The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents
Abstract
:1. Introduction
2. Results
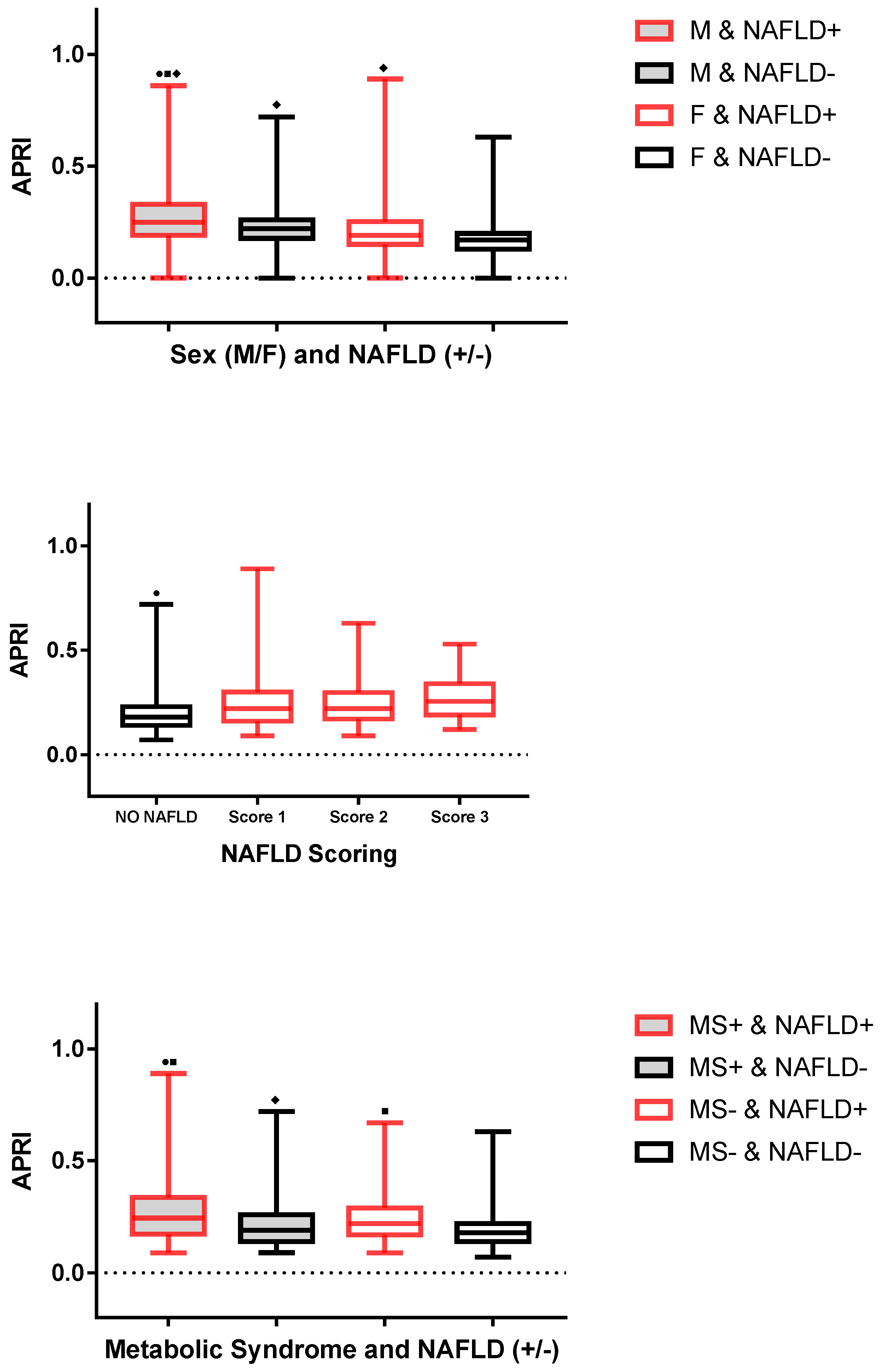
2.1. Comparison of Children and Adolescents with and without NAFLD
2.2. Correlations of APRI with Each Demographic, Biochemical and Clinical Parameter
2.3. Univariable Analysis of NAFLD Predictors
2.4. Multivariable Analysis of NAFLD Predictors
2.5. Accuracy of APRI as a Marker of NAFLD
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Diagnosis and Scoring of NAFLD
4.3. Anthropometric Measurements
4.4. Metabolic Variables
4.5. Evaluation of Blood Pressure
4.6. Definition of Metabolic Syndrome
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under curve |
| BMI | body mass index |
| CI | confidence interval |
| CRP | C-reactive protein |
| DBP | diastolic blood pressure |
| F | female |
| HDL-C | high-density lipoprotein cholesterol |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| IDF | International Diabetes Federation |
| IQR | interquartile range |
| LDL-C | low-density lipoprotein cholesterol |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatotic hepatitis |
| M | male |
| OR | odd ratio |
| ROC | receiver operating characteristic |
| SBP | systolic blood pressure |
| SD | standard deviation |
| SDS | standard deviation score |
| T-C | total cholesterol |
| TG | triglycerides |
| WC | waist circumference. |
References
- Nobili, V.; Alisi, A.; Newton, K.P.; Schwimmer, J.B. Comparison of the phenotype and approach to pediatric versus adult patients with nonalcoholic fatty liver disease. Gastroenterology 2016, 150, 1798–1810. [Google Scholar] [CrossRef] [Green Version]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Brunt, E.M. Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.P.; Schwimmer, B.J. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin. Liver Dis. 2016, 20, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Schwimmer, J.B.; Pardee, P.E.; Lavine, J.E.; Blumkin, A.K.; Cook, S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008, 118, 277–283. [Google Scholar] [CrossRef]
- Manco, M.; Bedogni, G.; Marcellini, M.; Devito, R.; Ciampalini, P.; Sartorelli, M.R.; Comparcola, D.; Piemonte, F.; Nobili, V. Waist circumference correlates with liver fibrosis in children with non-alcoholic steatohepatitis. Gut 2008, 57, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.S.; Monteiro, P.A.; Antunes Bde, M.; Seraphim, P.M.; Fernandes, R.A.; Christofaro, D.G.; Freitas Júnior, I.F. Intra-abdominal fat is related to metabolic syndrome and non-alcoholic fat liver disease in obese youth. BMC Pediatr. 2013, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelishadi, R.; Cook, S.R.; Adibi, A.; Faghihimani, Z.; Ghatrehsamani, S.; Beihaghi, A.; Salehi, H.; Khavarian, N.; Poursafa, P. Association of the components of the metabolic syndrome with non-alcoholic fatty liver disease among normal-weight, overweight and obese children and adolescents. Diabetol. Metab. Syndr. 2009, 1, 29. [Google Scholar] [CrossRef] [Green Version]
- Patton, H.M.; Yates, K.; Unalp-Arida, A.; Behling, C.A.; Huang, T.T.; Rosenthal, P.; Sanyal, A.J.; Schwimmer, J.B.; Lavine, J.E. Association between metabolic syndrome and liver histology among children with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2010, 105, 2093–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mencin, A.A.; Lavine, J.E. Advances in pediatric nonalcoholic fatty liver disease. Pediatr. Clin. N. Am. 2011, 58, 1375–1392. [Google Scholar] [CrossRef] [Green Version]
- Vajro, P.; Lenta, S.; Socha, P.; Dhawan, A.; McKiernan, P.; Baumann, U.; Durmaz, O.; Lacaille, F.; McLin, V.; Nobili, V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: Position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 700–713. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Unalp, A.; Van Natta, M.L.; Cummings, O.W.; Sandrasegaran, K.E.; Hameed, T.; Tonascia, J.; Chalasani, N. Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Amernia, B.; Moosavy, S.H.; Banookh, F.; Zoghi, G. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas, Iran. BMC Gastroenterol. 2021, 21, 453. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Leeming, D.J.; Goodman, Z.; Friedman, S.; Frederiksen, P.; Rasmussen, D.G.K.; Vig, P.; Seyedkazemi, S.; Fischer, L.; Torstenson, R.; et al. Comparison of ADAPT, FIB-4 and APRI as non-invasive predictors of liver fibrosis and NASH within the CENTAUR screening population. J. Hepatol. 2021, 75, 1292–1300. [Google Scholar] [CrossRef]
- De Matteis, C.; Cariello, M.; Graziano, G.; Battaglia, S.; Suppressa, P.; Piazzolla, G.; Sabbà, C.; Moschetta, A. AST to Platelet Ratio Index (APRI) is an easy-to-use predictor score for cardiovascular risk in metabolic subjects. Sci. Rep. 2021, 11, 14834. [Google Scholar] [CrossRef]
- Younes, R.; Caviglia, G.P.; Govaere, O.; Rosso, C.; Armandi, A.; Sanavia, T.; Pennisi, G.; Liguori, A.; Francione, P.; Gallego-Durán, R.; et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 786–794. [Google Scholar] [CrossRef]
- Catanzaro, R.; Aleo, A.; Sciuto, M.; Zanoli, L.; Balakrishnan, B.; Marotta, F. FIB-4 and APRI scores for predicting severe liver fibrosis in chronic hepatitis HCV patients: A monocentric retrospective study. Clin. Exp. Hepatol. 2021, 7, 111–116. [Google Scholar] [CrossRef]
- Drolz, A.; Wolter, S.; Wehmeyer, M.H.; Piecha, F.; Horvatits, T.; Schulze Zur Wiesch, J.; Lohse, A.W.; Mann, O.; Kluwe, J. Performance of non-invasive fibrosis scores in non-alcoholic fatty liver disease with and without morbid obesity. Int. J. Obes. 2021, 45, 2197–2204. [Google Scholar] [CrossRef]
- Sartorio, A.; Del Col, A.; Agosti, F.; Mazzilli, G.; Bellentani, S.; Tiribelli, C.; Bedogni, G. Predictors of non-alcoholic fatty liver disease in obese children. Eur. J. Clin. Nutr. 2007, 61, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Satiya, J.; Snyder, H.S.; Singh, S.P.; Satapathy, S.K. Narrative review of current and emerging pharmacological therapies for nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 60. [Google Scholar] [CrossRef]
- Denzer, C.; Thiere, D.; Muche, R.; Koenig, W.; Mayer, H.; Kratzer, W.; Wabitsch, M. Gender-Specific prevalences of fatty liver in obese children and adolescents: Roles of body fat distribution, sex steroids, and insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 3872–3881. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Tagi, V.M.; Samvelyan, S.; Chiarelli, F. Metabolic syndrome in children. Minerva Pediatr. 2020, 72, 312–325. [Google Scholar] [CrossRef]
- Serbis, A.; Giapros, V.; Galli-Tsinopoulou, A.; Siomou, E. Metabolic Syndrome in Children and Adolescents: Is There a Universally Accepted Definition? Does it Matter? Metab. Syndr. Relat. Disord. 2020, 18, 462–470. [Google Scholar] [CrossRef]
- Kim, C.H.; Younossi, Z.M. Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome. Clevel. Clin. J. Med. 2008, 75, 721–728. [Google Scholar] [CrossRef]
- Flisiak-Jackiewicz, M.; Bobrus-Chociej, A.; Wasilewska, N.; Lebensztejn, D.M. From Nonalcoholic Fatty Liver Disease (NAFLD) to Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)-New Terminology in Pediatric Patients as a Step in Good Scientific Direction? J. Clin. Med. 2021, 10, 924. [Google Scholar] [CrossRef]
- Trey, J.E.; Kushner, I. The acute phase response and the hematopoietic system: The role of cytokines. Crit. Rev. Oncol. Hematol. 1995, 21, 1–18. [Google Scholar] [CrossRef]
- Abd El Rihim, A.Y.; Omar, R.F.; Fathalah, W.; El Attar, I.; Hafez, H.A.; Ibrahim, W. Role of fibroscan and APRI in detection of liver fibrosis: A systematic review and meta-analysis. Arab J. Gastroenterol. 2013, 14, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH-current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Lee, C.K.; Perez-Atayde, A.R.; Mitchell, P.D.; Raza, R.; Afdhal, N.H.; Jonas, M.M. Serum biomarkers and transient elastography as predictors of advanced liver fibrosis in a United States cohort: The Boston children’s hospital experience. J. Pediatr. 2013, 163, 1058–1064.e2. [Google Scholar] [CrossRef] [PubMed]
- Hukkinen, M.; Kivisaari, R.; Lohi, J.; Heikkilä, P.; Mutanen, A.; Merras-Salmio, L.; Pakarinen, M.P. Transient elastography and aspartate aminotransferase to platelet ratio predict liver injury in paediatric intestinal failure. Liver Int. 2016, 36, 361–369. [Google Scholar] [CrossRef]
- Ozsu, E.; Yazıcıoğlu, B. Obese boys with low concentrations of high-density lipoprotein cholesterol are at greater risk of hepatosteatosis. Hormones 2019, 18, 477484. [Google Scholar] [CrossRef] [Green Version]
- Popa, A.; Bende, F.; Șirli, R.; Popescu, A.; Bâldea, V.; Lupușoru, R.; Cotrău, R.; Fofiu, R.; Foncea, C.; Sporea, I. Quantification of Liver Fibrosis, Steatosis, and Viscosity Using Multiparametric Ultrasound in Patients with Non-Alcoholic Liver Disease: A “Real-Life” Cohort Study. Diagnostics 2021, 11, 783. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.M.; Lee, G.; Jeon, S.K.; Joo, I. Quantitative Evaluation of Hepatic Steatosis Using Advanced Imaging Techniques: Focusing on New Quantitative Ultrasound Techniques. Korean J. Radiol. 2022, 23, 13–29. [Google Scholar] [CrossRef]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (6–20 years). Eur. J. Clin. Nutr. 2002, 56, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Saverymuttu, S.H.; Joseph, A.E.; Maxwell, J.D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br. Med. J. 1986, 292, 13–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, A.E.; Saverymuttu, S.H.; al-Sam, S.; Cook, M.G.; Maxwell, J.D. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin. Radiol. 1991, 43, 26–31. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- McCharty, H.D.; Jarret, K.V.; Crawley, H.F. The development of waist circumference percentiles in British children aged 5.0–16.9 y. Eur. J. Clin. Nutr. 2001, 55, 902–907. [Google Scholar] [CrossRef] [Green Version]

| Parameter | NAFLD − | NAFLD + | p |
|---|---|---|---|
| Sex (M/F) | 141/321 | 162/133 | <0.001 |
| Age (years) | 15.0 (13.4–16.5) [10.0–18.5] | 15.0 (12.5–16.6) [10.0–18.3] | 0.297 |
| BMI-SDS | 2.9 (2.5–3.2) [2.0–4.5] | 3.1 (2.7–3.5) [2.0–4.6] | <0.001 |
| WC (cm) | 111.0 (103.0–122.0) [69.0–160.0] | 121.0 (113.0–131.0) [93.0–166.0] | <0.001 |
| T-C (mg/dL) | 157.5 (141.0–180.2) [100.0–425.0] | 165.0 (143.0–186.0) [54.0–291.0] | 0.028 |
| HDL-C (mg/dL) | 42.0 (36.0–49.2) [17.0–118.0] | 40.0 (35.0–47.0) [19.0–77.0] | 0.005 |
| LDL-C (mg/dL) | 99.0 (83.0–118.0) [45.0–357.0] | 108.0 (87.0–126.0) [18.0–218.0] | 0.004 |
| Triglycerides (mg/dL) | 83.0 (65.0–110.2) [15.0–340.0] | 96.0 (77.0–126.0) [14.0–293.0] | <0.001 |
| Glucose (mg/dL) | 80.0 (77.0–85.0) [64.0–130.0] | 82.0 (78.0–86.0) [69.0–108.0] | 0.007 |
| Insulin (µIU/L) | 11.7 (7.8–16.8) [2.0–75.4] | 16.4 (10.9–22.0) [2.0–47.5] | <0.001 |
| HOMA-IR | 2.3 (1.5–3.3) [0.3–14.6] | 3.2 (2.2–4.5) [0.3–12.6] | <0.001 |
| APRI | 0.1 (0.1–0.2) [0.0–0.7] | 0.2 (0.1–0.3) [0.0–0.8] | 0.036 |
| Uric acid (mg/dL) | 5.8 (5.1–6.7) [0.7–10.5] | 6.6 (5.5–7.5) [0.2–10.1] | <0.001 |
| Total bilirubin (mg/dL) | 0.5 (0.4–0.7) [0.2–2.2] | 0.5 (0.4–0.7) [0.1–2.5] | 0.310 |
| CRP (mg/dL) | 0.3 (0.2–0.6) [0.0–8.7] | 0.4 (0.2–0.7) [0.0–4.0] | 0.095 |
| SBP (mmHg) | 120.0 (120.0–130.0) [100.0–170.0] | 130.0 (120.0–130.0) [90.0–180.0] | <0.001 |
| DBP (mmHg) | 80.0 (70.0–80.0) [50.0–110.0] | 80.0 (80.0–80.0) [60.0–110.0] | <0.001 |
| Metabolic syndrome (yes/no) | 111/351 | 100/195 | 0.004 |
| Parameter | r (95% CI) | p |
|---|---|---|
| Age (years) | 0.010 (−0.063–0.083) | 0.7768 |
| BMI SDS | 0.032 (−0.040–0.106) | 0.3658 |
| WC (cm) | 0.112 (−0.122–0.023) | 0.0020 |
| T-C (mg/dL) | −0.021 (−0.095–0.051) | 0.5483 |
| HDL-C (mg/dL) | −0.115 (−0.187–−0.042)) | 0.0015 |
| LDL-C (mg/dL) | 0.000 (−0.073–0.073) | 0.9970 |
| Triglycerides (mg/dL) | 0.061 (−0.011–0.134) | 0.0894 |
| Glucose (mg/dL) | 0.005 (−0.067–0.078) | 0.8763 |
| Insulin (µIU/L) | 0.028 (−0.045–0.101) | 0.4400 |
| HOMA-IR | 0.023 (−0.850–0.096) | 0.5224 |
| Uric acid (mg/dL) | 0.229 (0.158–0.297) | <0.0001 |
| Total bilirubin (mg/dL) | 0.138 (0.065–0.209) | 0.0001 |
| CRP (mg/dL) | −0.154 (−0.225–−0.082) | <0.0001 |
| SBP (mmHg) | 0.085 (0.011–0.157) | 0.0193 |
| DBP (mmHg) | 0.059 (−0.013–0.132) | 0.1016 |
| Predictor | OR (95% CI) | p |
|---|---|---|
| Female | 0.367 (0.270–0.497) | <0.001 |
| Age (years/10) | 1.000 (0.978–1.022) | 0.984 |
| BMI SDS | 2.164 (1.637–2.862) | <0.001 |
| WC (cm/10) | 1.002 (0.999–1.004) | 0.284 |
| T-C (mg/dL/10) | 1.000 (0.998–1.002) | 0.864 |
| HDL-C (mg/dL/10) | 0.997 (0.990–1.004) | 0.418 |
| LDL-C (mg/dL/10) | 1.000 (0.998–1.003) | 0.763 |
| Triglycerides (mg/dL/10) | 1.003 (1.001–1.006) | 0.018 |
| Glucose (mg/dL/10) | 1.000 (0.996–1.004) | 0.869 |
| Insulin (µIU/L/10) | 1.027 (1.011–1.044) | 0.001 |
| HOMA-IR | 1.351 (1.231–1.482) | <0.001 |
| APRI | 250.510 (47.891–1310.384) | <0.001 |
| Uric acid (mg/dL) | 1.438 (1.277–1.621) | <0.001 |
| Total bilirubin (mg/dL) | 0.833 (0.506–1.372) | 0.473 |
| CRP (mg/dL) | 0.958 (0.779–1.179) | 0.688 |
| SBP (mmHg/10) | 1.001 (0.998–1.003) | 0.593 |
| DBP (mmHg/10) | 1.001 (0.997–1.005) | 0.658 |
| Metabolic syndrome | 1.631 (1.180–2.255) | 0.003 |
| Predictor | OR (95% CI) | p | Coefficient |
|---|---|---|---|
| Female | 0.506 (0.353–0.726) | <0.001 | −0.681 |
| BMI SDS | 1.516 (1.081–2.126) | 0.016 | 0.416 |
| Triglycerides (mg/dL/10) | 1.007 (1.00–1.012) | 0.008 | 0.007 |
| HOMA-IR | 1.413 (1.226–1.629) | <0.001 | 0.346 |
| APRI | 68.853 (12.343–384.091) | <0.001 | 4.232 |
| Uric acid (mg/dL) | 1.088 (0.947–1.250) | 0.234 | 0.084 |
| Metabolic syndrome | 0.842 (0.571–1.242) | 0.386 | −0.172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.E.; Bondesan, A.; Rondinelli, E.; Cella, S.G.; Sartorio, A. The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents. Metabolites 2022, 12, 155. https://doi.org/10.3390/metabo12020155
Rigamonti AE, Bondesan A, Rondinelli E, Cella SG, Sartorio A. The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents. Metabolites. 2022; 12(2):155. https://doi.org/10.3390/metabo12020155
Chicago/Turabian StyleRigamonti, Antonello E., Adele Bondesan, Eugenia Rondinelli, Silvano G. Cella, and Alessandro Sartorio. 2022. "The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents" Metabolites 12, no. 2: 155. https://doi.org/10.3390/metabo12020155
APA StyleRigamonti, A. E., Bondesan, A., Rondinelli, E., Cella, S. G., & Sartorio, A. (2022). The Role of Aspartate Transaminase to Platelet Ratio Index (APRI) for the Prediction of Non-Alcoholic Fatty Liver Disease (NAFLD) in Severely Obese Children and Adolescents. Metabolites, 12(2), 155. https://doi.org/10.3390/metabo12020155







