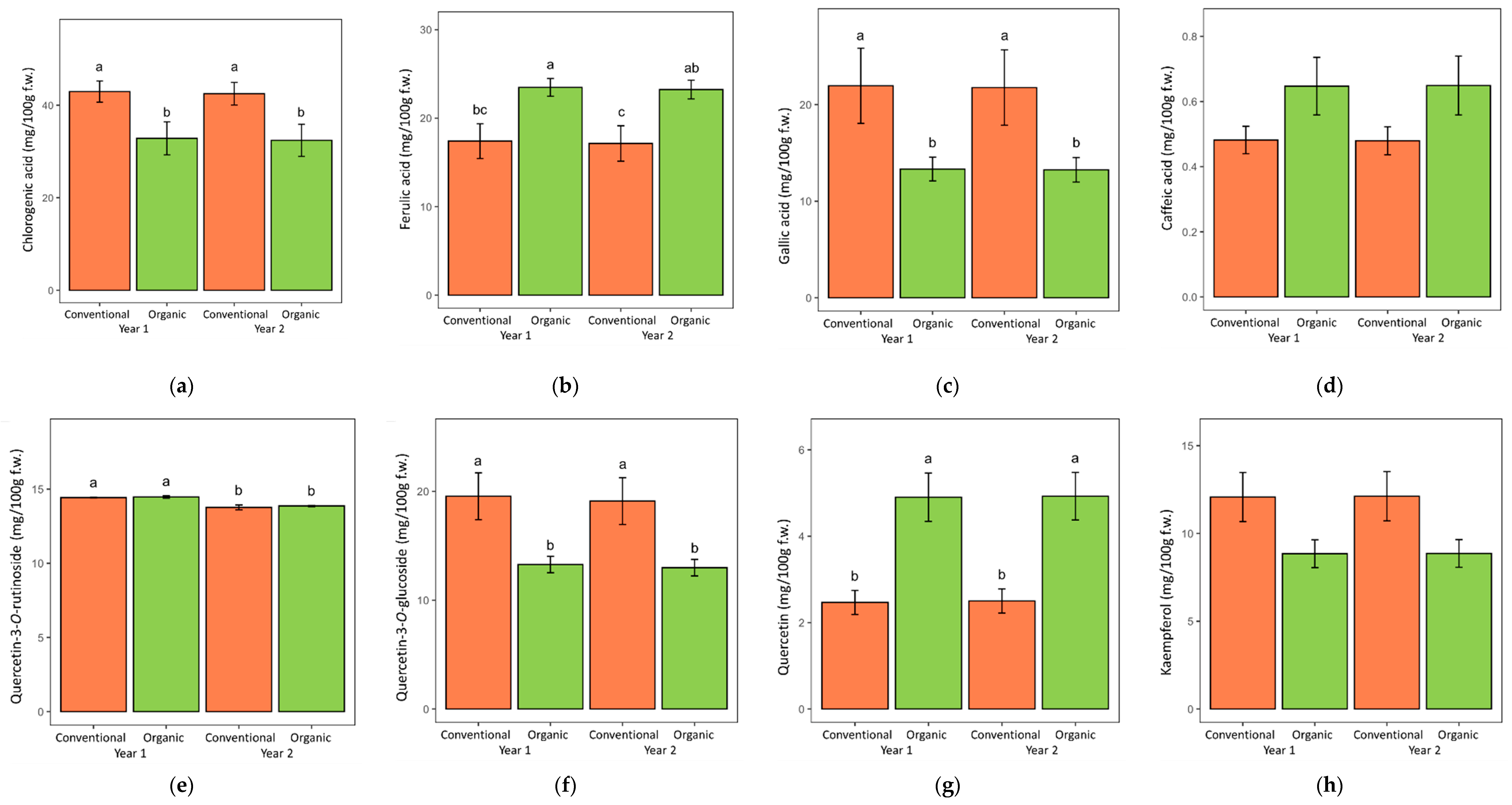
| Conventional | 130 ± 3 1 | 82.3 ± 2.4 | 42.7 ± 1.6 | 17.3 ± 1.4 | 21.9 ± 2.7 | 0.481 ± 0.03 | 48.0 ± 1.5 | 14.1 ± 0.1 | 19.3 ± 1.5 | 2.48 ± 0.19 | 12.1 ± 1.0 |
| Organic | 111 ± 2 | 69.9 ± 2.2 | 32.6 ± 2.5 | 23.4 ± 0.7 | 13.3 ± 0.9 | 0.648 ± 0.06 | 41.1 ± 1.1 | 14.2 ± 0.1 | 13.1 ± 0.5 | 4.91 ± 0.39 | 8.9 ± 0.6 |
| Year (YR) | |||||||||||
| Year 1 | 122 ± 3 | 76.5 ± 2.5 | 37.9 ± 2.3 | 20.4 ± 1.2 | 17.6 ± 2.1 | 0.565 ± 0.05 | 45.0 ± 1.4 | 14.4 ± 0.0 | 16.4 ± 1.2 | 3.68 ± 0.37 | 10.5 ± 0.8 |
| Year 2 | 120 ± 3 | 75.7 ± 2.6 | 37.4 ± 2.3 | 20.2 ± 1.2 | 17.5 ± 2.1 | 0.564 ± 0.05 | 44.1 ± 1.5 | 13.8 ± 0.1 | 16.1 ± 1.2 | 3.71 ± 0.37 | 10.5 ± 0.8 |
| ANOVA p-values | |||||||||||
| SYS | 0.0002 | 0.000 | 0.000 | 0.000 | 0.003 | 0.016 | 0.000 | 0.482 | 0.000 | 0.000 | 0.006 |
| YR | 0.641 | 0.788 | 0.866 | 0.858 | 0.963 | 0.998 | 0.610 | 0.000 | 0.818 | 0.947 | 0.979 |
| SYS × YR | 0.965 | 0.979 | 0.996 | 0.995 | 0.984 | 0.976 | 0.963 | 0.781 | 0.960 | 0.990 | 0.989 |
| Phenolics (Sum) | Phenolic Acids (Sum) | Chlorogenic Acid | Gallic Acid | Caffeic Acid | p-Coumaric Acid | Flavonoids (Sum) | Quercetin-3-O-Rutinoside | Quercetin-3-O-Glucoside | Quercetin | Kaempfe-Rol | Myricetin | |
| mg/100 g f.w. | ||||||||||||
| System (SYS) | ||||||||||||
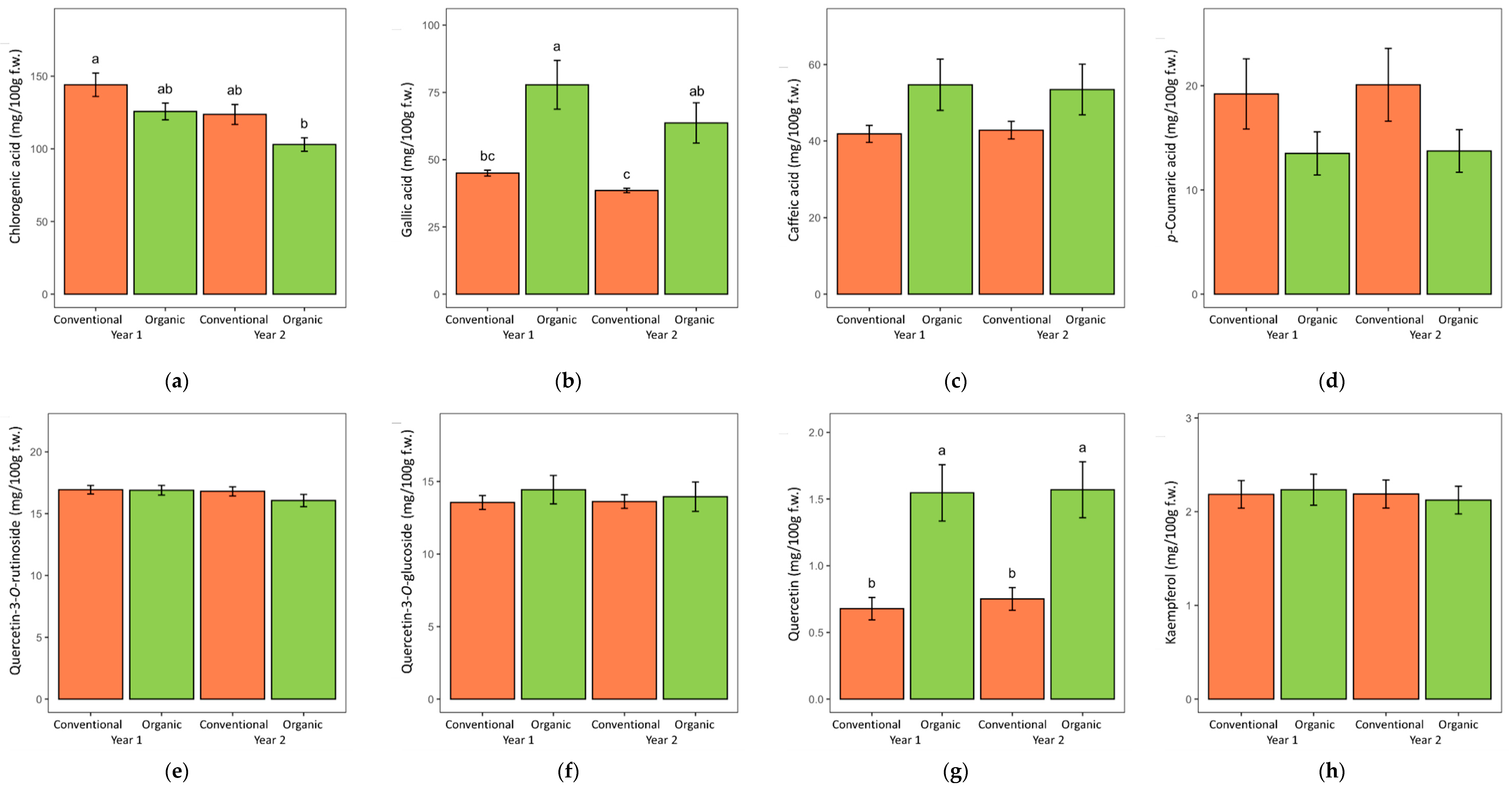
| Conventional | 274 ± 6 1 | 238 ± 6 | 134 ± 5 | 41.8 ± 0.9 | 42.3 ± 1.6 | 19.7 ± 2.4 | 36.2 ± 0.3 | 16.9 ± 0.3 | 13.6 ± 0.3 | 0.71 ± 0.06 | 2.19 ± 0.10 | 2.84 ± 0.21 |
| Organic | 291 ± 14 | 253 ± 14 | 114 ± 4 | 70.7 ± 5.9 | 54.1 ± 4.6 | 13.6 ± 1.4 | 37.9 ± 1.1 | 16.5 ± 0.3 | 14.2 ± 0.7 | 1.56 ± 0.15 | 2.18 ± 0.11 | 3.51 ± 0.45 |
| Year (YR) | ||||||||||||
| Year 1 | 298 ± 11 | 261 ± 11 | 135 ± 5 | 61.4 ± 5.3 | 48.3 ± 3.6 | 16.4 ± 2.0 | 37.4 ± 0.8 | 16.9 ± 0.3 | 14.0 ± 0.5 | 1.11 ± 0.13 | 2.21 ± 0.11 | 3.13 ± 0.37 |
| Year 2 | 266 ± 10 | 229 ± 9 | 113 ± 4 | 51.1 ± 4.3 | 48.1 ± 3.6 | 16.9 ± 2.1 | 36.8 ± 0.8 | 16.4 ± 0.3 | 13.8 ± 0.6 | 1.16 ± 0.13 | 2.16 ± 0.10 | 3.22 ± 0.35 |
| ANOVA p-values | ||||||||||||
| SYS | 0.238 | 0.263 | 0.004 | 0.000 | 0.011 | 0.029 | 0.107 | 0.337 | 0.385 | 0.000 | 0.962 | 0.188 |
| YR | 0.027 2 | 0.022 | 0.001 | 0.030 | 0.977 | 0.837 | 0.571 | 0.238 | 0.771 | 0.746 | 0.728 | 0.849 |
| SYS × YR | 0.609 | 0.631 | 0.857 | 0.408 | 0.806 | 0.905 | 0.443 | 0.391 | 0.697 | 0.869 | 0.711 | 0.834 |
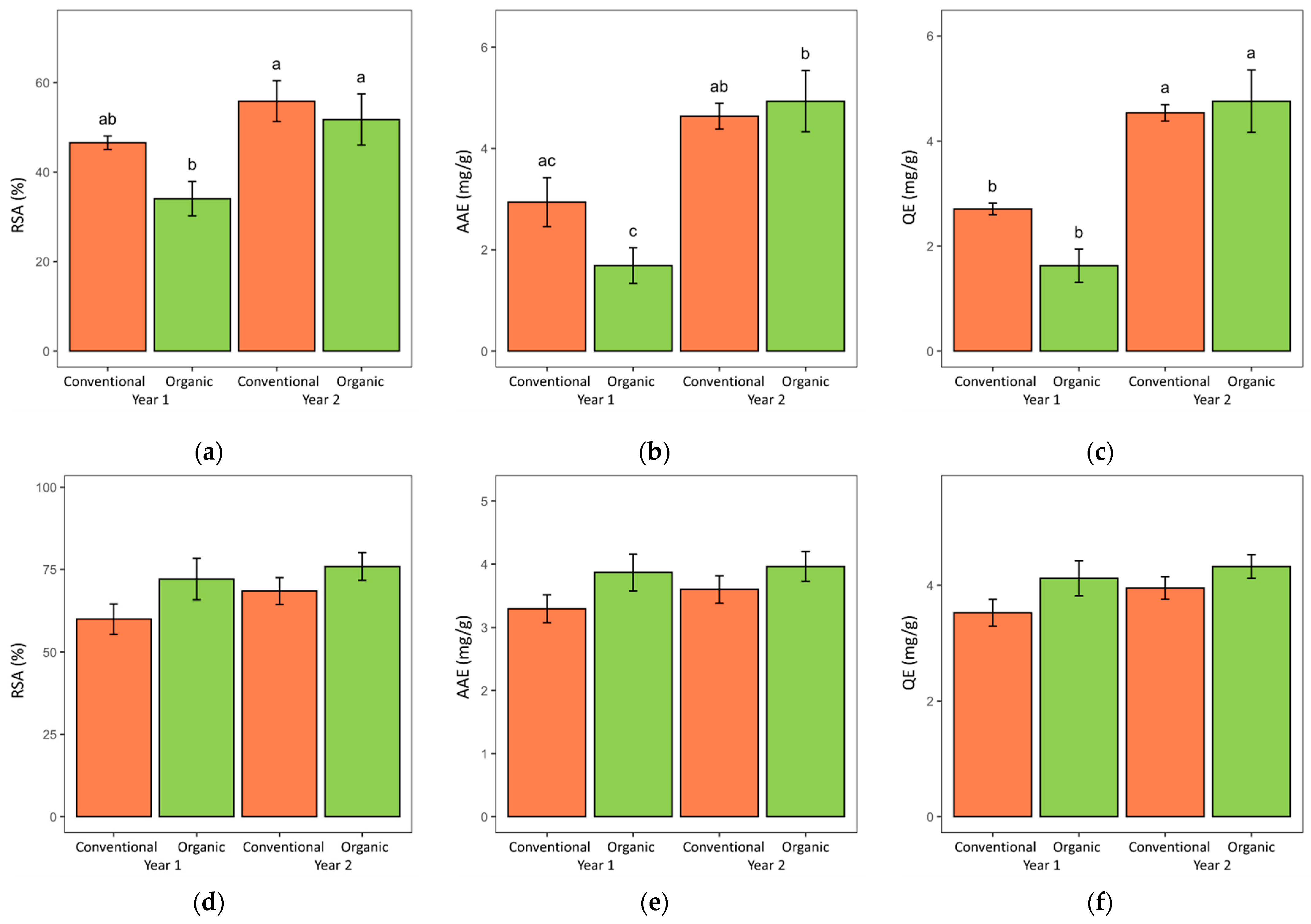
| Lovage | Valerian | |||||
|---|---|---|---|---|---|---|
| RSA 1 (%) | AAE 2 (mg/g) | QE 3 (mg/g) | RSA (%) | AAE (mg/g) | QE (mg/g) | |
| System (SYS) | ||||||
| Conventional | 51.2 ± 2.7 4 | 3.79 ± 0.36 | 3.62 ± 0.29 | 64.2 ± 3.2 | 3.45 ± 0.15 | 3.74 ± 0.16 |
| Organic | 42.9 ± 4.2 | 3.31 ± 0.59 | 3.19 ± 0.57 | 74.0 ± 3.6 | 3.91 ± 0.18 | 4.22 ± 0.18 |
| Year (YR) | ||||||
| Year 1 | 40.3 ± 2.7 | 2.31 ± 0.34 | 2.17 ± 0.23 | 66.1 ± 4.1 | 3.58 ± 0.19 | 3.82 ± 0.20 |
| Year 2 | 53.8 ± 3.5 | 4.79 ± 0.32 | 4.65 ± 0.29 | 72.2 ± 3.0 | 3.78 ± 0.16 | 4.14 ± 0.15 |
| ANOVA p-values | ||||||
| SYS | 0.053 | 0.295 | 0.234 | 0.059 | 0.069 | 0.055 |
| YR | 0.0035 | 0.000 | 0.000 | 0.222 | 0.421 | 0.199 |
| SYS × YR | 0.308 | 0.097 | 0.078 | 0.640 | 0.674 | 0.649 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Średnicka-Tober, D.; Hallmann, E.; Kopczyńska, K.; Góralska-Walczak, R.; Barański, M.; Grycz, A.; Seidler-Łożykowska, K.; Rembiałkowska, E.; Kazimierczak, R. Profile of Selected Secondary Metabolites and Antioxidant Activity of Valerian and Lovage Grown in Organic and Low-Input Conventional System. Metabolites 2022, 12, 835. https://doi.org/10.3390/metabo12090835
Średnicka-Tober D, Hallmann E, Kopczyńska K, Góralska-Walczak R, Barański M, Grycz A, Seidler-Łożykowska K, Rembiałkowska E, Kazimierczak R. Profile of Selected Secondary Metabolites and Antioxidant Activity of Valerian and Lovage Grown in Organic and Low-Input Conventional System. Metabolites. 2022; 12(9):835. https://doi.org/10.3390/metabo12090835
Chicago/Turabian StyleŚrednicka-Tober, Dominika, Ewelina Hallmann, Klaudia Kopczyńska, Rita Góralska-Walczak, Marcin Barański, Alicja Grycz, Katarzyna Seidler-Łożykowska, Ewa Rembiałkowska, and Renata Kazimierczak. 2022. "Profile of Selected Secondary Metabolites and Antioxidant Activity of Valerian and Lovage Grown in Organic and Low-Input Conventional System" Metabolites 12, no. 9: 835. https://doi.org/10.3390/metabo12090835
APA StyleŚrednicka-Tober, D., Hallmann, E., Kopczyńska, K., Góralska-Walczak, R., Barański, M., Grycz, A., Seidler-Łożykowska, K., Rembiałkowska, E., & Kazimierczak, R. (2022). Profile of Selected Secondary Metabolites and Antioxidant Activity of Valerian and Lovage Grown in Organic and Low-Input Conventional System. Metabolites, 12(9), 835. https://doi.org/10.3390/metabo12090835











