Ketogenic Diet and Vitamin D Metabolism: A Review of Evidence
Abstract
:1. Introduction
2. Ketogenic Diets (KDs)
2.1. Types and Macro- and Micronutrient Contents of KDs
2.2. Uses and Mechanistic Aspects of KDs
2.3. Potential Side Effects of KDs
3. Vitamin D
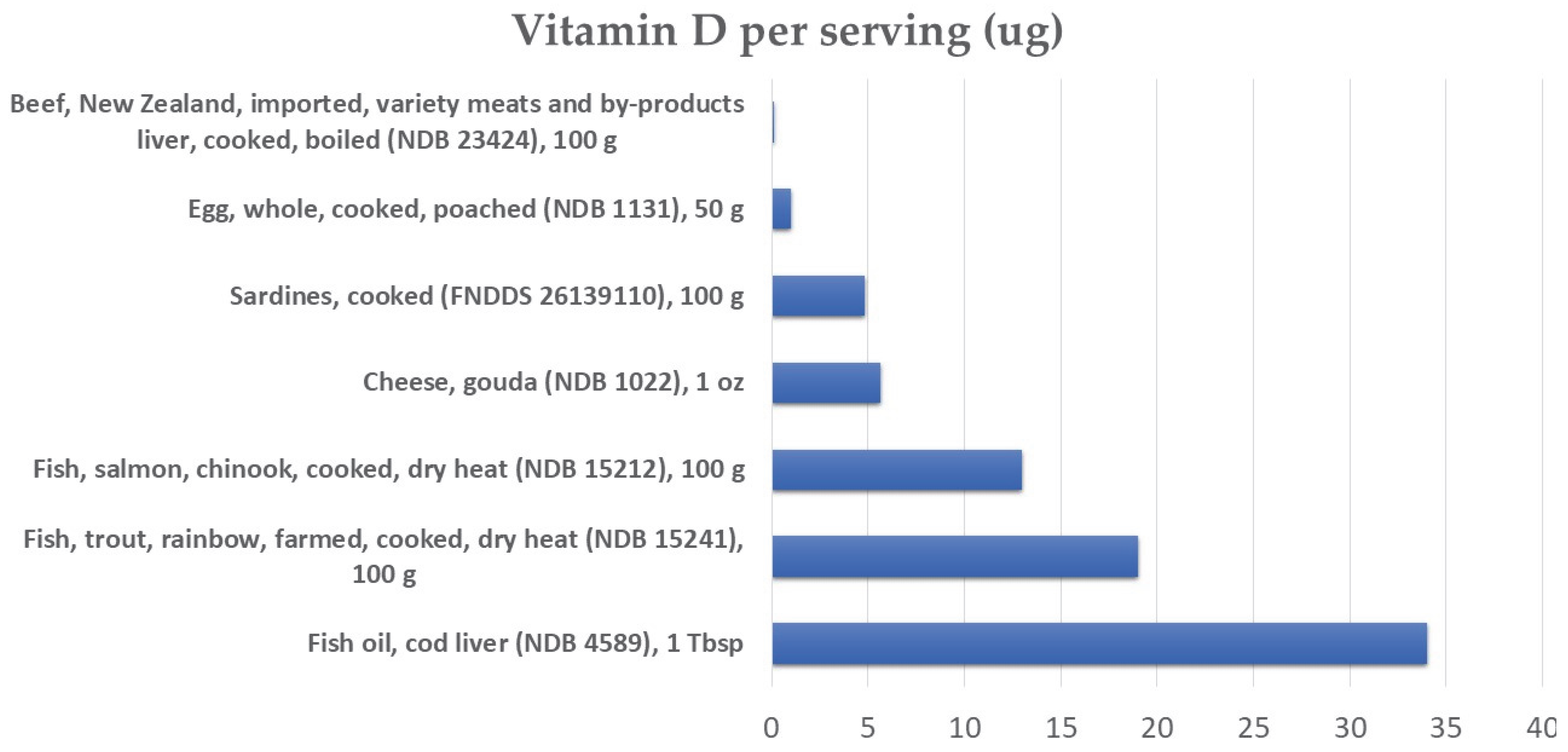
3.1. Sources of Vitamin D
3.2. Main Steps in the Metabolism of Vitamin D
3.3. Mechanism of Action of Vitamin D
3.4. Concentration of Circulating Vitamin D
4. Human Intervention Studies Relating KDs and Vitamin D
4.1. Intervention Studies in Healthy Adults and Patients with T2D
4.2. Intervention Studies in Patients with Epilepsy
5. Potential Effects of KDs on Vitamin D Levels and Metabolism
5.1. KD, Ketone Bodies and Vitamin D
5.2. KDs, Macronutrient Intake, and Vitamin D
5.3. KDs, Fat-Soluble Vitamin Status, and Vitamin D
5.4. KDs, Weight Loss, and Vitamin D
5.5. KDs, Hormonal Milieu, and Vitamin D
5.6. KDs, Gut Microbiota, and Vitamin D
6. Gene–Diet Interactions
7. Methodological Considerations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal Clinical Management of Children Receiving Dietary Therapies for Epilepsy: Updated Recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic Diet for Human Diseases: The Underlying Mechanisms and Potential for Clinical Implementations. Sig. Transduct. Target Ther. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.; Esteves, S.S.; da Costa Pereira, A.; Yancy Jr, W.S.; Nunes, J.P.L. Systematic Review and Meta-Analysis of Clinical Trials of the Effects of Low Carbohydrate Diets on Cardiovascular Risk Factors: Low Carbohydrate Diets and Cardiovascular Risk Factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Willard, K.-E.; Maki, K.C. Keto Is Trending: Implications for Body Weight and Lipid Management. Curr. Cardiol. Rep. 2022, 24, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. IJERPH 2022, 19, 10429. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.-Y.; Zhou, Z.-J.; Luo, R.; Gan, J.; Li, S.-P.; Mu, D.-Z.; Wan, C.-M. Safety and Tolerability of the Ketogenic Diet Used for the Treatment of Refractory Childhood Epilepsy: A Systematic Review of Published Prospective Studies. World J. Pediatr. 2017, 13, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.J.; Halstead, L.R.; DeVivo, D.C. Disordered Mineral Metabolism Produced by Ketogenic Diet Therapy. Calcif. Tissue Int. 1979, 28, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Eastep, J.; Chen, G. The Relationships of High-Fat Diet and Metabolism of Lipophilic Vitamins. Integr. Food Nutr. Metab. 2015, 2, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.-S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The Dependency of Vitamin D Status on Body Mass Index, Gender, Age and Season. Anticancer. Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- Colica, C.; Merra, G.; Gasbarrini, A.; De Lorenzo, A.; Cioccoloni, G.; Gualtieri, P.; Perrone, M.A.; Bernardini, S.; Bernardo, V.; Di Renzo, L.; et al. Efficacy and Safety of Very-Low-Calorie Ketogenic Diet: A Double Blind Randomized Crossover Study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2274–2289. [Google Scholar]
- Bolesławska, I.; Kowalówka, M.; Dobrzyńska, M.; Karaźniewicz-Łada, M.; Przysławski, J. Differences in the Concentration of Vitamin D Metabolites in Plasma Due to the Low-Carbohydrate-High-Fat Diet and the Eastern European Diet—A Pilot Study. Nutrients 2021, 13, 2774. [Google Scholar] [CrossRef]
- Lee, S.W.; Russell, J.; Avioli, L.V. 25-Hydroxycholecalciferol to 1,25-Dihydroxycholecalciferol: Conversion Impaired by Systemic Metabolic Acidosis. Science 1977, 195, 994–996. [Google Scholar] [CrossRef]
- Christodoulides, S.S.; Neal, E.G.; Fitzsimmons, G.; Chaffe, H.M.; Jeanes, Y.M.; Aitkenhead, H.; Cross, J.H. The Effect of the Classical and Medium Chain Triglyceride Ketogenic Diet on Vitamin and Mineral Levels: Vitamin and Mineral Levels on Ketogenic Diet. J. Hum. Nutr. Diet. 2012, 25, 16–26. [Google Scholar] [CrossRef]
- Skow, S.L.; Jha, R.K. A Ketogenic Diet Is Effective in Improving Insulin Sensitivity in Individuals with Type 2 Diabetes. CDR 2022, 18, e250422203985. [Google Scholar] [CrossRef]
- Mallard, S.R.; Howe, A.S.; Houghton, L.A. Vitamin D Status and Weight Loss: A Systematic Review and Meta-Analysis of Randomized and Nonrandomized Controlled Weight-Loss Trials. Am. J. Clin. Nutr. 2016, 104, 1151–1159. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral Supplementation with Probiotic L. Reuteri NCIMB 30242 Increases Mean Circulating 25-Hydroxyvitamin D: A Post Hoc Analysis of a Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhai, J.; Zhong, W.; Zhao, J.; Zhou, L.; Wang, B. Lactobacillus Rhamnosus GG Promotes Intestinal Vitamin D Absorption by Upregulating Vitamin D Transporters in Senile Osteoporosis. Calcif. Tissue Int. 2022, 111, 162–170. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Mychasiuk, R.; Rho, J.M. Genetic Modifications Associated with Ketogenic Diet Treatment in the BTBR T+Tf/J Mouse Model of Autism Spectrum Disorder: Genetic Modifications Associated with Ketogenic Diet Treatment. Autism Res. 2017, 10, 456–471. [Google Scholar] [CrossRef]
- Newmaster, K.; Zhu, Z.; Bolt, E.; Chang, R.J.; Day, C.; Mhanna, A.; Paudel, S.; Farooq, O.; Swaminathan, A.; Acharya, P.; et al. A Review of the Multi-Systemic Complications of a Ketogenic Diet in Children and Infants with Epilepsy. Children 2022, 9, 1372. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Muscaritoli, M. A Clinical Perspective of Low Carbohydrate Ketogenic Diets: A Narrative Review. Front. Nutr. 2021, 8, 642628. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, N.; Haghighatdoost, F.; Rahimlou, M.; Rodrigues, A.P.S.; Gaskarei, M.K.; Okhovat, P.; de Oliveira, C.; Silveira, E.A.; Sarrafzadegan, N. The Effect of Ketogenic Diet on Shared Risk Factors of Cardiovascular Disease and Cancer. Nutrients 2022, 14, 3499. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Kossoff, E.H. The Ketogenic Diet. Neurologist 2005, 11, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.G.C.; Chee, C.M.; Lutchka, L.; Rychik, J.; Stallings, V.A. Selenium Deficiency Associated with Cardiomyopathy: A Complication of the Ketogenic Diet. Epilepsia 2003, 44, 618–620. [Google Scholar] [CrossRef] [Green Version]
- El-Rashidy, O.F.; Youssef, M.M.; Elgendy, Y.G.; Mohsen, M.A.; Morsy, S.M.; Dawh, S.A.; Saad, K. Selenium and Antioxidant Levels in Children with Intractable Epilepsy Receiving Ketogenic Diet. Acta Neurol. Belg. 2020, 120, 375–380. [Google Scholar] [CrossRef]
- Prudencio, M.B.; de Lima, P.A.; Murakami, D.K.; de Brito Sampaio, L.P.; Damasceno, N.R.T. Micronutrient Supplementation Needs More Attention in Patients with Refractory Epilepsy under Ketogenic Diet Treatment. Nutrition 2021, 86, 111158. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Laffel, L. Ketone Bodies: A Review of Physiology, Pathophysiology and Application of Monitoring to Diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Jackson, C.F.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic Diets for Drug-Resistant Epilepsy. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Thurman, D.J.; Begley, C.E.; Carpio, A.; Helmers, S.; Hesdorffer, D.C.; Mu, J.; Touré, K.; Parko, K.L.; Newton, C.R. The Primary Prevention of Epilepsy: A Report of the Prevention Task Force of the International League Against Epilepsy. Epilepsia 2018, 59, 905–914. [Google Scholar] [CrossRef]
- Masino, S.A.; Li, T.; Theofilas, P.; Sandau, U.S.; Ruskin, D.N.; Fredholm, B.B.; Geiger, J.D.; Aronica, E.; Boison, D. A Ketogenic Diet Suppresses Seizures in Mice through Adenosine A1 Receptors. J. Clin. Investig. 2011, 121, 2679–2683. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; He, X.; Luan, G.; Li, T. Role of DNA Methylation and Adenosine in Ketogenic Diet for Pharmacoresistant Epilepsy: Focus on Epileptogenesis and Associated Comorbidities. Front. Neurol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Lucia, A.; Naclerio, F. Effects of Combining a Ketogenic Diet with Resistance Training on Body Composition, Strength, and Mechanical Power in Trained Individuals: A Narrative Review. Nutrients 2021, 13, 3083. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Swain, J.F.; Feldman, H.A.; Wong, W.W.; Hachey, D.L.; Garcia-Lago, E.; Ludwig, D.S. Effects of Dietary Composition on Energy Expenditure During Weight-Loss Maintenance. JAMA 2012, 307, 2627–2634. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a High-Protein Ketogenic Diet on Hunger, Appetite, and Weight Loss in Obese Men Feeding Ad Libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Jeon, S.-M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic Diet in the Treatment of Cancer—Where Do We Stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef]
- Barrea, L.; Caprio, M.; Tuccinardi, D.; Moriconi, E.; Di Renzo, L.; Muscogiuri, G.; Colao, A.; Savastano, S.; on behalf of the Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Could Ketogenic Diet “Starve” Cancer? Emerging Evidence. Crit. Rev. Food Sci. Nutr. 2022, 62, 1800–1821. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between Hyperinsulinemia and Increased Risk of Cancer Death in Nonobese and Obese People: A Population-Based Observational Study: Cancer Mortality and Hyperinsulinemia without Obesity. Int. J. Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Liśkiewicz, A.D.; Kasprowska, D.; Wojakowska, A.; Polański, K.; Lewin–Kowalik, J.; Kotulska, K.; Jędrzejowska–Szypułka, H. Long-Term High Fat Ketogenic Diet Promotes Renal Tumor Growth in a Rat Model of Tuberous Sclerosis. Sci. Rep. 2016, 6, 21807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Zhu, J.; DeLuca, H.F. Vitamin D 25-Hydroxylase—Four Decades of Searching, Are We There Yet? Arch. Biochem. Bioph. 2012, 523, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Goodman, D.S. The Turnover and Transport of Vitamin D and of a Polar Metabolite with the Properties of 25-Hydroxycholecalciferol in Human Plasma. J. Clin. Investig. 1971, 50, 2159–2167. [Google Scholar] [CrossRef]
- Fraser, D.R.; Kodicek, E. Unique Biosynthesis by Kidney of a Biologically Active Vitamin D Metabolite. Nature 1970, 228, 764–766. [Google Scholar] [CrossRef]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and Pathophysiologic Roles of Extra Renal CYP27b1: Case Report and Review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; Updated 31 December 2021; MDText.Com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Gronemeyer, H.; Gustafsson, J.-Å.; Laudet, V. Principles for Modulation of the Nuclear Receptor Superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Mongioì, L.M.; Cimino, L.; Condorelli, R.A.; Magagnini, M.C.; Barbagallo, F.; Cannarella, R.; La Vignera, S.; Calogero, A.E. Effectiveness of a Very Low Calorie Ketogenic Diet on Testicular Function in Overweight/Obese Men. Nutrients 2020, 12, 2967. [Google Scholar] [CrossRef]
- Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss Is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, S.; Buscemi, C.; Corleo, D.; De Pergola, G.; Caldarella, R.; Meli, F.; Randazzo, C.; Milazzo, S.; Barile, A.M.; Rosafio, G.; et al. Obesity and Circulating Levels of Vitamin D before and after Weight Loss Induced by a Very Low-Calorie Ketogenic Diet. Nutrients 2021, 13, 1829. [Google Scholar] [CrossRef]
- Almsaid, H.; Khalfa, H.M. The Effect of Ketogenic Diet on Vitamin D3 and Testosterone Hormone in Patients with Diabetes Mellitus Type 2. Curr. Issues Pharm. Med. Sci. 2020, 33, 202–205. [Google Scholar] [CrossRef]
- De Pergola, G.; Zupo, R.; Lampignano, L.; Paradiso, S.; Murro, I.; Cecere, A.; Bartolomeo, N.; Ciccone, M.M.; Giannelli, G.; Triggiani, V. Effects of a Low Carb Diet and Whey Proteins on Anthropometric, Hematochemical, and Cardiovascular Parameters in Subjects with Obesity. EMIDDT 2020, 20, 1719–1725. [Google Scholar] [CrossRef]
- Bergqvist, A.C.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive Bone Mineral Content Loss in Children with Intractable Epilepsy Treated with the Ketogenic Diet. Am. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef] [Green Version]
- Bergqvist, A.G.C.; Schall, J.I.; Stallings, V.A. Vitamin D Status in Children with Intractable Epilepsy, and Impact of the Ketogenic Diet. Epilepsia 2007, 48, 66–71. [Google Scholar] [CrossRef]
- Simm, P.J.; Bicknell-Royle, J.; Lawrie, J.; Nation, J.; Draffin, K.; Stewart, K.G.; Cameron, F.J.; Scheffer, I.E.; Mackay, M.T. The Effect of the Ketogenic Diet on the Developing Skeleton. Epilepsy Res. 2017, 136, 62–66. [Google Scholar] [CrossRef]
- Svedlund, A.; Hallböök, T.; Magnusson, P.; Dahlgren, J.; Swolin-Eide, D. Prospective Study of Growth and Bone Mass in Swedish Children Treated with the Modified Atkins Diet. Eur. J. Paed. Neur. 2019, 23, 629–638. [Google Scholar] [CrossRef]
- Molteberg, E.; Taubøll, E.; Kverneland, M.; Iversen, P.O.; Selmer, K.K.; Nakken, K.O.; Hofoss, D.; Thorsby, P.M. Substantial Early Changes in Bone and Calcium Metabolism among Adult Pharmacoresistant Epilepsy Patients on a Modified Atkins Diet. Epilepsia 2022, 63, 880–891. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.I.; Song, K.; Choi, H.S.; Suh, J.; Kim, S.H.; Chae, H.W.; Kang, H.-C.; Lee, J.S.; Kim, H.D.; et al. Association of Hypercalciuria with Vitamin D Supplementation in Patients Undergoing Ketogenic Dietary Therapy. Front. Nutr. 2022, 9, 970467. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F.; Malumbres-Chacón, M.; Moreno-Gónzalez, P.; Aguilera-Albesa, S.; Yoldi-Petri, M.E. Vitamin D Deficiency in Children with Epilepsy Taking Valproate and Levetiracetam as Monotherapy. Epilepsy Res. 2018, 139, 80–84. [Google Scholar] [CrossRef]
- Sauveur, B.; Garabedian, M.; Fellot, C.; Mongin, P.; Balsan, S. The Effect of Induced Metabolic Acidosis on Vitamin D3 Metabolism in Rachitic Chicks. Calc. Tissue Res. 1977, 23, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Hussain, A.; Iqbal, A.; Kumar, V. Correlation between Vitamin D Deficiency and Diabetic Ketoacidosis. Cureus 2019, 11, e4497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, A.; Gleize, B.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.-J.; Reboul, E. Fatty Acids Affect Micellar Properties and Modulate Vitamin D Uptake and Basolateral Efflux in Caco-2 Cells. J. Nutr. Biochem. 2013, 24, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Lichtenstein, A.H.; Dolnikowski, G.; Palermo, N.J.; Rasmussen, H. Dietary Fat Increases Vitamin D-3 Absorption. J Acad. Nutr. Diet. 2015, 115, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hylemon, P.B. Bile Acids Are Nutrient Signaling Hormones. Steroids 2014, 86, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Futawaka, K.; Koyama, R.; Fukuda, Y.; Hayashi, M.; Imamoto, M.; Miyawaki, T.; Kasahara, M.; Tagami, T.; Moriyama, K. Vitamin D3/VDR Resists Diet-Induced Obesity by Modulating UCP3 Expression in Muscles. J. Biomed. Sci. 2016, 23, 56. [Google Scholar] [CrossRef] [Green Version]
- Wilkens, M.R.; Firmenich, C.S.; Schnepel, N.; Muscher-Banse, A.S. A Reduced Protein Diet Modulates Enzymes of Vitamin D and Cholesterol Metabolism in Young Ruminants. J. Steroid Biochem. Mol. Biol. 2019, 186, 196–202. [Google Scholar] [CrossRef]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.-J.; Reboul, E. Fat-Soluble Vitamin Intestinal Absorption: Absorption Sites in the Intestine and Interactions for Absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef]
- Maurya, V.K.; Aggarwal, M. Factors Influencing the Absorption of Vitamin D in GIT: An Overview. J. Food. Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef]
- Sánchez-Martínez, R.; Castillo, A.I.; Steinmeyer, A.; Aranda, A. The Retinoid X Receptor Ligand Restores Defective Signalling by the Vitamin D Receptor. EMBO Rep. 2006, 7, 1030–1034. [Google Scholar] [CrossRef] [Green Version]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of Vitamin D in Obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A.G. 25-Hydroxyvitamin D Response to Graded Vitamin D 3 Supplementation Among Obese Adults. J. Clin. End. Metab. 2013, 98, 4845–4851. [Google Scholar] [CrossRef] [Green Version]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of Vitamin D-Metabolizing Enzymes in Human Adipose Tissue—The Effect of Obesity and Diet-Induced Weight Loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2019, 55, 368. [Google Scholar] [CrossRef] [Green Version]
- Bär, L.; Feger, M.; Fajol, A.; Klotz, L.-O.; Zeng, S.; Lang, F.; Hocher, B.; Föller, M. Insulin Suppresses the Production of Fibroblast Growth Factor 23 (FGF23). Proc. Natl. Acad. Sci. USA 2018, 115, 5804–5809. [Google Scholar] [CrossRef] [Green Version]
- Kuro-o, M.; Moe, O.W. FGF23-AKlotho as a Paradigm for a Kidney-Bone Network. Bone 2017, 100, 4–18. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdostrp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2021, 14, 191. [Google Scholar] [CrossRef]
- Gokhale, S.; Bhaduri, A. Provitamin D3 Modulation through Prebiotics Supplementation: Simulation Based Assessment. Sci. Rep. 2019, 9, 19267. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, C.; Meroni, E.; Casiraghi, M.C.; Tagliabue, A.; De Giorgis, V.; Erba, D. One Month of Classic Therapeutic Ketogenic Diet Decreases Short Chain Fatty Acids Production in Epileptic Patients. Front. Nutr. 2021, 8, 613100. [Google Scholar] [CrossRef]
- Sepulveda-Villegas, M.; Elizondo-Montemayor, L.; Trevino, V. Identification and Analysis of 35 Genes Associated with Vitamin D Deficiency: A Systematic Review to Identify Genetic Variants. J. Steroid Biochem. Mol. Biol. 2020, 196, 105516. [Google Scholar] [CrossRef]
- Alathari, B.E.; Aji, A.S.; Ariyasra, U.; Sari, S.R.; Tasrif, N.; Yani, F.F.; Sudji, I.R.; Lovegrove, J.A.; Lipoeto, N.I.; Vimaleswaran, K.S. Interaction between Vitamin D-Related Genetic Risk Score and Carbohydrate Intake on Body Fat Composition: A Study in Southeast Asian Minangkabau Women. Nutrients 2021, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Alathari, B.E.; Bodhini, D.; Jayashri, R.; Lakshmipriya, N.; Shanthi Rani, C.S.; Sudha, V.; Lovegrove, J.A.; Anjana, R.M.; Mohan, V.; Radha, V.; et al. A Nutrigenetic Approach to Investigate the Relationship between Metabolic Traits and Vitamin D Status in an Asian Indian Population. Nutrients 2020, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Xenos, K.; Papasavva, M.; Raptis, A.; Katsarou, M.-S.; Drakoulis, N. Vitamin D Supplementation and Genetic Polymorphisms Impact on Weight Loss Diet Outcomes in Caucasians: A Randomized Double-Blind Placebo-Controlled Clinical Study. Front. Med. 2022, 9, 811326. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, Y.; Sarafidou, T.; Kotsa, K.; Papadimitriou, M.; Goutzelas, Y.; Stamatis, C.; Bagiatis, V.; Tsekmekidou, X.; Yovos, J.G.; Mamuris, Z. VDR TaqI Is Associated with Obesity in the Greek Population. Gene 2013, 512, 237–239. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood Biomarkers of Vitamin D Status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [Green Version]
- Luttmann-Gibson, H.; Mora, S.; Camargo, C.A.; Cook, N.R.; Demler, O.V.; Ghoshal, A.; Wohlgemuth, J.; Kulkarni, K.; Larsen, J.; Prentice, J.; et al. Serum 25-Hydroxyvitamin D in the VITamin D and OmegA-3 TriaL (VITAL): Clinical and Demographic Characteristics Associated with Baseline and Change with Randomized Vitamin D Treatment. Contemp. Clin. Trials 2019, 87, 105854. [Google Scholar] [CrossRef]
- O’Neill, C.; Kazantzidis, A.; Ryan, M.; Barber, N.; Sempos, C.; Durazo-Arvizu, R.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef]

| Ref | n | Age Range, Mean ± SD (y) | Females/Males | Weight Status | Obesity | Health Status | Study Design | Type of Intervention | Vitamin D Supplement | Duration | Baseline Vitamin D Status | Weight Loss | Effect on Vitamin D | Other Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy adults | ||||||||||||||
| Colica, 2017 [11] | 40 (20 per group) | 18–65 45.4 ± 14.2 | Both (% NA) | BMI (Mean ± SD): 30.4 ± 2.6 | 50% obese | Healthy | Double-blind randomized crossover study; placebo-controlled | VLCKD1: Females: 450–500 kcal, 35–45% PROT, 45–50% fat and 15% CHO(< 20 g). Males: 650–700 kcal, 50–55% PROT, 35–40% FAT, 10% CHO (< 20 g). 50% PROT from synthetic amino acids VLCKD2: Females: 450–500 kcal, 25–35% PROT, 45–50% FAT, 20–25% CHO Males: 650–700 kcal, 45–50% PROT, 35–40% FAT, 15–20% CHO. <10% saturated fat in all diets | Multivitamin, multimineral (not specified) | 3 weeks (after a 3 weeks washout) | Serum 25-(OH)2-vitamin D3 Total sample: 21.74 ± 2.38 ng/mL VLCKD1: 21.89 ± 3.88 ng/mL VLCKD2: 22.28 ± 2.69 ng/mL | Yes | ↑ 25.7% serum 25-(OH)2-Vitamin D3 with VLCKD2 | VLCKD1: ↓ BMI, ↓abdominal fat, ↓ peripheral fat, ↓ HOMA, ↓ glucose, ↓ insulin, ↑ AST, ↑ uric acid, ↑ creatinine VLCKD2: ↓ BMI, ↓abdominal fat, ↓ peripheral fat, ↓ HOMA, ↓ glucose, ↓ insulin |
| Mongioì 2020 [50] | 40 | 45.8 ± 2.42 | 40 males | BMI (Mean ± SD): 37.5± 1.1 | 85% obese 10% overweight | Healthy | Prospective study | CHO <30 g/day Fat 44%, PROT 43% Gradual increase in provided energy VLCKD Phase 1: 600–800 kcal LCKD Phase 2: 800–1000 kcal LCD Phase 3: 1200–1500 kcal Maintenance Phase 4: 1500 and 2000 Kcal | Vitamins (B, C, E), minerals, and omega-3 fatty acids | VLCKD for at least 8 weeks Mean duration 13.5± 0.83 weeks | 19.9± 1.1 ng/mL | Yes | ↑ 19.9± 1.1 to 38.5± 1.8 | ↓ Glucose homeostasis ↓ total cholesterol, LDL, TGC, lipids, ↑ HDL-cholesterol, ↓PSA ↑LH, ↑TT No changes in creatinine, uric acid |
| Perticone 2019 [51] | 56 28 in the VLCKD group | 46.8 ± 11.0 | 24 females, 32 males | BMI (Mean ± SD): 39.65 ± 9.7 | 100% obese | healthy | Clinical trial | VLCKD Phase 1–3: 600–800 kcal (<50 g CHO daily, 10 g of olive oil/day). Phase 4–5 1000–1500 kcal/day | Multivitamin supplement (not specified) | 12 months | 17.8 ± 5.6 ng/mL 25(OH)D (all) 18.4 ± 5.9 25(OH)D (VLCKD arm) | Yes | ↑ 18.4 ± 5.9 to 29.3 ± 6.8 Vitamin D did not increase in the MedDiet group | ↓ CRP, ↓ HOMA |
| dePergola 2020 [54] | 22 | 45 ± 13.9 | NA | BMI (Mean ± SD): 31.3 ± 6.2 | 100% obese or overweight | Healthy | Clinical trial | Low-carbohydrate diet with whey protein 1400–1800 kcal FAT: 50–55%, PROT: 25%, CHO: 15–20% of total calories + nutritional supplement with 18 g of whey proteins (4 g of L-leucine), 4 g of carbohydrates, 1.4 g of lipids, 331 mg of cocoa polyphenols, and several vitamins | 5 μg vit D | 6 weeks | 22.5 (12–26) | Yes | ↑ 22.5 (12–26) to 26 (22–35) | ↓ Diastolic blood pressure, triglycerides, total cholesterol, pre-albumin, insulin, HOMA, FT3, c-IMT ↑ FMD |
| Buscemi 2021 [52] | 31 | 18–65 43 ± 11 (intervention) | 24 females, 7 males, 20 control group (25% males) | (Mean ± SD): 39.4 ± 6.3 | 100% obese | Healthy | Placebo-controlled clinical trial | First 20 ± 3 days: VLDKD with industrial meal replacements 600–800 kcal/day, CHO < 50 g; then conventional meals were introduced while maintaining the same nutritional intake | No | 10–12 weeks | 21.6± 9.9 ng/mL 25(OH)D 29.7 ± 6.7 ng/mL 25(OH)D (control group) Patients with obesity had a higher habitual intake of vitamin D | Yes | ↑ 21.6 to 25.8± 10.4 ng/ml | - |
| Adults with T2D | ||||||||||||||
| Almseid 2020 [53] | 30 patients with T2D on KD, 30 patients with T2D not on KD, 30 controls | 30–41 | NA | NA | NA | T2D | Case-control study | KD | NA | NA | NA | Yes | ↑ Vitamin D3 in patients with T2D on KD (mean ± SE 53.5 ± 0.32) as compared with a control group (mean± SE 57 ± 0.24) and with patients with T2D not on KD (mean ± SE 25.1 ± 1.55) | ↑ TT in patients with T2D on KD (mean± SE 427.4 ± 2.52) vs. control group (mean ± SE 422.2 ± 0.24) and patients with T2D not on KD (mean± SE 151.4 ± 1.41); no differences in LDL-cholesterol or HDL-cholesterol |
| Ref | n | Age Range, Mean ± SD (y) | Females/Males | Weight Status | Health Status | Study Design | Type of Intervention | Vitamin D Supplement | Duration | Baseline Vitamin D Status | Weight Loss | Effect on Vitamin D | Other Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children with epilepsy | |||||||||||||
| Hahn 1979 [7] | 5 15 controls | 10.4 ± 1.5 | 3 girls, 2 boys | NA | Patients on anticonvulsant therapy | Placebo-controlled pilot study | Anticonvulsant therapy + KD | Yes | Anticonvulsant drug therapy = 7.4 years; triglyceride ketogenic diet therapy = 2.5 years | 14.1 ± 2.5 ng/mL | NA | ↑ After supplementation | Decrease in bone mass was observed in the KD group; mean bone mass in the KD + vitamin D group increased by 8.1–0.9% (p < 0.001) over 12 months |
| Bergqvist 2007 [56] | 45 | 5.1 ± 2.7 years | 73% (33 33 boys, 27% (12) girls | Weight for age (Z-score) −0.4 ± 1.6 BMI for age (Z-score) −0.3 ± 2.1 (mean± SD) | Epilepsy | Clinical trial | Treatment with the ketogenic diet (KD) | vitamin D (in 14 patients) | 15 months | Before KD therapy, 4% had deficient and 51% had insufficient serum 25-OHD levels. | NA | ↑ After 3 months and then ↓ | ↓ PTH |
| Bergqvist 2008 [55] | 25 | 5–21 7.3 ± 1.9 | 9 girls, 16 boys | BMI: 16.8 ± 4.4 BMI-for-age z score −0.06 ± 1.6 | Epilepsy | Clinical trial | KD 4:1 (g FAT:PROT) | Yes | 15 months | 54% intake < AI 25-OH D 27.2 ± 13.6 ng/mL 1,25-(OH)2D 25.5 ± 8.3 ng/mL 73% had suboptimal levels (<32 ng/mL) | Yes | ↑ In the first 3 months and then stable | ↓Whole-body and spine BMC-for-age (0.6 z score/y), ↓ whole-body and spine BMC-for- height (0.7 z score/y and 0.4 z score/y, respectively), ↓ height (0.5 z score/y). |
| Simm 2017 [57] | 29 | 3.3–17.8 6.4 | 15 females, 14 males | NA | Epilepsy | Prospective, longitudinal study | PROT:RDA Energy, PROT and FAT:CHO-PROT ratios were adjusted to address weight gain and loss and optimize ketosis | Yes | mean 2.1 years range 0.5–6.5 years | 82 nmol/L (range 42–133); 5 patients <50 nmol/L | NA | There were no associations between vitamin D and BMD changes over time | ↓ BMD 0.16 SD (relative to age-matched referent children) for every year; ↑ mean urinary calcium/creatinine ratios were elevated (0.77) |
| Svedlund 2019 [58] | 38 | 6.1 ± 4.8 | 21 females, 17 males | BMI SDS (median) 0.2 (min-max) 3.3-4.5 | Epilepsy, glucose transporter type 1 deficiency syndrome, pyruvate dehydrogenasecomplex deficiency | Prospective longitudinal study | Modified Atkins diet | Yes (14 patients) | 24 months | No patient was vitamin D deficient (<12 ng/mL); 8% had an insufficient 25(OH)D level (<20 ng/mL) | No ↑BMI SDS | ↑ In the first 6 months and then stable | No effects were observed for bone mass (total body, lumbar spine and hip) or fat mass. |
| Lee, 2021 [60] | 49 | 0.0–11.7 4.3 ± 3.2 | 18 girls 31 boys | BMI: 16.4 ± 2.3 Weight SDS: −0.09 ± 1.31 (−3.20–2.95) | Epilepsy | Noncontrolled intervention | KD 3:1 (g fat to nonfat) | Yes D3 (50.8 IU/kg) | 12 months | 22.4 ± 9.0 42.9% deficiency | NA | ↑ In the first 3 months and then stable (not statistically checked) | OR for hypercalciuria was 0.945 (95% confidence interval, 0.912–0.979; p = 0.002) per 1.0 ng/mL increment in 25-OH-D3 level. |
| Adults with epilepsy | |||||||||||||
| Molteberg 2021 [59] | 53 | Mean 37.5 | 33 female 20 male 13 female control 15 male control | BMI (Median): 26.8 (18.7–41.7) | Epilepsy | Placebo- controlled clinical trial | Treatment with a modified Atkins diet, max 16 g of CHO/d (e.g., 5% CHO, 70% FAT, and 25% PROT) Control group: habitual diet, typical Norwegian diet with 43–44%CHO, 34% FAT, 18% PROT | Yes 5–7.5μg | 12 weeks | 25-OH vit D 60 nmol/l 1,25-OH vit D97 pmol/L | Yes | ↑ 25-OH vit D ↓ 1,25-OH2 vit D | ↓ PTH, Ca, CTX- 1, P1NP and leptin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detopoulou, P.; Papadopoulou, S.K.; Voulgaridou, G.; Dedes, V.; Tsoumana, D.; Gioxari, A.; Gerostergios, G.; Detopoulou, M.; Panoutsopoulos, G.I. Ketogenic Diet and Vitamin D Metabolism: A Review of Evidence. Metabolites 2022, 12, 1288. https://doi.org/10.3390/metabo12121288
Detopoulou P, Papadopoulou SK, Voulgaridou G, Dedes V, Tsoumana D, Gioxari A, Gerostergios G, Detopoulou M, Panoutsopoulos GI. Ketogenic Diet and Vitamin D Metabolism: A Review of Evidence. Metabolites. 2022; 12(12):1288. https://doi.org/10.3390/metabo12121288
Chicago/Turabian StyleDetopoulou, Paraskevi, Sousana K. Papadopoulou, Gavriela Voulgaridou, Vasileios Dedes, Despoina Tsoumana, Aristea Gioxari, George Gerostergios, Maria Detopoulou, and George I. Panoutsopoulos. 2022. "Ketogenic Diet and Vitamin D Metabolism: A Review of Evidence" Metabolites 12, no. 12: 1288. https://doi.org/10.3390/metabo12121288
APA StyleDetopoulou, P., Papadopoulou, S. K., Voulgaridou, G., Dedes, V., Tsoumana, D., Gioxari, A., Gerostergios, G., Detopoulou, M., & Panoutsopoulos, G. I. (2022). Ketogenic Diet and Vitamin D Metabolism: A Review of Evidence. Metabolites, 12(12), 1288. https://doi.org/10.3390/metabo12121288










