Abstract
Celiac disease is a chronic autoimmune disorder involving the small intestine, characterized by villous atrophy, crypt hyperplasia and an increase in intraepithelial lymphocytes. Due to both calcium malabsorption and immune activation, a high prevalence of bone mass derangement is evident in this condition, regardless of the presence of overt malabsorption. Alterations of mineral metabolism are also frequently described, and in this review, the modifications of serum levels of vitamin D are analyzed, according to the available literature on this topic. In untreated patients, secondary hyperparathyroidism is responsible for the hyperconversion of 25-vitamin D into 1,25-vitamin D making mandatory the determination of serum levels of both vitamin metabolites to avoid a wrong diagnosis of vitamin D deficit. A gluten-free diet allows for a normalization of bone and mineral metabolism, reverting these abnormalities and raising some doubts on the need for vitamin supplementation in all the patients. Data available do not support this wide indication, and a complete evaluation of bone and mineral metabolism should be performed to select patients who need this therapeutic approach.
1. Introduction
Celiac disease (CD) is a chronic autoimmune disorder of the small intestine caused by the intake of gluten in genetically predisposed subjects. The gluten-evoked immune response alters enteric mucosa architecture, determining villous atrophy, crypt hyperplasia and an increased number of intraepithelial lymphocytes [1]. Mucosal lesions compromise the absorbing capacity due to a reduction in intestinal surface area and cause, among others, calcium malabsorption and vitamin D metabolism alterations [2,3]. Together with the persistent inflammation [4], intestinal malabsorption is considered a pivotal mechanism for bone mass and mineral metabolism impairment. The impairment of calcium absorption and the consequent endocrine alterations of the secretion of parathyroid hormone and vitamin D metabolism deeply modify bone homeostasis, causing an increased bone resorption stimulating bone turnover. In this review, we have analyzed the evidence describing the impairment of bone and mineral metabolism, and we have suggested both a correct timing for their measurement and a correct strategy to select patients who need a vitamin D supplementation along their life. This is still an unmet need, as guidelines for the management of osteoporosis in CD patients lack specific suggestions on bone metabolism alteration approaches [5,6].
2. Bone and Mineral Metabolism in CD Patients at Diagnosis and after GFD
It is estimated that three-quarters of patients with overt malabsorption at diagnosis and half of patients with minor symptoms suffer from significant alterations of bone mineralization [7], but alterations of calcium metabolism are present in all the patients. In comparison with CD patients at diagnosis, the prevalence of bone loss in patients following a strict GFD is significantly lower [7], suggesting a therapeutic role of GFD on this consequence of CD. It is known that fracture risk is significantly increased in postmenopausal women [8], and when bone loss is present, it is very difficult to induce its reversal with drug treatment. Therefore, the importance of bone loss prevention, through an active search for bone derangement in all patients suffering from conditions responsible for a secondary osteoporosis, is evident. In CD patients diagnosed during childhood, a strict GFD from childhood normalizes BMD [9], and a significant bone mass gain could be evident, even after a six-month period of GFD [10]. Unfortunately, this is not the same in CD patients diagnosed in adulthood [7], making the adoption of strategies aimed at the prevention of osteoporosis very important.
Calcium represents one of the main regulators of muscular contraction, and its serum levels are finely monitored and balanced to support cardiac muscle activity. Unless severe malabsorption symptoms characterize the disease at diagnosis [11], reduced serum calcium levels are unfrequently detected in untreated CD patients. The increased attention to CD diagnosis and the adoption of screening tests for first-degree relatives of CD patients elicits the recognition of patients in an early phase of the disease, generally characterized by minor symptoms caused by subclinical malabsorption and a low degree of severity of bone derangement. Parathyroid hormone (PTH) and vitamin D represent the main actors of the regulation of calcium metabolism, and their serum level alterations in untreated CD patients suggest that calcium malabsorption is a pivotal mechanism in the pathophysiology of bone loss. All the papers dealing with bone mass and mineral metabolism in untreated CD patients agree with this issue.
Vitamin D is a secosteroid that is detectable in blood in two main forms: 25-hydroxyvitamin D, that is, 25(OH)-vitamin D, an indicator of the entity of vitamin D storage; and 1,25-dihydroxyvitamin D, that is, 1,25(OH)2-vitamin D, the active metabolite, regulating calcium balance and modulating the immune system.
In untreated CD patients, a complex network of events is arranged to rapidly compensate serum calcium reduction and prevent fecal losses: in Figure 1, the cascade of endocrine reaction to hypocalcemia in untreated CD patients is shown [7].
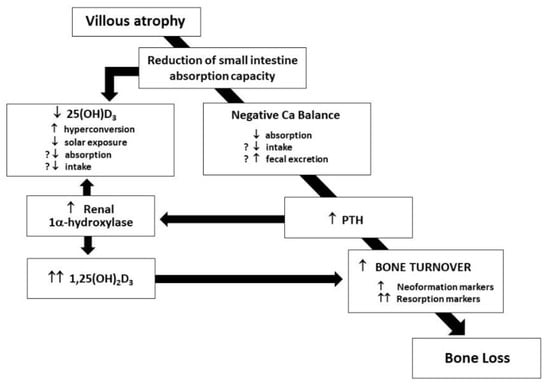
Figure 1.
Alterations of calcium balance in untreated CD patients.
The release of PTH and its effect on the kidney, stimulating 1-α-hydroxylase activation, and in turn, enhancing the conversion of 25-vitamin D in 1,25-vitamin D with the consequent effect of activated vitamin D on intestinal calcium absorption through an increased vitamin D-dependent active transport, is evident. Even if enterocytes express a normal number of vitamin D receptors [3], negligible cytoplasmatic levels of vitamin D-dependent calcium-binding protein at enterocyte level [12] frustrate such a compensatory effort, and in fact, the activation of the complex network expresses only its negative effects on bone mineralization, played by the direct proresorptive effect of increased levels of both PTH and 1,25-vitamin D [13].
Accordingly, in untreated CD, serum levels of 25 vitamin D are very frequently low; on the contrary, serum levels of 1,25-vitamin D are frequently high. Many papers have clearly shown the characteristic behavior of these two metabolites. High levels of 1,25-vitamin D at diagnosis are frequently associated with high levels of PTH [4,14], and a strict GFD generally normalizes these alterations [4,14,15,16,17]. GFD-related improvement of PTH levels seem strictly related to the reversal of mucosal lesions, as hyperparathyroidism persists both in patients with refractory CD [10] and patients with a low compliance to GFD [18], and is associated with alterations of vitamin D metabolite levels [16]. However, one year of GFD duration is not always sufficient to normalize PTH levels [18], while a longer period was clearly shown to be effective [4,14,15,16,19]. It is worth noting that in patients with CD at diagnosis, the previous use of calcium and vitamin D supplements may be effective in the prevention of hyperparathyroidism [20]: on clinical grounds, this is an important issue, as these pivotal alterations of bone and mineral metabolism may be masked by the supplementation. Physicians could be misled.
In untreated patients with CD, circulating levels of the active metabolite of vitamin D show a pattern of modification similar to PTH. In the presence of high levels of PTH, due to its effect on renal 1-α-hydroxylase, high levels of 1,25-vitamin D are expected, and many papers confirm this issue. In particular, it was previously shown that serum levels of PTH and 1,25-vitamin D are strictly correlated even in patients on long term GFD [21]. In patients on GFD for a median 2-year period, we showed significantly lower levels of serum levels of 1,25-vitamin D in comparison with patients at diagnosis [14,22]. As expected, these results were confirmed in patients on GFD for longer periods of time [4,16,19] and the duration of GFD seems crucial for the complete normalization. More interestingly, it was shown that the reduction of serum levels of 1,25-vitamin D is very rapid, beginning after just three months of GFD [23].
The reversal of altered calcium balance also reflects its effects on 25-vitamin D serum levels, which increase after the introduction of GFD. In a cohort of 44 CD patients on long-term GFD, we recently showed a normalization of 25-vitamin D levels, independently from the presence of bone loss [24]. This is a largely confirmed result: all available papers agree on this issue. Low levels of 25-vitamin D in untreated CD could be caused by a reduction in intestinal absorption, a reduction in dietary intake and a reduction in exposure to sunlight [25]. However, a reduction in 25-vitamin D half-life in patients with malabsorption was also shown [26].
Ultimately, the presence of vitamin D deficiency in untreated CD patients should be considered very rare, as 25-vitamin D levels are low, but are always accompanied by high levels of 1,25-vitamin D. On the contrary, the presence of vitamin D deficiency in treated CD patients represents a more important condition on clinical grounds, deserving a different diagnostic and therapeutic approach. Unfortunately, very few papers report the prevalence of vitamin D deficiency in treated adult CD patients. On the basis of previous results, the prevalence of 25-vitamin D deficiency is expected to decrease following a strict GFD, and this was evident, in particular, in a 5-year longitudinal follow-up study on a cohort of untreated patients: the prevalence of 68% at baseline was 54% after one year of GFD and fell down to 8% after 5 years of diet [26].
It is, however, conceivable that both age at diagnosis of CD and age at the time of vitamin D measurement may condition the modification of vitamin D levels during the CD patient’s lifespan, but we have no information on this issue. Recent papers reported a prevalence of 25-vitamin D deficiency in treated CD patients of 25% [27] and 28% [28], but we have no information on the age at measurement and on the duration of GFD of these subgroups of patients. Only one study, describing a prevalence of 41%, reported the absence of a significant difference between patients with and without 25-vitamin D deficiency [29]. A critical point is, therefore, the discrepancy between the frequent normalization of serum indices of bone and mineral metabolism and the difficult normalization of BMD levels in treated CD, even after long periods of GFD.
Several studies suggested a role of vitamin D receptor genotypes in the pathophysiology of both bone loss and vitamin D serum alterations in patients with CD [30,31,32]. Among the high number of single-nucleotide polymorphisms (SNPs), Fok I (SNP rs 2228570), Bsm I (SNP rs1544410), Apa I (SNP rs7975232) and Taq I (SNP rs731236) are the most studied. A recent meta-analysis excluded the causal relationship between VDR polymorphism and the alteration of bone and mineral metabolism [33].
3. Awareness of the Persistence of Nutritional Deficiencies in CD Patients following GFD
GFD represents the treatment of CD, but both in children [34] and in adults [35,36], it was shown that it is not sufficient to prevent nutritional deficiency. Recently, the adoption of GFD was considered a very healthy measure for patients affected by a series of conditions different from CD [37] as well as for healthy subjects, according to an unproven hypothesis attributing the ability to guarantee wellness to gluten-free products [38].
Di Nardo et al., in their systematic review dealing with nutritional deficiencies in childhood CD, showed an increased risk of excessive fat and an insufficient intake of vitamin D, besides fibers, iron and calcium [34]. The daily intake of vitamins B1, B2 and B6 as well as folate proved to be significantly lower in CD on GFD than in the general German population [39]. Vitamin B deficiencies should be considered secondary to the ingestion of gluten-free cereals containing lower amounts of folate than gluten-containing cereals [40]. It should be also considered that the folic acid content of quinoa is 78.1 mg/100 g, while amaranth contains 102 mg/100 g, which is substantially higher than wheat, containing an level of folate of 40 mg/100 g [41]. It was recently shown that in comparison with gluten-containing products, gluten-free equivalents are characterized by lower levels of vitamin D, vitamin E, vitamin B12, folate, iron, magnesium, potassium and sodium [42]. Similar considerations could be made for gluten-free breads: only 1 product out of 20 tested was proven to be fortified with iron, calcium, thiamin and niacin, while 3 out of 10 were fortified only with calcium and iron. The intake of minerals in CD patients on GFD was shown to be insufficient, in particular for calcium, iron, manganese and zinc [40], due to the low mineral concentration of these minerals in gluten-free raw materials. It was recently shown that mineral deficiency in CD patients on a long-term GFD is detectable in 10% of patients, in particular magnesium and calcium for both males and females, as well as iron for females and zinc for male patients [43]. Apart from iron deficiency, the presence of other mineral deficiencies is frequently underestimated in CD patients, and a series of metabolic consequences could be lost in the clinical follow up. For instance, this is the case of zinc, as its deficiency may impair protein synthesis and hamper growth [44].
From these studies clearly emerges the information that even if patient adherence to GFD is judged to be perfect, the lack of fortification increases the risk of micronutrient deficiency. Accordingly, follow-up visits of CD patients should be also aimed at the prevention, or at least at the early detection, of these complications.
Recently, among both healthcare professionals and medical students, an assessment of the knowledge of the risk of nutritional deficiencies in CD patients following a strict GFD was performed [45]. Results showed that only 37% of interviewed healthcare professionals provided at least 60% of correct answers, suggesting a very low level of awareness of the long-term risk of nutritional deficiencies in CD patients, strictly adhering to GFD. These results underline the need for ad hoc educational programs finalized to a deep learning of the nutritional requirements of CD patients in their real life.
Moreover, the need for an expert dietitian in monitoring GFD in CD was recently evaluated [46]. This is a very important issue, as the replacement of wheat, rye and barley with gluten-free equivalents or grains such as maize, millet, rice, teff and tapioca may expose CD patients to an increased intake of fibers and an increased intake of fat [47]; this different diet increases the risk of metabolic disorders such as obesity, diabetes, hypertension and cardiovascular complications. An increased fat intake may expose patients to a reduced absorption of magnesium [48]. The increased intake of fibers in CD patients may also contribute to the persistence of gas-related symptoms, such as bloating, flatulence and abdominal distention [49], evoking the coexistence of other disorders, in particular functional bowel disorders. Accordingly, the role of dietitians in the follow-up of CD patients should be emphasized and strongly suggested in order to prevent the occurrence of gas-related symptoms and perform a sort of direction of the diet.
4. When Is It Correct to Supplement Vitamin D in CD Patients?
There are many concerns about nutritional supplementation in CD patients, and guidelines are frequently characterized by substantial differences. A recent Consensus Conference underlined the need for further studies aimed at the clarification of the role of vitamin D in the pathophysiology of CD and the need for supplementation [50]. The case of vitamin D is, however, not isolated, as the same considerations could be expressed for calcium supplementation. A correct intake of calcium for the maintenance of optimal bone health and a balance of mineral metabolism depends on the age of the subjects. Adolescents and old subjects need a high amount of calcium due to the rapid growth and the reduced intestinal calcium absorption capacity, respectively. However, the Food and Agriculture Organization (FAO) recommends a calcium intake of 800–1000 mg/day in men and women over 50 years of age [51]. The Institute of Medicine of the United States National Academy of Sciences suggests 1000 mg for 19–50-year-old women and 19–70-year-old men and 1200 mg/day for postmenopausal women and men over 70 years old [52]. The increase in calcium supplementation to over 1200 mg/day may predispose one to an increased risk of urinary stones, but the risk of this complication should be monitored in all the subjects following a pharmacological calcium supplementation.
Serum levels of 25-vitamin D below the value of 30 nmol/L are considered correlated to an increased risk of bone derangement, and values higher than 50 nmol/L or 20 ng/mL are considered appropriate for bone health [53,54,55,56]. A lack of standardization is, however, evident for vitamin D measurement, and comparisons between methods have reported discrepancies of immunoassays in comparison with LC-MS/MS methods [57]. This is still an unmet need hampering clinical research: all the efforts to overcome this impasse are welcome.
An appropriate vitamin D supplementation should consider age and gender, but also skin type, season and geographic area. The USA Institute of Medicine and the European Food Safety Authority (EFSA) agreed to consider 15 microg of vitamin D as a recommended dietary allowance (RDA) [51,57]. In particular, expressed in IU/day, an RDA of 600 IU/day is suggested for adults in the age range of 50–70 years and 800 IU/day is suggested for adults aged >70 years. The goal of vitamin D supplementation should be the achievement of serum 25 vitamin D levels of 50 nmol/L, an effective value for bone health maintenance in more than 97% of North America’s people [58,59].
4.1. Vitamin D Supplementation in Untreated CD Patients
As far as CD patients are concerned, the increased conversion of 25-vitamin D into 1,25-vitamin D is a crucial point and should be particularly stressed. In untreated CD patients, low levels of 25-vitamin D should not be necessarily interpreted as the expression of vitamin deficiency: the hyperconversion to 1,25-vitamin D, secondary to hyperparathyroidism, is the cause of low 25-vitamin D. Consequently, 25-vitamin D supplementation in CD patients at diagnosis could not only result in a useless therapeutic measure, but even in a dangerous treatment, as excessively elevated serum levels of 1,25-vitamin D are characterized by a proresorptive effect, as they enhance bone loss [60].
The indication of vitamin D supplementation in untreated adult CD patients should arise from the evaluation of circulating levels of both vitamin D metabolites, together with the measurement of PTH levels. Low levels of 25-vitamin D associated with high levels of 1,25-vitamin D and PTH should be not approached with supplementation of drugs aimed at the increase in 25-vitamin D, as this measure may enhance the already-stimulated hyperconversion to 1,25-vitamin D and may increase the risk of 1,25-vitamin D-mediated bone resorption. Moreover, it was clearly shown that 25-vitamin D supplementation as an add-on therapy to GFD does not improve bone mass gain in comparison with GFD alone [23], making this therapeutic measure even more useless.
On the contrary, two different approaches could be suggested. Due to the rapid reversal of GFD-mediated modification of vitamin D metabolite levels [23], a conservative approach is justified, associated with a re-evaluation of serum levels of 25-vitamin D, 1,25-vitamin D and PTH after 3 months of GFD [22], to check for the positive effect of the diet.
An alternative approach deduces its rationale on the presence of calcium balance impairment. In untreated CD, the modification of vitamin D and PTH serum levels is the expression of the impaired calcium balance independently from the actual detection of hypocalcemia. Accordingly, oral supplementation of calcium could represent a useful, preliminary therapeutic approach. Villous atrophy may hamper the efficacy of this measure, as untreated CD patients show a reduced calcium absorption, which improves on GFD [14]. However, the description of a rapid improvement of serum levels of both 25-vitamin D and 1,25-vitamin D after three months of GFD [23] suggests that the effect of calcium supplementation on the improvement of secondary hyperparathyroidism is low at the beginning of GFD. Instead, the effect could be evident within the third month of the diet. Moreover, the presence of normal levels of vitamin D and PTH during calcium and vitamin D supplementation begun before diagnosis in untreated CD patients suggests a positive effect of this therapeutic measure, besides the presence of villous atrophy [20].
4.2. Vitamin D Supplementation in Treated CD Patients
In patients with treated CD, the need for vitamin D supplementation seems less urgent, especially in those patients showing a progressive normalization of biomarkers of calcium balance after the beginning of GFD. If patients follow a strict GFD, it is very improbable that alterations of calcium balance may originate from mechanisms related to gluten toxicity. However, recently, nutritional and biochemical parameters of a group of CD patients on GFD recruited through a patient’s association were compared to a group of non-celiac adult volunteers [61]. In both the studied groups, vitamin D intake did not reach the recommended intake, and similar results were obtained for energy, folates, calcium, iodine, zinc and magnesium. In particular, a very low vitamin D intake was reported-around 22% of the recommended intake- in both CD and non-CD subjects, without differences between males and females. Plasma levels of vitamin D were found between 10 and 30 ng/mL in 35% of CD patients. These results were not different when compared to the control group. These results strengthen the need for the measurement of circulating levels of vitamin D, in order both to select patients with vitamin D deficiency and to address them to a nutritional intake evaluation.
The age of the patients becomes crucial: a correct suggestion should consider how menopause age is drawing closer. In patients younger than 45 years, before the perimenopausal period, the persistence of vitamin D deficiency or its recurrence should be investigated towards, first of all, the identification of incorrect adherence to GFD, and secondly, the identification of other conditions responsible for this alteration. In Table 1, autoimmune conditions associated with CD and considered at high risk of osteoporosis are reported.

Table 1.
Autoimmune conditions associated with CD and considered at risk of osteoporosis.
Under this light, one of the main actors of this coexisting alteration is represented by a thyroid disorder [29], which is very frequently associated to CD. In patients with Hashimoto’s thyroiditis and Grave’s disease, reduced levels of vitamin D were shown [55,62]. Vitamin D deficiency is considered to be responsible for the impaired T-cell suppression causing the release of proinflammatory cytokines and the consequent inflammatory-related alteration of thyroid structure, and therefore, function [63]. Many immune cells, such as macrophages, lymphocytes and dendritic cells, express vitamin D receptors and may convert 25-vitamin D into 1,25-vitamin D [64], which inhibits Th1 cell proliferation and Th1 cell cytokine production and decreases HLA class II antigen surface expression and B cell apoptosis [62,64,65,66]. Oral supplementation of 2000 IU/day vitamin D (more than twofold higher than RDA) is associated with a reduction in antithyroid autoantibodies suggesting a protective effect [67]. Moreover, it should be emphasized that in CD patients, intestinal malabsorption frequently causes iron deficiency, worsening thyroid function due to the impairment of heme-dependent TPO [68].
Another condition to rule out, if alterations of calcium balance are not dependent on gluten toxicity, is sarcoidosis [69]. It was recently reported by a systematic review and meta-analysis that the risk of sarcoidosis in CD patients is higher than subjects without CD with a pooled OR higher than 7 [70].
5. Conclusions
Alterations of bone and mineral metabolism are very frequent in CD patients and in comparison with the general population; the risk of fractures is significantly higher in this condition. Accordingly, besides a prompt diagnosis of CD, a prompt evaluation of bone mineral density and calcium metabolism is also mandatory in newly diagnosed patients to prevent the consequences of bone derangement and reduce the risk of fractures. A strict adherence to GFD proved to be effective in the reduction of risk fractures, even if in patients diagnosed after the achievement of the peak of bone mass, the reversal of bone loss is more difficult, in comparison with patients starting GFD in younger age.
The diet-induced improvement of calcium absorption, after the reversal of intestinal mucosa lesions, represents an important mechanism determining bone mineral density increase and making calcium supplementation less urgent. The need for vitamin D supplementation should be accurately evaluated because the beginning of a strict GFD may be rapidly and effectively adequate to revert calcium balance abnormalities detected at diagnosis, and consequently, to normalize serum levels of both 25-vitamin D and 1,25-vitamin D.
The relapse of alterations of calcium balance in patients on long-term GFD may represent a clinical sign of low adherence to GFD or the occurrence of another condition responsible for vitamin D deficiency. In this latter case, the mere correction of calcium balance will hesitate in a delay of an undiagnosed condition, together with patient exposition to its consequences and complications. Thyroid function should be promptly evaluated in case of vitamin D deficiency relapse.
The research agenda to elucidate vitamin D status in CD is still full of topics: first of all, studies dealing with vitamin D status in postmenopausal CD patients on long-term GFD are needed to ascertain whether this peculiar period of women physiology modifies the indication to vitamin D supplementation. Moreover, information is needed on the effect of vitamin D on immune response in CD patients, analyzing a possible effect on the RANK/RANKL/OPG system, known to be involved in the pathophysiology of bone loss. Finally, some information is needed on the pleiotropic effect of vitamin D, involving many endocrine and immune pathways, in relation of a possible proresorptive effect of the imbalance of the activity of other glands.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corazza, G.R.; Di Sabatino, A.; Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Corazza, G.R.; Di Stefano, M.; Maurino, E.; Bai, J.C. Bone and coeliac disease: Diagnosis and treatment. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 453–465. [Google Scholar] [CrossRef]
- Colston, K.W.; Mackay, A.G.; Finlayson, C.; Wu, J.C.J.; Maxwell, J.D. Localisation of vitamin D receptor in normal human duodenum and in patients with coeliac disease. Gut 1994, 35, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Taranta, A.; Fortunati, D.; Longo, M.; Rucci, N.; Iacomino, E.; Aliberti, F.; Facciuto, E.; Migliaccio, S.; Bardella, M.T.; Dubini, A.; et al. Imbalance of osteoclastogenesis-regulating factors in patients with celiac disease. J. Bone Miner. Res. 2004, 19, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Gaywood, I.; Scott, B.B. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. Gut 2000, 46 (Suppl. I), i1–i8. [Google Scholar] [CrossRef]
- American Gastroenterological Association Medical Position Statement: Guidelines on Osteoporosis in Gastrointestinal Diseases. Gastroenterology 2003, 124, 91–794.
- Di Stefano, M.; Mengoli, C.; Bergonzi, M.; Corazza, G.R. Bone mass and mineral metabolism alterations in adult celiac disease: Pathophysiology and clinical approach. Nutrients 2013, 5, 4786–4799. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Abrahamsen, B.; Al-Daghri, N.M.; Brandi, M.L.; Cannata-Andia, J.; Cortet, B.; Dimai, H.P.; Ferrari, S.; et al. European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Identification and management of patients at increased risk of osteoporotic fracture: Outcomes of an ESCEO expert consensus meeting. Osteoporos. Int. 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- Molteni, N.; Caraceni, M.P.; Bardella, M.T.; Ortolani, S.; Gandolini, G.G.; Bianchi, P.A. Bone mineral density in adult celiac patients and the effect of gluten-free diet from childhood. Am. J. Gastroenterol. 1990, 85, 51–53. [Google Scholar]
- Gerenli, N.; Dursun, F.; Celtik, C.; Kirmizibekmez, H. Significant improvement in bone mineral density in pediatric celiac disease: Even at six months with gluten-free diet. J. Ped. Endocrinol. Metab. 2021, 34, 341–348. [Google Scholar] [CrossRef]
- Corazza, G.R.; Di Sario, A.; Cecchetti, L.; Tarozzi, C.; Corrao, G.; Bernardi, M.; Gasbarrini, G. Bone mass and metabolism in patients with celiac disease. Gastroenterology 1995, 109, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Staun, M.; Jarnum, S. Measurement of the 10.000-molecular weight calcium-binding protein in small intestinal biopsy specimens from patients with malabsorption syndrome. Scand. J. Gastroenterol. 1988, 23, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Maierhofer, W.J.; Gray, R.W.; Cheung, H.S.; Lemann, J., Jr. Bone resorption stimulated by elevated serum levels of 1,25-(OH)2-vitamin D concentrations in healthy men. Kidney Int. 1983, 24, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Molteni, N.; Bardella, M.T.; Vezzoli, G.; Pozzoli, E.; Bianchi, P. Intestinal calcium absorption as shown by stable strontium test in celiac disease before and after gluten-free diet. Am. J. Gastroenterol. 1995, 90, 2025–2028. [Google Scholar]
- Keaveny, A.P.; Freaney, R.; McKenna, M.J.; Masterson, J.; O’Donoghue, D.P. Bone remodeling indices and secondary hyperparathyroidism in celiac disease. Am. J. Gastroenterol. 1996, 91, 1226–1231. [Google Scholar]
- Corazza, G.R.; Di Sario, A.; Cecchetti, L.; Jorizzo, A.; Di Stefano, M.; Minguzzi, L.; Brusco, G.; Bernardi, M.; Gasbarrini, G. Influence of pattern of clinical presentation and of gluten-free diet on bone mass and metabolism in adult coeliac disease. Bone 1996, 18, 525–530. [Google Scholar] [CrossRef]
- Valdimarsson, T.; Toss, G.; Lofman, O.; Strom, M. Three years’ follow up of bone density in adult coeliac disease: Significance of secondary hyperparathyroidism. Scand. J. Gastroenterol. 2000, 35, 274–280. [Google Scholar] [CrossRef]
- Mautalen, C.; Gonzalez, D.; Mazure, R.; Vazquez, H.; Lorenzetti, M.P.; Maurino, E.; Niveloni, S.; Pedreira, S.; Smecuol, E.; Boerr, L.A.; et al. Effect of treatment on bone mass, mineral metabolism, and body composition in untreated celiac disease patients. Am. J. Gastroenterol. 1997, 92, 313–318. [Google Scholar]
- Stein, E.M.; Rogers, H.; Leib, A.; McMahon, D.J.; Young, P.; Nishijama, K.; Guo, X.E.; Lewis, S.; Green, P.; Shane, E. Abnormal skeletal strength and microarchitecture in women with celiac disease. J. Clin. Endocrinol. Metab. 2015, 100, 2347–2353. [Google Scholar] [CrossRef]
- Selby, P.L.; Davies, M.; Adams, J.E.; Mawer, E.B. Bone loss in celiac disease is related to secondary hyperparathyroidism. J. Bone Miner. Res. 1999, 14, 652–657. [Google Scholar] [CrossRef]
- Corazza, G.R.; Di Stefano, M.; Jorizzo, R.A.; Cecchetti, L.; Minguzzi, L.; Gasbarrini, G. Propeptyde of type I procollagen is predictive of post-treatment bone mass gain in adult celiac disease. Gastroenterology 1997, 113, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Bergonzi, M.; Benedetti, I.; De Amici, M.; Torre, C.; Brondino, N.; Miceli, E.; Pagani, E.; Marseglia, G.L.; Corazza, G.R.; et al. Alterations of inflammatory and matrix production indices in celiac disease with low bone mass on long-term gluten-free diet. J. Clin. Gastroenterol. 2019, 53, e221–e226. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, B.; Boivin, M.; Brossard, J.H.; Lepage, R.; Picard, D.; Rousseau, L.; D’Amour, P. Normal parathyroid function with decreased bone minerl density in treated celiac disease. Can. J. Gastroenterol. 2001, 15, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Mawer, E.B.; Krawitt, E.L. Comparative absorption of vitamin D3 and 25-hydroxyvitaminD3 in intestinal disease. Gut 1980, 21, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, A.J.; Watson, G.; Compston, J.E. Changes in plasma half-life and clearance of 3H-25-hydroxyvitaminD3 in patients with intestinal malabsorption. Gut 1982, 23, 1068–1071. [Google Scholar] [CrossRef]
- Kempainnen, T.; Kroger, H.; Janatuinen, E.; Arnala, I.; Lamberg-Allardt, C.; Karkkainen, M. Bone recovery after a gluten-free diet: A 5-year follow-up study. Bone 1999, 25, 355–360. [Google Scholar] [CrossRef]
- Tavakkoli, A.; DiGiacomo, D.; Green, P.H.; Lebwohl, B. VitaminD status and concomitant autoimmunity in celiac disease. J. Clin. Gastroenterol. 2013, 47, 515–519. [Google Scholar] [CrossRef]
- Ciacci, C.; Bilancio, G.; Russo, I.; Iovino, P.; Cavallo, P.; Santonicola, A.; Bucci, C.; Cirillo, M.; Zingone, F. 25-hydroxyvitaminD, 1,25-dihydroxyvitaminD, and peripheral bone densitometry in adults with celiac disease. Nutrients 2020, 12, 929. [Google Scholar] [CrossRef]
- Sarela, S.; Thompson, D.V.; Nagrant, B.; Thakkar, P.; Clarke, K. A retrospective chart review evaluating the association of psychological disorders and vitamin D deficiency with celiac disease. Minerva Gastroenterol. Dietol. 2016, 62, 240–244. [Google Scholar]
- Marild, K.; Tapia, G.; Haugen, M.; Dahl, S.R.; Cohen, A.S.; Lundqvist, M.; Lie, B.A.; Stene, L.C.; Stordal, K. Matenal and neonatal vitamin D status, genotype and childhood celiac disease. PLoS ONE 2017, 12, e0179080. [Google Scholar] [CrossRef]
- San Pedro, J.I.; Bilbao, J.R.; Perez De Nunclares, G.; Vitoria, J.C.; Martul, P.; Castano, L. Heterogeneity of vitamin D receptor gene association with celiac disease and type I diabetes mellitus. Autoimmunity 2005, 38, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, H.; Suk, E.K.; Janisiw, M.; Stain, C.; Mayr, W.R.; Panzer, S. Calcaneal ultrasound attenuation and vitamin D receptor genotypes in celiac disease. Scand. J. Gastroenterol. 2000, 35, 172–176. [Google Scholar] [PubMed]
- Lu, C.; Zhou, W.; He, X.; Zhou, X.; Yu, C. Vitamin D status and vitamin D receptor genotypes in celiac disease: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional deficiencies in children with celiac disease resulting from a gluten-free diet: A systematic review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, A.; Hutchinson, J.M.; Ching, E.; Marwaha, A.; Verdu, E.; Armstring, D.; Pinto-Sanchez, M.I. Micronutrient deficiencies are frequent in adult patients with and without celiac disease on a gluten-free diet, regardless of duration and adherence to the diet. Nutrition 2022, 103–104, 111809. [Google Scholar] [CrossRef] [PubMed]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef] [PubMed]
- Passali, M.; Josefsen, K.; Frederiksen, J.L.; Antvorskov, J.C. Current evidence on the efficacy of gluten-free diet in multiple sclerosis, psoriasis, type I diabetes and autoimmune thyroid disease. Nutrients 2020, 12, 23164. [Google Scholar] [CrossRef]
- Croall, I.D.; Trott, N.; Rej, A.; Aziz, I.; O’Brien, D.J.; George, H.A.; Hossain, M.Y.; Marks, L.J.S.; Richardson, J.I.; Rigby, R.; et al. A Population Survey of Dietary Attitudes towards Gluten. Nutrients 2019, 11, 1276. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease. Results from a German dietary survey. Digestion 2013, 87, 240e6. [Google Scholar] [CrossRef]
- Hallert, C.; Grant, C.; Grehn, S.; Granno, C.; Hulten, S.; Midhagen, G.; Strom, M.; Svensson, M.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiency: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Iron deficiency anemia in celiac disease. World. J. Gastroenterol. 2015, 21, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Katsikeros, R.; Manton, N.; Krebs, N.F.; Hambridge, K.M.; Butler, R.N.; Davidson, G.P. Zinc homeostasis and gut function in children with celiac disease. Am. J. Clin. Nutr. 2011, 94, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Dembrinski, L.; Mazur, A.; Dabrowski, M.; Jackowska, T. Knowledge of medical students and medical professionals regarding nutritional deficiencies in patients with celiac disease. Nutrients 2021, 13, 1771. [Google Scholar] [CrossRef] [PubMed]
- Gladys, K.; Dardzinska, J.; Guzek, M.; Adrych, K.; Kochen, Z.; Malgorzewicz, S. Expanded role of a dietitian in monitoring a gluten-free diet in patients with celiac disease: Implications for clinical practice. Nutrients 2021, 13, 1859. [Google Scholar] [CrossRef] [PubMed]
- Kupper, C. Dietary guidelines and implementation for celiac disease. Gastroenterology 2005, 128 (suppl. S1), S121–S127. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Intestinal absorption and factors influencing bioavailability of magnesium. An update. Curr. Nutr. Food Sci. 2017, 13, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Stasi, E.; Marafini, I.; Caruso, R.; Soderino, F.; Angelucci, E.; Del Vecchio Blanco, G.; Paoluzi, O.A.; Calabrese, E.; Sedda, S.; Zorzi, F.; et al. Frequency and cause of persistent symptoms in celiac disease patients on a long-term gluten-free diet. J. Clin. Gastroenterol. 2016, 50, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazzaretti-Castro, M.; et al. Consensus statements from 2nd International Conference on controversies in Vitamin D. Rev. Endocr. Metab. Dis. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; McKenzie, J.E.; McDonald, S.; Baram, L.; Page, M.J.; Allman-Farinelli, M.; Raubenheimer, D.; Bero, L.A. Assessment of the methods used to develop vitamin D and calcium recommendations. A systematic review of bone health guidelines. Nutrients 2021, 13, 2423. [Google Scholar] [CrossRef] [PubMed]
- Human Vitamin and Mineral Requirements Report of a Joint FAO/WHO Expert Consultation. Bangkok, Thailand. 2001. Available online: https://www.fao.org/3/y2809e/y2809e.pdf (accessed on 29 September 2022).
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Elsenberg, E.H.A.M.; Ten Bockel, E.; Huijgen, H.; Heijboer, A.C. Standardization of automated 25-hydroxyvitamin D assays: How successful is it? Clin. Biochem. 2017, 50, 1126–1130. [Google Scholar] [CrossRef]
- Thomas, S.D.C.; Need, A.G.; Tucker, G.; Slobodian, P.O.; O’Loughlin, P.D.; Nordin, B.E.C. Suppression of parathyroid hormine and bone resorption by calcium carbonate and calcium citrate in postmenopausal women. Calcif. Tissue Int. 2008, 83, 81–84. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guidelines. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Bouillon, R.; Muls, E.; De Moor, P. Influence of thyroid function on the serum concentration of 1,25-dihydroxyvitaminD3. J. Clin. Endocrinol. Metab. 1980, 51, 793–797. [Google Scholar] [CrossRef]
- Ballestrero-Fernandez, C.; Varela-Moreiras, G.; Ubeda, N.; Alonso-Aperte, E. Nutritional status in Spanish adults with celiac disease following a long-term gluten-free diet is similar to non-celiac. Nutrients 2021, 13, 1626. [Google Scholar] [CrossRef]
- Kim, D. The role of vitamin D in thyroid disease. Int. J. Mol. Sci. 2017, 18, 1949. [Google Scholar] [CrossRef] [PubMed]
- Moscogiuri, G.; Tirabassi, G.; Bizzarro, G.; Orio, F.; Paschou, S.; Vryonidou, A.; Balercia, G.; Shoenfeld, Y.; Colao, A. Vitamin D and thyroid disease: To D or not to D? Eur. J. Clin. Nutr. 2014, 69, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.M.; Gorman, S.; Gedenhuys, S.; Hart, P.H. Vitamin D and immunity. F1000 Prime. Rep. 2014, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanach, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Vanoirbeek, E.; Krishnan, A.; Eelen, G.; Verlinden, L.; Bouillon, R.; Feldman, A.; Verstuyf, A. The anti-cancer and anti-inflammatory actions of 1,25(OH)2D3. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 593–604. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Vitamin D on Thyroid Autoimmunity in Levothyroxine-Treated Women with Hashimoto’s Thyroiditis and Normal Vitamin D Status. Exp. Clin. Endocrinol. Diabetes. 2017, 125, 229–233. [Google Scholar] [CrossRef]
- O’Kane, M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, iodine status and concentrations of thyroid hormones: A systematic review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef]
- Bell, N.H.; Stern, P.H.; Pantzer, E.; Sinha, T.K.; DeLuca, H.F. Evidence that increased circulating 1 alpha, 25-dihydroxyvitaminD is the probable cause for abnormal calcium metabolism in sarcoidosis. J. Clin. Investig. 1979, 64, 218–225. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Panjawatanan, P.; Corral, J.E.; Lukens, F.J.; Ungprasert, P. Celiac disease and risk of sarcoidosis: A systematic review and meta-analysis. J. Evid. Based Med. 2019, 12, 194–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).