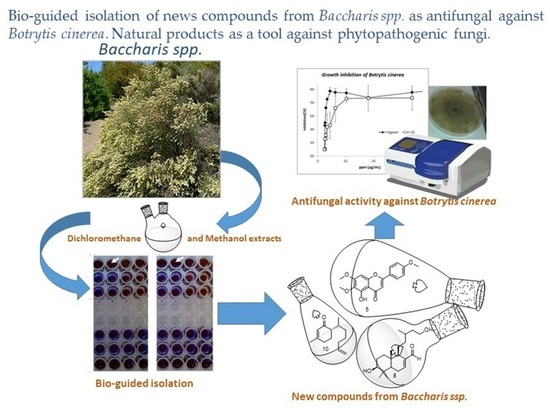
Bio-Guided Isolation of New Compounds from Baccharis spp. as Antifungal against Botrytis cinerea
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure
2.2. Isolates and Cultures
2.3. Extraction and Bio-Guided Isolation
2.4. In Vitro Antifungal Assay
2.4.1. Preparation of Pure Compounds Stock and Broth Microdilution Method Bioassay
2.4.2. Bio-Guided Antifungal Assays: Use of Resazurin as Inhibition Indicator
2.4.3. Poisoned Food Medium Assay
2.4.4. Statistical Analysis
3. Results
Bio-Guided Isolation and Identification of New Compounds
4. Antifungal Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bremer, K.; Jansen, R.K.; Karis, P.O.; Källersjö, M.; Keeley, S.C.; Kim, K.-J.; Michaels, H.J.; Palmer, J.D.; Wallace, R.S. A Review of the Phylogeny and Classification of the Asteraceae. Nord. J. Bot. 1992, 12, 141–148. [Google Scholar] [CrossRef]
- Bremer, K. Asteraceae, Cladistics & Classification; Timber Press, Inc.: Portland, OR, USA, 1994. [Google Scholar]
- Abad, M.J.; Bermejo, P. Baccharis (Compositae): A Review Update. Arkivoc 2007, 7, 76–96. [Google Scholar] [CrossRef] [Green Version]
- Verdi, L.G.; Brighente, I.M.C.; Pizzolatti, M.G. Gênero Baccharis (Asteraceae): Aspectos Químicos, Económicos e Biológicos. Quim. Nov. 2005, 28, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Ramos Campos, F.; Bressan, J.; Godoy Jasinski, V.C.; Zuccolotto, T.; da Silva, L.E.; Bonancio Cerqueira, L. Baccharis (Asteraceae): Chemical Constituents and Biological Activities. Chem. Biodivers. 2016, 13, 1–17. [Google Scholar] [CrossRef]
- Borella, J.C.; Duarte, D.P.; Novaretti, A.A.G.; Menezes, A., Jr.; França, S.C.; Rufato, C.B.; Santos, P.A.S.; Veneziani, R.C.S.; Lopes, N.P. Variabilidade Sazonal Do Teor de Saponinas de Baccharis trimera (Less.) DC (Carqueja) e Isolamento de Flavona. Rev. Bras. Farmacogn. 2006, 16, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Dos, S.; Grecco, S.; Dorigueto, A.C.; Landre, I.M.; Soares, M.G.; Martho, K.; Lima, R.; Pascon, R.C.; Vallim, M.A.; Capello, T.M.; et al. Structural Crystalline Characterization of Sakuranetin—An Antimicrobial Flavanone from Twigs of Baccharis retusa (Asteraceae). Molecules 2014, 19, 7528–7542. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos Ribeiro, P.R.; de Castro Girão, D.; Ávila Pimenta, A.T.; Braz-Filho, R.; Silva Guedes, M.L.; Silveira, E.R.; Sousa Lima, M.A. Clerodane and Patchoulene Terpenes as New Constituents from Baccharis salzmannii DC. Biochem. Syst. Ecol. 2013, 50, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Fullas, F.; Hussam, R.; Bordas, E.; Pezzuto, J.M.; SoeJarto, D.D.; Douglas Kmghom, A. Gaudichadiosides A–E, Five Novel Diterpene Glycoside Constituents from the Sweet-Tasting Plant Baccharis gaudichaudiana. Tetrahedron 1991, 47, 8515–8522. [Google Scholar] [CrossRef]
- Akaike, S.; Sumino, M.; Sekine, T.; Seo, S.; Kimura, N.; Ikegami, F. A New Ent-Clerodane Diterpene from the Aerial Parts of Baccharis gaudichaudiana. Chem. Pharm. Bull. 2003, 51, 197–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loayza, I.; Abujder, D.; Aranda, R.; Jakupovic, J.; Collin, G.U.Y.; Li, H.I.; Deslauriers, N.E.; Jeand, F. Essential Oils of Baccharis salicifolia, B. Latifolia and B. dracuncufolia. Phytochemistry 1995, 38, 381–389. [Google Scholar] [CrossRef]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Review: Alternatives to Synthetic Fungicides for Botrytis cinerea Management in Vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; de Souza, E.B.; Rocha, M.F.G.; Albuquerque, M.R.J.R.; Bandeira, P.N.; dos Santos, H.S.; de Paula Cavalcante, C.S.; Oliveira, S.S.; Aragão, P.R.; de Morais, S.M.; et al. Chemical Composition, Antioxidant, Antifungal and Hemolytic Activities of Essential Oil from Baccharis trinervis (Lam.) Pers. (Asteraceae). Ind. Crops Prod. 2016, 84, 108–115. [Google Scholar] [CrossRef]
- Giuliano, D.A.; Nesom, G.L. A New Section of Baccharis (Asteraceae: Astereae), and Notes on Allied Taxa. SIDA Contrib. Bot. 2017, 20, 1481–1484. Available online: https://www.jstor.org/stable/41961006 (accessed on 22 December 2021).
- Vásquez, J.; Alarcón, J.C.; Jiménez, S.L.; Jaramillo, G.I.; Gómez-Betancur, I.C.; Rey-Suárez, J.P.; Jaramillo, K.M.; Muñoz, D.C.; Marín, D.M.; Romero, J.O. Main Plants Used in Traditional Medicine for the Treatment of Snake Bites n the Regions of the Department of Antioquia, Colombia. J. Ethnopharmacol. 2015, 170, 158–166. [Google Scholar] [CrossRef]
- Chaverri, C.; Cicció, J.F. Essential Oils of Baccharis trinervis (Asteraceae) from Costa Rica. Rev. Biol. Trop. 2017, 65, 1307. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, M.R.J.R.; Souza, E.B.; Lins, M.U.D.S.; Nogueira, N.A.P.; Lemos, T.L.G.; Silveira, E.R.; Pessoa, O.D.L. Composition and Antimicrobial Activity of the Essential Oil from Aerial Parts of Baccharis trinervis (Lam.) Pers. Arkivoc 2004, 6, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.; Velasco, J.; Rojas, L.B.; Díaz, T.; Carmona, J.; Morales, A. Chemical Composition and Antibacterial Activity of the Essential Oil of Baccharis latifolia Pers. and B. prunifolia H. B. & K. (Asteraceae). Nat. Prod. Commun. 2007, 2, 1245–1248. [Google Scholar]
- Sharp, H.; Bartholomew, B.; Bright, C.; Latif, Z.; Sarker, S.D.; Nash, R.J. 6-Oxygenated Flavones from Baccharis trinervis (Asteraceae). Biochem. Syst. Ecol. 2001, 29, 105–107. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Uchida, K.; Ueno, A.; Satake, M.; Shimomura, K. Neo-Clerodane Type Diterpenes from Baccharis trinervis. Phytochemistry 1993, 34, 1377–1384. [Google Scholar] [CrossRef]
- Rojas, J.; Velasco, J.; Morales, A.; Rojas, L.; Díaz, T.; Rondón, M.; Carmona, J. Chemical Composition and Antibacterial Activity of the Essential Oil of Baccharis trinervis (Lam.) Pers. (Asteraceae) Collected in Venezuela. Nat. Prod. Commun. 2008, 3, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Caneschi, C.A.; Martins, F.J.; Larrudé, D.G.; Romani, E.C.; Brandão, M.A.F.; Raposo, N.R.B. In Vitro Antifungal Activity of Baccharis trimera Less (DC) Essential Oil against Dermatophytes. Trop. J. Pharm. Res. 2015, 14, 2083–2089. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, T.G.; da Silva, J.C.P.; Carneiro, J.N.P.; do Amaral, W.; Deschamps, C.; de Araújo, J.P.; da Costa, J.G.M.; de Oliveira Almeida, W.; da Silva, L.E.; Coutinho, H.D.M.; et al. Phytochemical Characterization and Inhibition of Candida sp. by the Essential Oil of Baccharis trimera (Less.) DC. Arch. Microbiol. 2021, 203, 3077–3087. [Google Scholar] [CrossRef]
- Carrizo, S.L.; Zampini, I.C.; Sayago, J.E.; Simirgiotis, M.J.; Bórquez, J.; Cuello, A.S.; Isla, M.I. Antifungal Activity of Phytotherapeutic Preparation of Baccharis Species from Argentine Puna against Clinically Relevant Fungi. J. Ethnopharmacol. 2020, 251, 112553. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, C.; da Silva Ribeiro, R.T.; Schwambach, J. Control of Postharvest Fungal Rots in Grapes through the Use of Baccharis trimera and Baccharis dracunculifolia Essential Oils. Crop Prot. 2019, 125, 104912. [Google Scholar] [CrossRef]
- Tomazoni, Z.E.; Ribeiro, R.T.S.; Pauletti, G.F.; Gonçalves Soares, G.L.; Schwambach, J. Inhibition of Alternaria Stem Canker on Tomato by Essential Oils from Baccharis Species. J. Environ. Sci. Health Part B 2019, 54, 781–790. [Google Scholar] [CrossRef]
- Luchesi, L.A.; Paulus, D.; Busso, C.; Frata, M.T.; Oliveira, J.B. Chemical Composition, Antifungal and Antioxidant Activity of Essential Oils from Baccharis dracunculifolia and Pogostemon cablin against Fusarium graminearum. Nat. Prod. Res. 2022, 36, 849–852. [Google Scholar] [CrossRef]
- Stegmayer, M.I.; Fernández, L.N.; Álvarez, N.H.; Seimandi, G.M.; Reutemann, A.G. In Vitro Antifungal Screening of Argentine Native or Naturalized Plants against the Phytopathogen Monilinia fructicola. Comb. Chem. High Throughput Screen 2022, 25, 1158–1166. [Google Scholar]
- Tomazoni, Z.E.; Griggio, S.G.; Broilo, P.E.; da Silva Ribeiro, R.T.; Gonçalves Soares, G.L.; Schwambach, J. Screening for Inhibitory Activity of Essential Oils on Fungal Tomato Pathogen Stemphylium solani Weber. Biocatal. Agric. Biotechnol. 2018, 16, 364–372. [Google Scholar] [CrossRef]
- Lam-Gutiérrez, A.; Winkler, R.; Garrido-Ramírez, E.R.; Rincón-Rosales, R.; Gutiérrez-Miceli, F.A.; Peña-Ocaña, B.A.; Guzmán-Albores, J.M.; Ruíz-Valdiviezo, V.M. Antifungal Activity of Root Extracts from Baccharis Salicina on Germination of Uredospores of Hemileia vastatrix. Int. J. Agric. Biol. 2021, 25, 1075–1084. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey Mould of Strawberry, a Devastating Disease Caused by the Ubiquitous Necrotrophic Fungal Pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A.L. Botrytis cinerea: The Cause of Grey Mould Disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Van Kan, J.A.L. Licensed to Kill: The Lifestyle of a Necrotrophic Plant Pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- O’Neill, T.E.; Li, H.; Colquhoun, C.D.; Johnson, J.A.; Webster, D.; Gray, C.A. Optimisation of the Microplate Resazurin Assay for Screening and Bioassay-Guided Fractionation of Phytochemical Extracts against Mycobacterium tuberculosis. Phytochem. Anal. 2014, 25, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, L.R.; Butassi, E.; Svetaz, L.; Zacchino, S.A.; Palermo, J.A.; Sánchez, M. Antifungal terpenoids from Hyalis argentea var. latisquama. J. Nat. Prod. 2014, 77, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Zacchino, S.A.; Gupta, M. Manual de Técnicas “In Vitro” para la Detección de Compuestos Antifúngicos; Corpus Editorial y Distribuidora: Rosario, Argentina, 2007; ISBN 9789509030404. [Google Scholar]
- Fai, P.B.; Grant, A. A rapid resazurin bioassay for assessing the toxicity of fungicides. Chemosphere 2009, 74, 1165–1170. [Google Scholar] [CrossRef]
- Zhang, H.X.; Du, G.H.; Zhang, J.T. Assay of mitochondrial functions by resazurin in vitro. Acta Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar]
- Pinedo-Rivilla, C.; Moraga, J.; Pérez-Sasián, G.; Peña-Hernández, A.; Collado, I.G.; Aleu, J. Biocatalytic preparation of chloroindanol derivatives. Antifungal activity and detoxification by the phytopathogenic fungus Botrytis cinerea. Plants 2020, 9, 1648. [Google Scholar] [CrossRef]
- CLSI Document M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, Approved Standard—Second Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Feresin, G.E.; Tapia, A.; Gimenez, A.; Gutierrez Ravelo, A.; Zacchino, S.; Sortino, M.; Schmeda-Hirschmann, G. Constituents of the Argentinian medicinal plant Baccharis grisebachii and their antimicrobial activity. J. Ethnopharmacol. 2003, 89, 73–80. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, H.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany, 1970; pp. 23–25, 37–38. [Google Scholar]
- Balaei-Kahnamoei, M.; Eftekhari, M.; Ardekani, M.R.S.; Akbarzadeh, T.; Saeedi, M.; Jamalifar, H.; Safavi, M.; Sam, S.; Zhalehjoo, N.; Khanavi, M. Phytochemical constituents and biological activities of Salvia macrosiphon Boiss. BMC Chem. 2021, 15, 4. [Google Scholar] [CrossRef]
- Xie, H.; Liang, Y.; Ito, Y.; Wang, X.; Chen, R.; He, J.; Li, H.; Zhang, T. Preparative isolation and purification of four flavonoids from Daphne genkwa Sieb. et Zucc. by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2360–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porras, G.; Bacsa, J.; Tang, H.; Quave, C.L. Characterization and structural analysis of genkwanin, a natural product from Callicarpa americana. Crystals 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Rodriguez, J.P.; Lee, K.H.; Park, J.Y.; Kang, K.S.; Hahm, D.H.; Huh, C.K.; Lee, S.C.; Lee, S. Determination of flavonoids from Cirsium japonicum var. maackii and their inhibitory activities against aldose reductase. Appl. Biol. Chem. 2017, 60, 487–496. [Google Scholar] [CrossRef]
- Labbe, C.; Rovirosa, J.; Faini, F.; Mahu, M.; San-Martin, A.; Castillo, M. Secondary Metabolites from Chilean Baccharis Species. J. Nat. Prod. 1986, 49, 517–518. [Google Scholar] [CrossRef]
- Savona, G.; Dommisee, R.A. Additional Proof for the Structure of the New Flavone Galangustin (Bucegin) Obtained by 13C-NMR Spectroscopy. Bull. Soc. Chim. Belg. 1983, 92, 497–498. [Google Scholar]
- Shukla, Y.N.; Tandon, J.S.; Dhar, M.M. Lyonogenin—A new dihydrochalkone from Lyonia formosa. Indian J. Chem. 1973, 2, 720–722. [Google Scholar]
- Bohlmann, F.; Zdero, C. New sesquiterpenes from Senecio oxyodontus. Phytochemistry 1978, 17, 1591–1593. [Google Scholar] [CrossRef]
- Dos Santos, M.S.; da Silva, J.; Menezes, A.P.S.; de Barros, F.M.C.; Lemes, M.L.B.; Rossatto, R.R.; Feistel, C.; de Almeida, I.D.; Grivicich, I.; Prado, L.; et al. Biotoxicological Analyses of Trimeroside from Baccharis trimera Using a Battery of in Vitro Test Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Silvia, G.R.A.; Gabriela, T.T.; Maribel, H.R.; Nayeli, M.B.; Luis, T.E.J.; Alejandro, Z.; Manasés, G.C. Effect of Terpenoids and Flavonoids Isolated from Baccharis conferta Kunth on TPA-Induced Ear Edema in Mice. Molecules 2020, 25, 1379. [Google Scholar] [CrossRef] [Green Version]
- Jisaka, M.; Kawanaka, M.; Sugiyama, H.; Takegawa, K.; Huffman, M.A.; Ohigashi, H.; Koshimizu, K. Studies on the Constituents of Baccharis genistelloides. Chem. Pharm. Bull. 1992, 56, 845–846. [Google Scholar]
- Brahmachari, G. Nevadensin: Isolation, chemistry and bioactivity. Int. J. Green Pharm. 2010, 4, 213–219. [Google Scholar] [CrossRef]
- Benali, T.; Jaouadi, I.; Ghchime, R.; El Omari, N.; Harboul, K.; Hammani, K.; Rebezov, M.; Shariati, M.A.; Mubarak, M.S.; Simal-Gandara, J.; et al. The Current State of Knowledge in Biological Properties of Cirsimaritin. Antioxidants 2022, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Paredes, J.D.; Villegas Gabutti, C.M.; Gamarra-Luques, C.; Bórquez, J.; Luna, L.; Wendel, G.H.; Maria, A.O.; et al. Antioxidant, gastroprotective, cytotoxic activities and uhplc pda-q orbitrap mass spectrometry identification of metabolites in Baccharis grisebachii decoction. Molecules 2019, 24, 1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serino, E.; Chahardoli, A.; Badolati, N.; Sirignano, C.; Jalilian, F.; Mojarrab, M.; Farhangi, Z.; Rigano, D.; Stornaiuolo, M.; Shokoohinia, Y.; et al. Salvigenin, a trimethoxylated flavone from achillea wilhelmsii c. Koch, exerts combined lipid-lowering and mitochondrial stimulatory effects. Antioxidants 2021, 10, 1042. [Google Scholar] [CrossRef]
- Qin, G.; Yi, S. Genkwanin improves inflammatory injury in rats with septic lung injury by regulating NF-κB signaling pathway. Qual. Assur. Saf. Crops Foods 2022, 14, 66–73. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Bao, Y.; Yu, H.; Chen, Q.-J.; Lu, F.; Zhai, S.; Zhang, C.-F.; Li, F.; Wang, C.-Z.; Yuan, C.-S. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int. Immunopharmacol. 2020, 83, 106384. [Google Scholar] [CrossRef]
- Cottiglia, F.; Loy, G.; Garau, D.; Floris, C.; Caus, M.; Pompei, R.; Bonsignore, L. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine 2001, 8, 302–305. [Google Scholar] [CrossRef]
- Martini, N.D.; Katerere, D.R.; Eloff, J.N. Seven flavonoids with antibacterial activity isolated from Combretum erythrophyllum. S. Afr. J. Bot. 2004, 70, 310–312. [Google Scholar] [CrossRef] [Green Version]
- Ben Sghaier, M.; Skandrani, I.; Nasr, N.; Franca, M.G.D.; Chekir-Ghedira, L.; Ghedira, K. Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: A structure-activity relationship study. Environ. Toxicol. Pharmacol. 2011, 32, 336–348. [Google Scholar] [CrossRef]
- Harkati, B.; Akkal, S.; Bayat, C.; Laouer, H.; Dijoux Franca, M.G. Secondary metabolites from Scorzonera undulata ssp. deliciosa (Guss.) Maire (Asteracae) and their antioxidant activities. Rec. Nat. Prod. 2010, 4, 171–175. [Google Scholar]
- Brahim, H.; Salah, A.; Christine, B.; Hocine, L.; Dijoux-Franca, M.G. Evaluation of antioxidant activity, free radical scavenging and cuprac of two compounds isolated from Scorzonera undulata ssp. deliciosa. Adv. Environ. Biol. 2013, 7, 591–594. [Google Scholar]
- Bohlmann, F.; Zdero, C. New Norsesquiterpenes from Senecio digitalifolius. Phytochemistry 1978, 17, 759–761. [Google Scholar] [CrossRef]
- Mendoza, L.; Sepúlveda, C.; Melo, R.; Cotoras, M. Characterization of the antifungal activity against Botrytis cinerea of sclareol and 13-epi-sclareol, two labdane-type diterpenoids. J. Chil. Chem. Soc. 2015, 60, 3024–3028. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Galan, R.H. The Antifungal Activity of Widdrol and Its Biotransformation by Botrytis cinerea. J. Agric. Food Chem. 2006, 54, 7517–7521. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Rivilla, C.; Collado, I.G.; Aleu, J. Metabolism of Antifungal Thiochroman-4-ones by Trichoderma viride and Botrytis cinerea. J. Nat. Prod. 2018, 81, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Rivilla, C.; Bustillo, A.J.; Hernández-Galán, R.; Aleu, J.; Collado, I.G. Asymmetric preparation of antifungal 1-(4′-chlorophenyl)-1-cyclopropyl methanol and 1-(4′-chlorophenyl)-2-phenylethanol. Study of the detoxification mechanism by Botrytis cinerea. J. Mol. Catal. B Enzym. 2011, 70, 61–66. [Google Scholar] [CrossRef]








| Species | Part of the Plant | Extract | MIC (μg/mL) |
|---|---|---|---|
| Baccharis prunifolia | Leaves | Dichloromethane | 125 |
| Methanol | 125 | ||
| Baccharis trinervis | Leaves | Dichloromethane | 125 |
| Methanol | 250 | ||
| Baccharis zumbadorensis | Leaves | Dichloromethane | 125 |
| Methanol | 250 | ||
| Irgasan | 0.23 |
| 7A | 8 | 11 | ||||||
|---|---|---|---|---|---|---|---|---|
| δH Mult(J in Hz) | δC | δH Mult(J in Hz) | δC | δH Mult(J in Hz) | δC | |||
| 1 | - | 130.3 | 1 | 1.65, m | 27.2 | 1 | - | 201.3 |
| 2 | 7.38, d (8.8) | 127.7 | 2 | 1.65, m | 38.5 | 2 | 5.85, m | 127.0 |
| 3 | 6.96, d (8.8) | 114.2 | 3 | 3.27, dd (3.9, 11.2) | 78.8 | 3 | - | 161.4 |
| 4 | - | 160.1 | 4 | - | 38.6 | 4 | 2.29, m | 30.4 |
| 5 | 6.96, d (8.8) | 114.2 | 5 | 1.20, m | 49.0 | 5 | a 1.78 m | 22.6 |
| b 1.92, m | ||||||||
| 6 | 7.38, d (8.8) | 127.7 | 6 | 2.35, m | 24.9 | 6 | 2.12, m | 49.8 |
| α | 5.37, m | 79.0 | 7 | 6.79, t | 151.7 | 7 | 2.30, m | 30.7 |
| β | 3.11, m | 43.1 | 8 | - | 144.4 | 8 | 1.30, m | 34.5 |
| 2.81, m | ||||||||
| 1′ | - | 103.3 | 9 | 1.95, m | 50.4 | 9 | a 1.31 | 23.4 |
| b 1.49 | ||||||||
| 2′ | - | 164.4 | 10 | - | 36.6 | 10 | 1.44, m | 33.2 |
| 3′ | 5.99, s | 96.7 | 11 | 1.48, m | 23.9 | 11 | 3.70, m | 72.2 |
| 4′ | - | 164.1 | 12 | 1.95, m 1.20, m | 37.1 | 12 | 3.64–3.43, m | 66.8 |
| 5′ | 5.98, s | 95.3 | 13 | 1.52, m | 30.3 | 13 | - | - |
| 6′ | - | 163.3 | 14 | 1.65, m 1.20, m | 38.5 | 14 | 0.80, d (7.0) | 15.8 |
| C=O | - | 196.0 | 15 | 3.67, m | 61.3 | 15 | 1.93, s | 24.1 |
| OCH3 | 3.84, s | 55.4 | 16 | 0.91, d (7.9) | 19.6 | - | - | |
| OH | 12.05, s | - | 17 | 9.39, s | 194.6 | - | - | |
| - | - | - | 18 | 1.01, s | 27.9 | - | - | |
| - | - | - | 19 | 0.91, s | 15.2 | - | - | |
| - | - | - | 20 | 0.80, s | 14.3 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, A.A.; Ruano-González, A.; Ezzanad, A.; Pinedo-Rivilla, C.; Sánchez-Maestre, R.; Amaro-Luis, J.M. Bio-Guided Isolation of New Compounds from Baccharis spp. as Antifungal against Botrytis cinerea. Metabolites 2022, 12, 1292. https://doi.org/10.3390/metabo12121292
Pinto AA, Ruano-González A, Ezzanad A, Pinedo-Rivilla C, Sánchez-Maestre R, Amaro-Luis JM. Bio-Guided Isolation of New Compounds from Baccharis spp. as Antifungal against Botrytis cinerea. Metabolites. 2022; 12(12):1292. https://doi.org/10.3390/metabo12121292
Chicago/Turabian StylePinto, Ana A., Antonio Ruano-González, Abdellah Ezzanad, Cristina Pinedo-Rivilla, Rosario Sánchez-Maestre, and Juan Manuel Amaro-Luis. 2022. "Bio-Guided Isolation of New Compounds from Baccharis spp. as Antifungal against Botrytis cinerea" Metabolites 12, no. 12: 1292. https://doi.org/10.3390/metabo12121292
APA StylePinto, A. A., Ruano-González, A., Ezzanad, A., Pinedo-Rivilla, C., Sánchez-Maestre, R., & Amaro-Luis, J. M. (2022). Bio-Guided Isolation of New Compounds from Baccharis spp. as Antifungal against Botrytis cinerea. Metabolites, 12(12), 1292. https://doi.org/10.3390/metabo12121292