Development of an Endoscopic Auto-Fluorescent Sensing Device to Aid in the Detection of Breast Cancer and Inform Photodynamic Therapy
Abstract
:1. Introduction
2. Methods
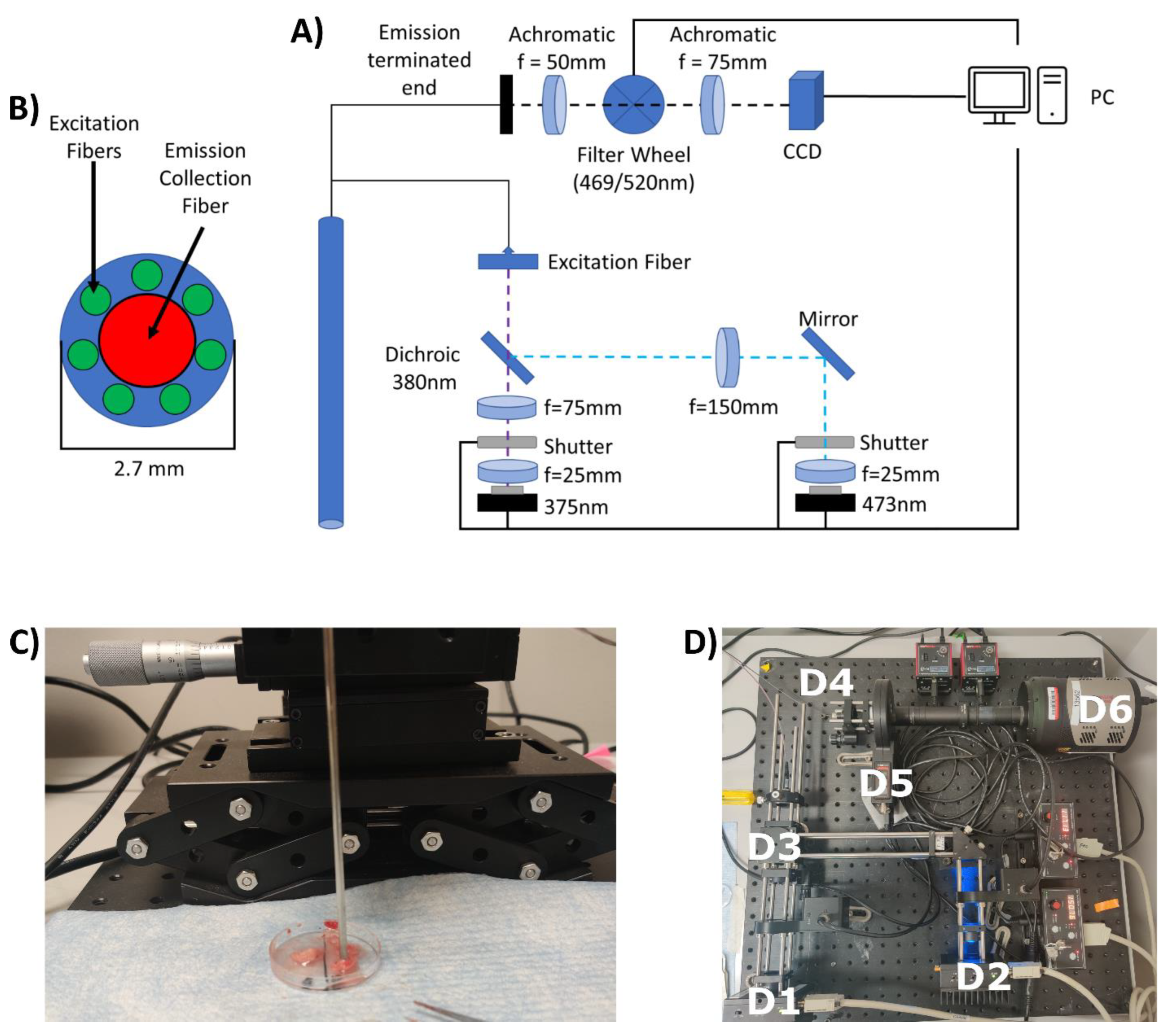
2.1. Construction of the Endoscopic Imaging System
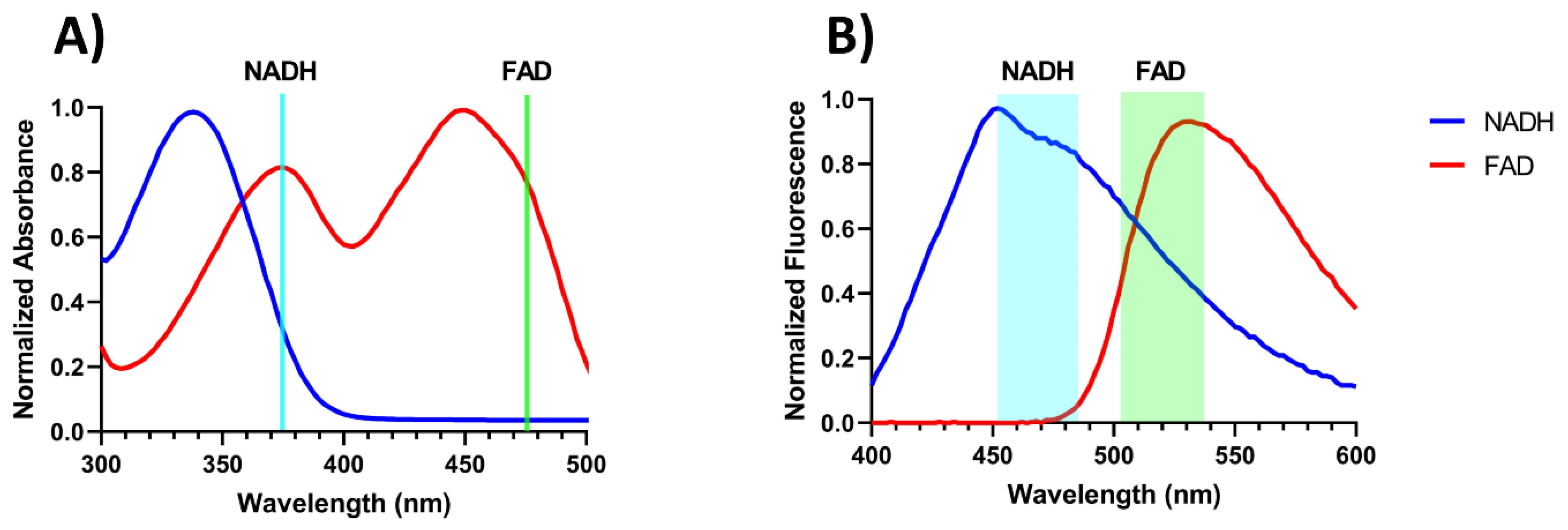
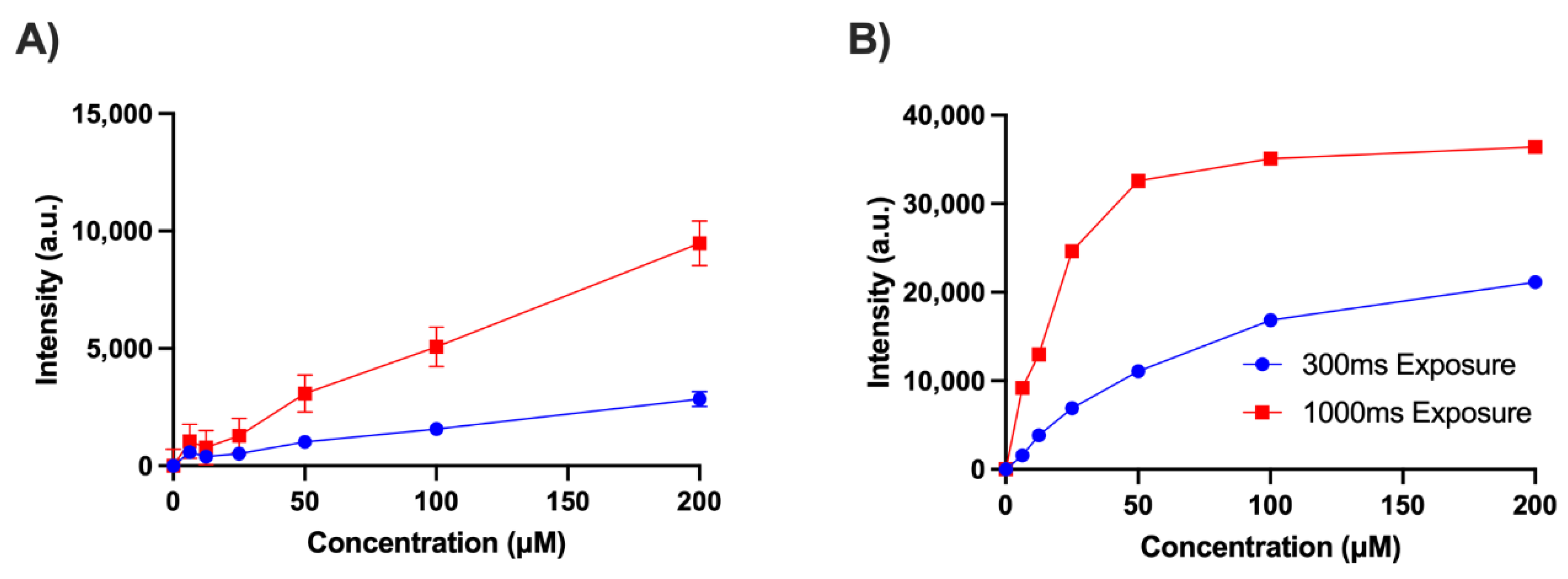
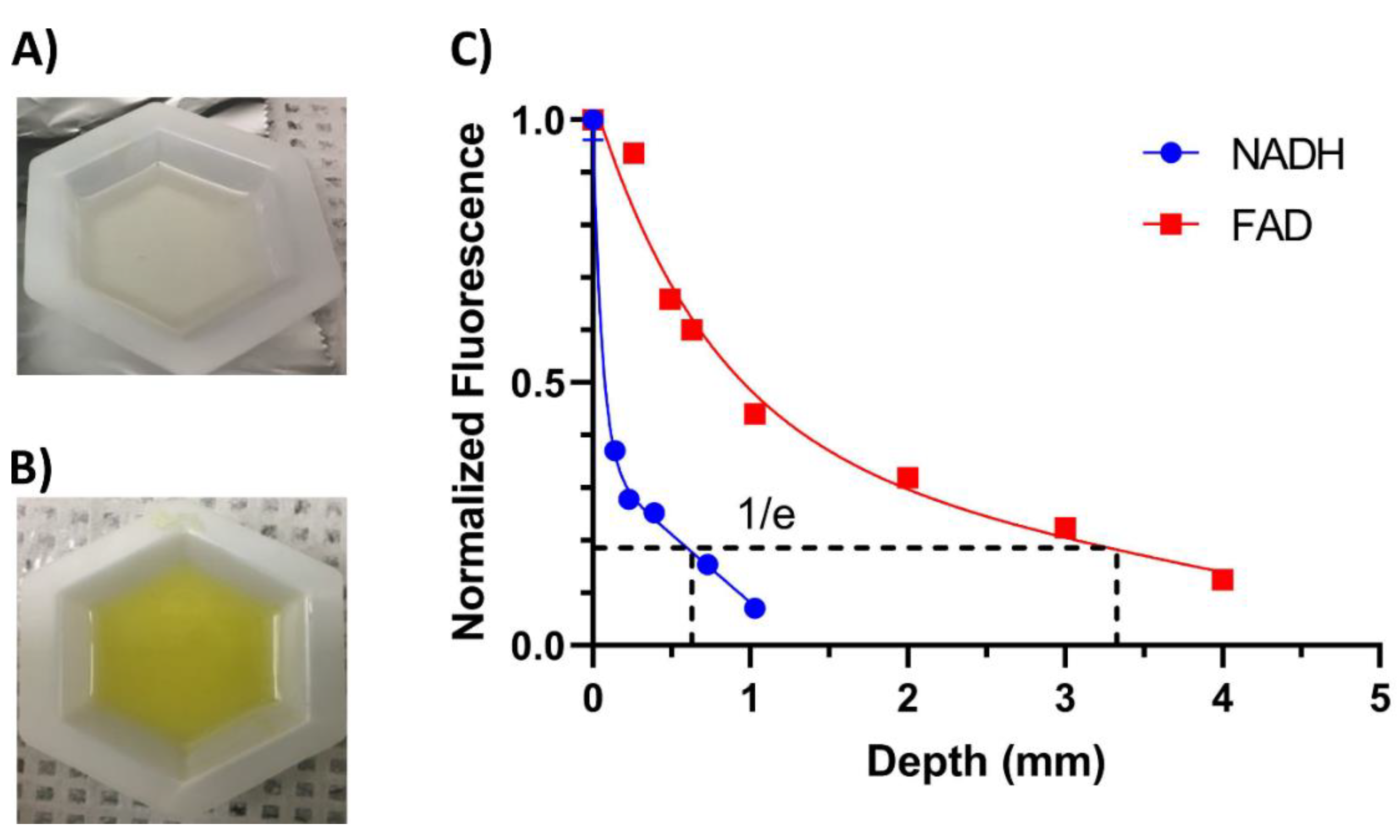
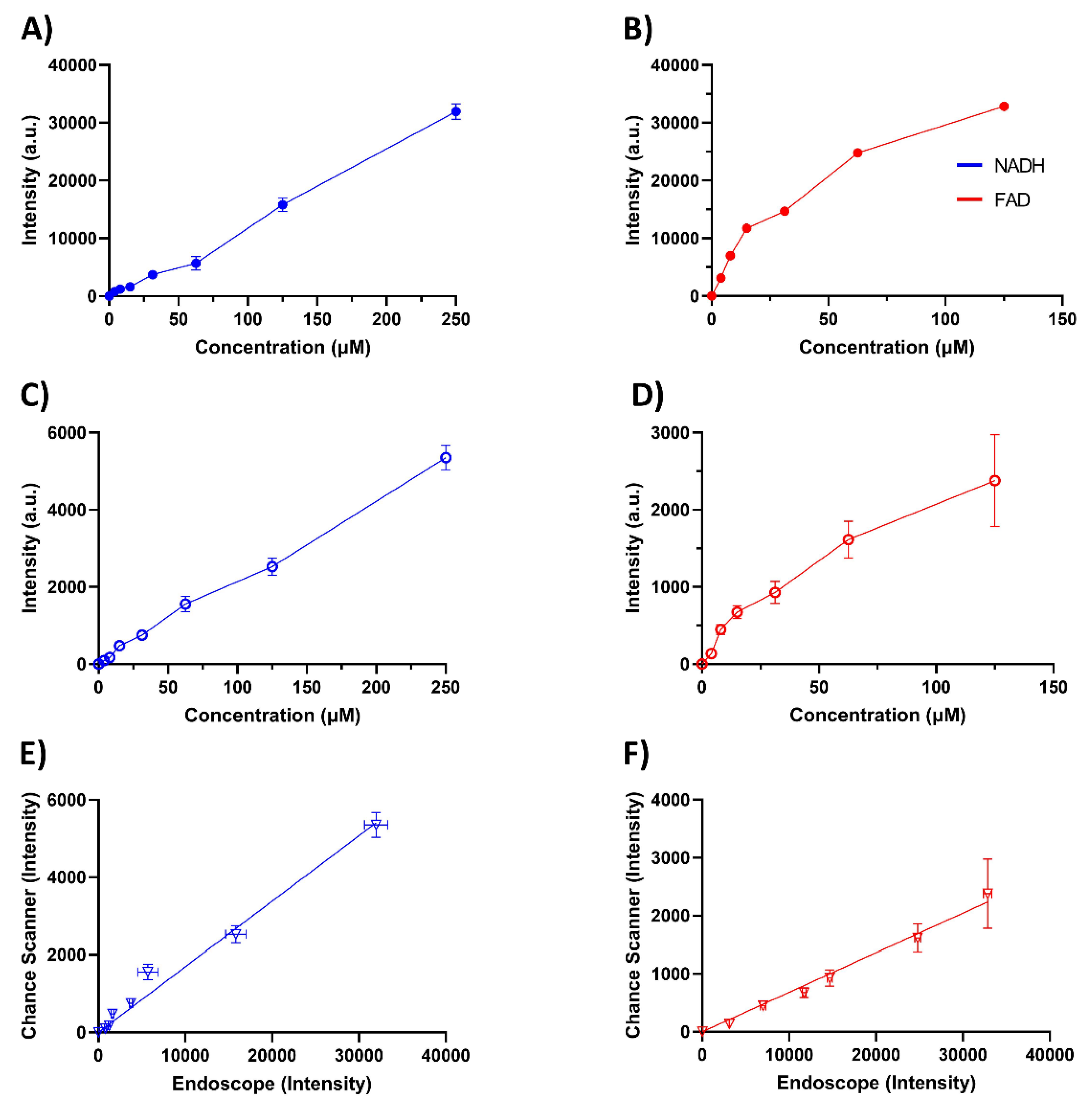
2.2. Validation of the Endoscope Using Phantoms
2.3. Validation and Application of Endoscope Ex Vivo
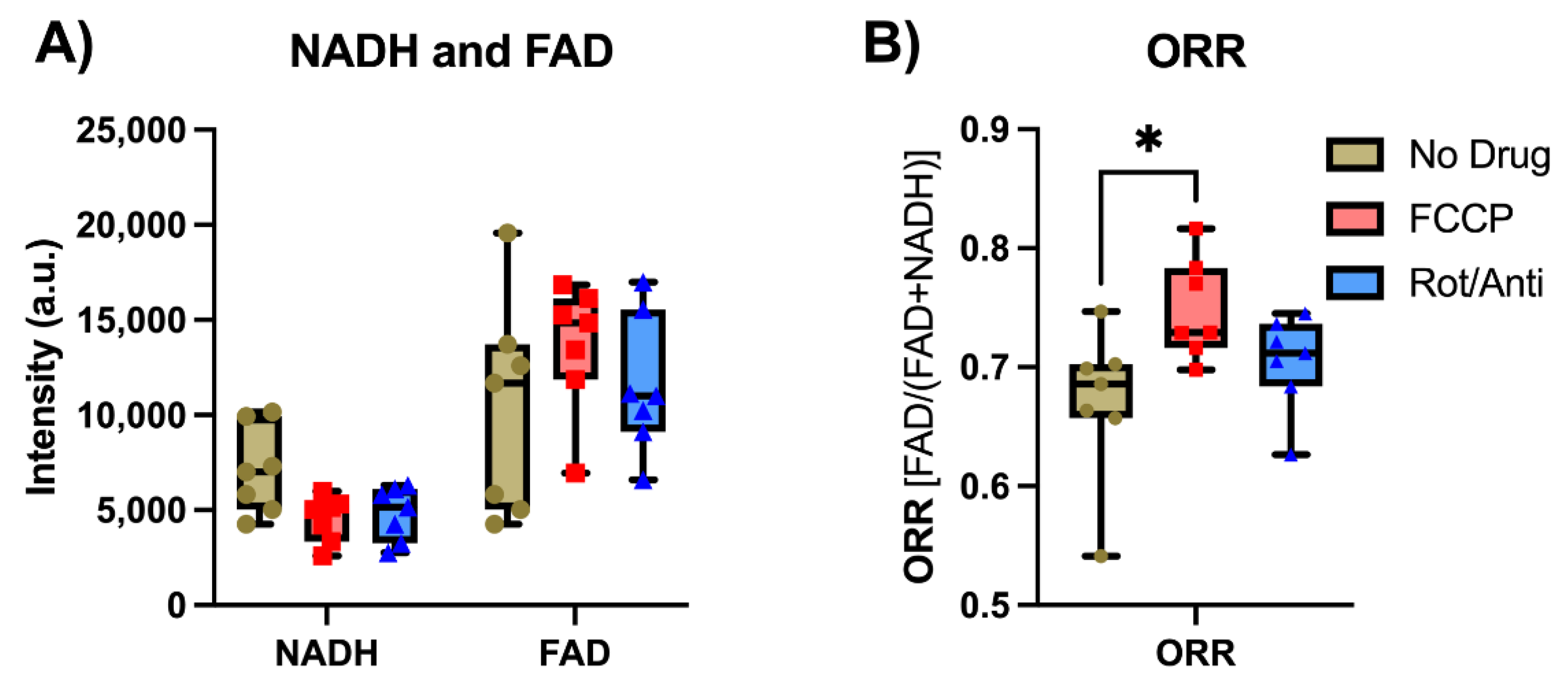
2.3.1. Ex Vivo Validation by Metabolic Modulation
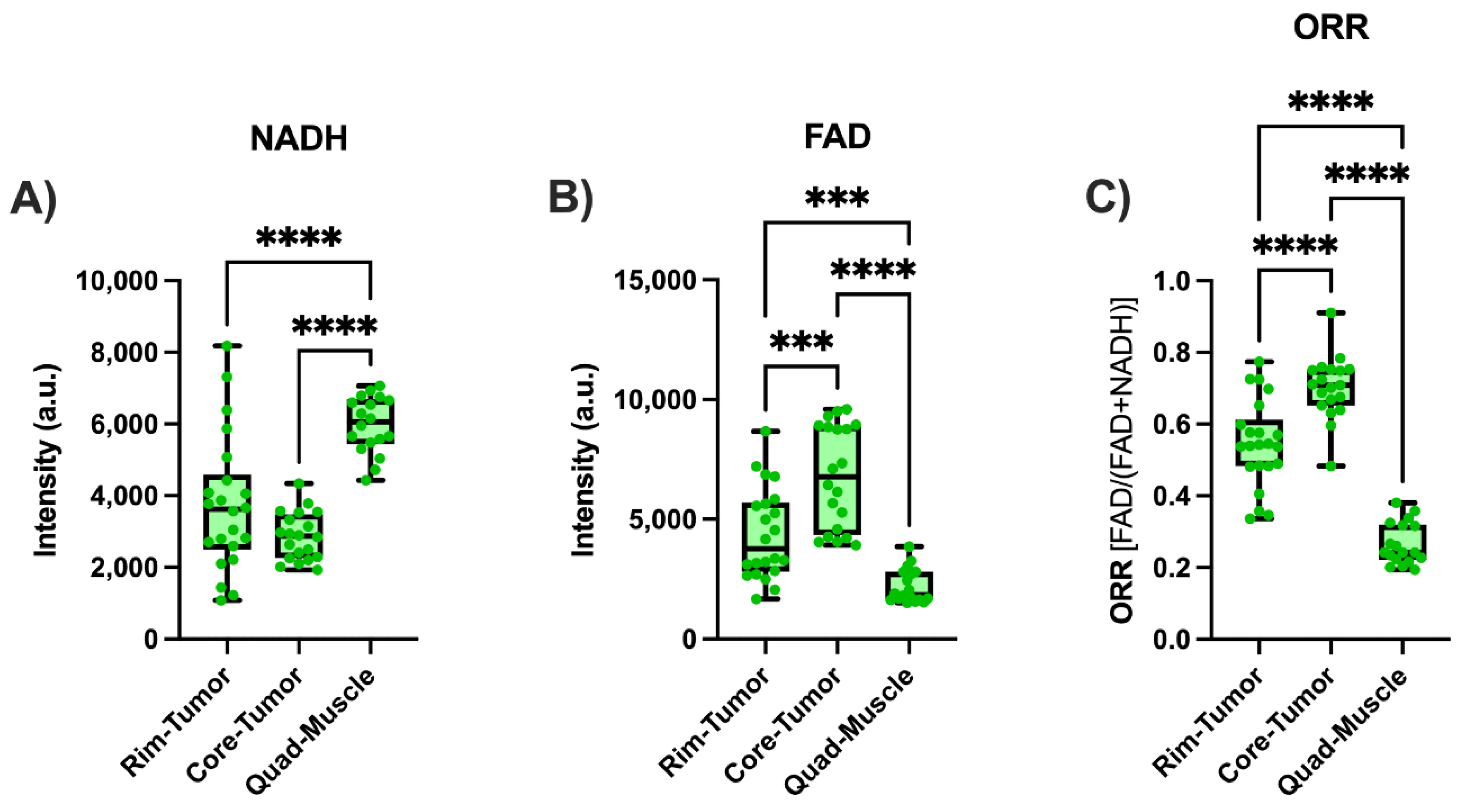
2.3.2. Ex vivo Tumor Imaging
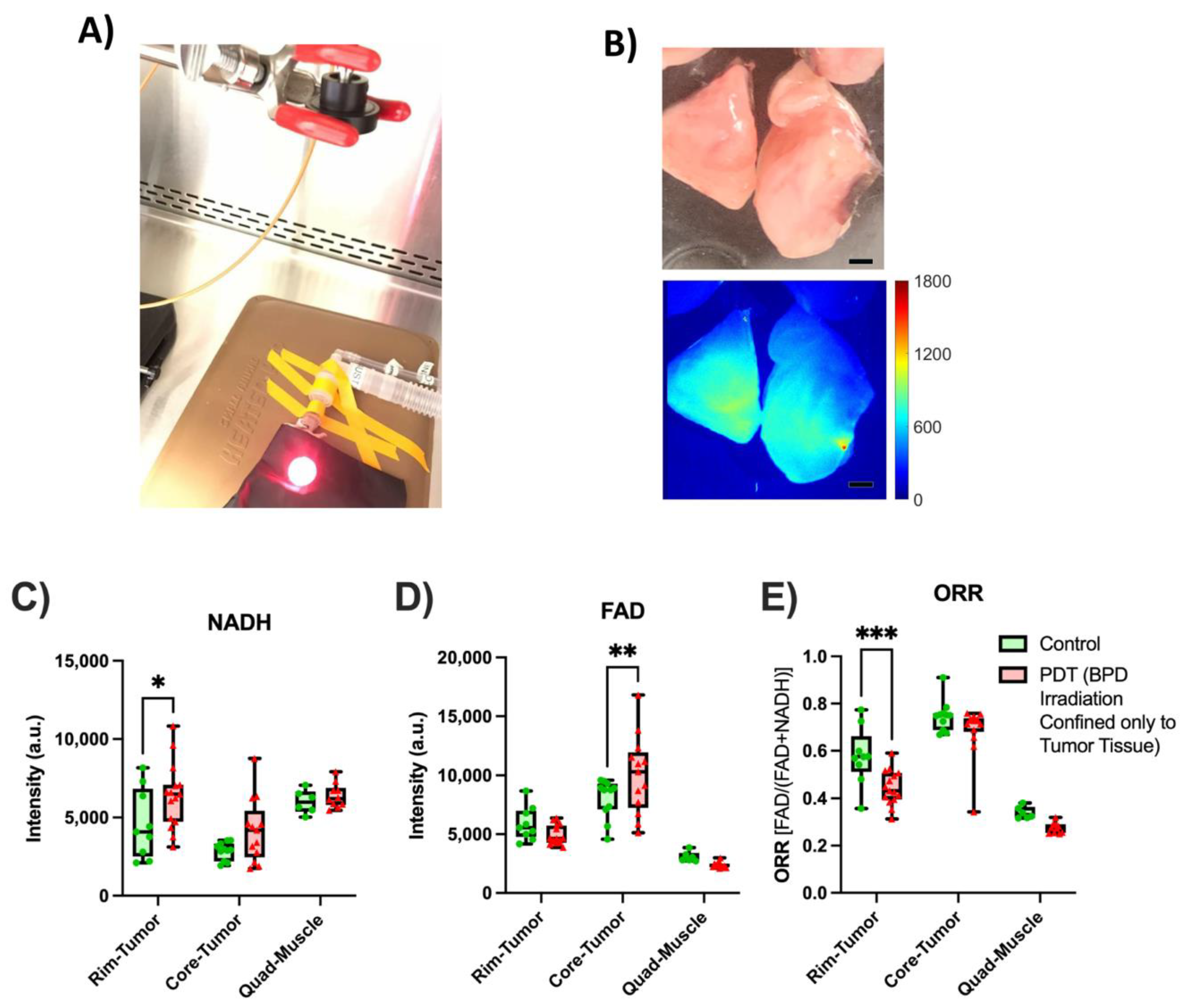
2.3.3. PDT Treatment and Subsequent Metabolic Imaging
2.4. Data Processing
3. Results
4. Discussion
4.1. Device Validation with Phantoms
4.2. Ex Vivo Tissue Measurements
4.3. Limitations and Future Work
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Ciatto, S.; Martinelli, F.; Bonardi, R.; Duffy, S. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann. Oncol. 2009, 20, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.B.; Phil, A.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.; van der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.L.L.; Glatz, J.; Kranendonk, M.E.G.; van Scheltinga, A.G.T.; Jansen, L.; de Vries, J.; Hooge, M.N.L.-D.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab–IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin. Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef] [Green Version]
- Halperin, L.; Olk, R.J.; Soubrane, G.; Coscas, G. Safety of Fluorescein Angiography during Pregnancy. Am. J. Ophthalmol. 1990, 109, 563–566. [Google Scholar] [CrossRef]
- Chance, B. Spectra and Reaction Kinetics of Respiratory Pigments of Homogenized and Intact Cells. Nature 1952, 169, 215–221. [Google Scholar] [CrossRef]
- Chance, B.; Cohen, P.; Jobsis, F.; Schoener, B. Intracellular Oxidation-Reduction States in Vivo. Science 1962, 137, 499–508. [Google Scholar] [CrossRef]
- Dumollard, R.; Marangos, P.; Fitzharris, G.; Swann, K.; Duchen, M.; Carroll, J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 2004, 131, 3057–3067. [Google Scholar] [CrossRef] [Green Version]
- Podsednik, A.; Jacob, A.; Li, L.Z.; Xu, H.N. Relationship between Optical Redox Status and Reactive Oxygen Species in Cancer Cells. React. Oxyg. Species 2020, 9, 95–108. [Google Scholar] [CrossRef]
- Alhallak, K.; Rebello, L.G.; Muldoon, T.J.; Quinn, K.P.; Rajaram, N. Optical redox ratio identifies metastatic potential-dependent changes in breast cancer cell metabolism. Biomed. Opt. Express 2016, 7, 4364–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quistorff, B.; Haselgrove, J.C.; Chance, B. High spatial resolution readout of 3-D metabolic organ structure: An automated, low-temperature redox ratio-scanning instrument. Anal. Biochem. 1985, 148, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Ranji, M.; Nioka, S.; Xu, H.N.; Wu, B.; Li, L.Z.; Jaggard, D.L.; Chance, B. Fluorescent Images of Mitochondrial Redox States Inin Situmouse Hypoxic Ischemic Intestines. J. Innov. Opt. Health Sci. 2009, 2, 365–374. [Google Scholar] [CrossRef]
- La Cour, M.F.; Mehrvar, S.; Heisner, J.S.; Motlagh, M.M.; Medhora, M.; Ranji, M.; Camara, A.K.S. Optical metabolic imaging of irradiated rat heart exposed to ischemia–reperfusion injury. J. Biomed. Opt. 2018, 23, 016011. [Google Scholar] [CrossRef]
- Mehrvar, S.; la Cour, M.F.; Medhora, M.; Camara, A.K.S.; Ranji, M. Optical Metabolic Imaging for Assessment of Radiation-Induced Injury to Rat Kidney and Mitigation by Lisinopril. Ann. Biomed. Eng. 2019, 47, 1564–1574. [Google Scholar] [CrossRef]
- Li, L.Z.; Xu, H.N.; Ranji, M.; Nioka, S.; Chance, B. Mitochondrial Redox Imaging for Cancer Diagnostic and Therapeutic Studies. J. Innov. Opt. Health Sci. 2009, 2, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Xu, H.N.; Vinnedge, L.P.; Feng, M.; Li, L.Z. Optical Redox Imaging Detects the Effects of DEK Oncogene Knockdown on the Redox State of MDA-MB-231 Breast Cancer Cells. Mol. Imaging Biol. 2019, 21, 410–416. [Google Scholar] [CrossRef]
- Xu, H.N.; Nioka, S.; Glickson, J.D.; Chance, B.; Li, L.Z. Quantitative mitochondrial redox imaging of breast cancer metastatic potential. J. Biomed. Opt. 2010, 15, 036010. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.N.; Tchou, J.; Chance, B.; Li, L.Z. Imaging the Redox States of Human Breast Cancer Core Biopsies. In Oxygen Transport to Tissue XXXIV; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; Volume 765, pp. 343–349. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.N.; Tchou, J.; Feng, M.; Zhao, H.; Li, L.Z. Optical redox imaging indices discriminate human breast cancer from normal tissues. J. Biomed. Opt. 2016, 21, 114003. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhou, R.; Xu, H.N.; Moon, L.; Zhong, T.; Kim, E.J.; Qiao, H.; Reddy, R.; Leeper, D.; Chance, B.; et al. Quantitative magnetic resonance and optical imaging biomarkers of melanoma metastatic potential. Proc. Natl. Acad. Sci. USA 2009, 106, 6608–6613. [Google Scholar] [CrossRef] [Green Version]
- Li, L.Z.; Zhou, R.; Zhong, T.; Moon, L.; Kim, E.J.; Hui, Q.; Pickup, S.; Hendrix, M.J.; Leeper, D.; Chance, B.; et al. Predicting Melanoma Metastatic Potential by Optical and Magnetic Resonance Imaging. In Oxygen Transport to Tissue XXVIII; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 599, pp. 67–78. [Google Scholar] [CrossRef]
- Xu, H.N.; Zheng, G.; Nioka, S.; Chance, B.; Li, L.Z. High-Resolution Simultaneous Mapping of Mitochondrial Redox State and Glucose Uptake in Human Breast Tumor Xenografts. In Oxygen Transport to Tissue XXXIII; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 737, pp. 175–179. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.N.; Feng, M.; Moon, L.; Dolloff, N.G.; El-Deiry, W.; Li, L.Z. Redox Imaging of the P53-Dependent Mitochondrial Redox State in Colon Cancer Ex Vivo. J. Innov. Opt. Health Sci. 2013, 6, 1350016. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, Y.; Tang, Q.; Li, Z.; Horng, H.; Li, J.; Wu, Z.; Chen, Y.; Li, H. Quantitative evaluation of redox ratio and collagen characteristics during breast cancer chemotherapy using two-photon intrinsic imaging. Biomed. Opt. Express 2018, 9, 1375–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef] [Green Version]
- Levitt, J.M.; McLaughlin-Drubin, M.E.; Münger, K.; Georgakoudi, I. Automated Biochemical, Morphological, and Organizational Assessment of Precancerous Changes from Endogenous Two-Photon Fluorescence Images. PLoS ONE 2011, 6, e24765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.N.; Nioka, S.; Li, L.Z. Imaging heterogeneity in the mitochondrial redox state of premalignant pancreas in the pancreas-specific PTEN-null transgenic mouse model. Biomark. Res. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.Z.; Sun, N. Autofluorescence perspective of cancer diagnostics. In Natural Biomarkers for Cellular Metabolism: Biology, Techniques, and Applications (Autofluorescence Perspective of Cancer Diagnostics); CRC Press: New York, NY, USA, 2014; pp. 273–297. [Google Scholar]
- Gil, D.A.; Deming, D.A.; Skala, M.C. Patient-derived cancer organoid tracking with wide-field one-photon redox imaging to assess treatment response. J. Biomed. Opt. 2021, 26, 036005. [Google Scholar] [CrossRef]
- Favreau, P.F.; He, J.; Gil, D.A.; Deming, D.A.; Huisken, J.; Skala, M.C. Label-free redox imaging of patient-derived organoids using selective plane illumination microscopy. Biomed. Opt. Express 2020, 11, 2591–2606. [Google Scholar] [CrossRef]
- Mehrvar, S.; Rymut, K.T.; Foomani, F.H.; Mostaghimi, S.; Eells, J.T.; Ranji, M.; Gopalakrishnan, S. Fluorescence Imaging of Mitochondrial Redox State to Assess Diabetic Wounds. IEEE J. Transl. Eng. Health Med. 2019, 7, 1800809. [Google Scholar] [CrossRef]
- Liang, W.; Hall, G.; Messerschmidt, B.; Li, M.-J.; Li, X. Nonlinear optical endomicroscopy for label-free functional histology in vivo. Light. Sci. Appl. 2017, 6, e17082. [Google Scholar] [CrossRef] [Green Version]
- Romano, R.A.; Rosa, R.G.T.; Salvio, A.G.; Jo, J.A.; Kurachi, C. Multispectral autofluorescence dermoscope for skin lesion assessment. Photodiagnosis Photodyn. Ther. 2020, 30, 101704. [Google Scholar] [CrossRef]
- Meena, B.L.; Agarwal, A.; Pantola, C.; Pandey, K.; Pradhan, A. Concentration of FAD as a marker for cervical precancer detection. J. Biomed. Opt. 2019, 24, 035008. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Sorvina, A.; Morrison, J.L.; Darby, J.R.T.; Brooks, D.A.; Plush, S.E.; Vahid, S.A. Development of an optical fiber-based redox monitoring system for tissue metabolism. J. Biophotonics 2022, 15, e202100304. [Google Scholar] [CrossRef] [PubMed]
- Kanniyappan, U.; Xu, H.N.; Tang, Q.; Gaitan, B.; Liu, Y.; Li, L.Z.; Chen, Y. Novel fiber optic-based needle redox imager for cancer diagnosis (SPIE BiOS). In Optical Biopsy XVI: Toward Real-Time Spectroscopic Imaging and Diagnosis; SPIE: Bellingham, DC, USA, 2018. [Google Scholar] [CrossRef]
- Udovich, J.A.; Kirkpatrick, N.D.; Kano, A.; Tanbakuchi, A.; Utzinger, U.; Gmitro, A.F. Spectral background and transmission characteristics of fiber optic imaging bundles. Appl. Opt. 2008, 47, 4560–4568. [Google Scholar] [CrossRef]
- Willems, S.M.; van Deurzen, C.; van Diest, P. Diagnosis of breast lesions: Fine-needle aspiration cytology or core needle biopsy? A review. J. Clin. Pathol. 2011, 65, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-L.; Hong, J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg. 2014, 3, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.H.; Kim, E.-K.; Kim, M.J.; Lee, J.Y.; Oh, K.K. Missed Breast Cancers at US-guided Core Needle Biopsy: How to Reduce Them. RadioGraphics 2007, 27, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, L.; Torricelli, A.; Pifferi, A.; Taroni, P.; Danesini, G.M.; Cubeddu, R. Bulk optical properties and tissue components in the female breast from multiwavelength time-resolved optical mammography. J. Biomed. Opt. 2004, 9, 1137–1143. [Google Scholar] [CrossRef]
- Sandell, J.L.; Zhu, T.C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Kanick, S.C.; Krishnaswamy, V.; Gamm, U.A.; Sterenborg, H.J.C.M.; Robinson, D.J.; Amelink, A.; Pogue, B.W. Scattering phase function spectrum makes reflectance spectrum measured from Intralipid phantoms and tissue sensitive to the device detection geometry. Biomed. Opt. Express 2012, 3, 1086–1100. [Google Scholar] [CrossRef] [Green Version]
- Validation of analytical procedures: Text and methodology Q2(R1). In ICH Harmonized Tripartite Guideline; International Council for Harmonisation: Geneva, Switzerland, 2005.
- Zhang, Z.; Blessington, D.; Li, H.; Busch, T.M.; Glickson, J.; Luo, Q.; Chance, B.; Zheng, G. Redox ratio of mitochondria as an indicator for the response of photodynamic therapy. J. Biomed. Opt. 2004, 9, 772–778. [Google Scholar] [CrossRef]
- Chance, B.; Schoener, B.; Oshino, R.; Itshak, F.; Nakase, Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J. Biol. Chem. 1979, 254, 4764–4771. [Google Scholar] [CrossRef]
- Inglut, C.T.; Gaitan, B.; Najafali, D.; Lopez, I.A.; Connolly, N.P.; Orsila, S.; Perttilä, R.; Woodworth, G.F.; Chen, Y.; Huang, H.-C. Predictors and Limitations of the Penetration Depth of Photodynamic Effects in the Rodent Brain. Photochem. Photobiol. 2019, 96, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Rizvi, I.; Liu, J.; Anbil, S.; Kalra, A.; Lee, H.; Baglo, Y.; Paz, N.; Hayden, D.; Pereira, S.; et al. Photodynamic Priming Mitigates Chemotherapeutic Selection Pressures and Improves Drug Delivery. Cancer Res. 2018, 78, 558–571. [Google Scholar] [CrossRef] [Green Version]
- Pigula, M.; Huang, H.-C.; Mallidi, S.; Anbil, S.; Liu, J.; Mai, Z.; Hasan, T. Size-dependent Tumor Response to Photodynamic Therapy and Irinotecan Monotherapies Revealed by Longitudinal Ultrasound Monitoring in an Orthotopic Pancreatic Cancer Model. Photochem. Photobiol. 2018, 95, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Baglo, Y.; Liang, B.J.; Robey, R.; Ambudkar, S.V.; Gottesman, M.M.; Huang, H.-C. Porphyrin-lipid assemblies and nanovesicles overcome ABC transporter-mediated photodynamic therapy resistance in cancer cells. Cancer Lett. 2019, 457, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Kwok, S.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef] [Green Version]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Wagnieres, G.A.; Star, W.M.; Wilson, B.C. In Vivo Fluorescence Spectroscopy and Imaging for Oncological Applications. Photochem. Photobiol. 1998, 68, 603–632. [Google Scholar] [CrossRef]
- Brazard, J.; Usman, A.; Lacombat, F.; Ley, C.; Martin, M.M.; Plaza, P. New Insights into the Ultrafast Photophysics of Oxidized and Reduced FAD in Solution. J. Phys. Chem. A 2011, 115, 3251–3262. [Google Scholar] [CrossRef]
- Li, G.; Glusac, K.D. The Role of Adenine in Fast Excited-State Deactivation of FAD: A Femtosecond Mid-IR Transient Absorption Study. J. Phys. Chem. B 2009, 113, 9059–9061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubista, M.; Sjöback, R.; Eriksson, S.; Albinsson, B. Experimental correction for the inner-filter effect in fluorescence spectra. Analyst 1994, 119, 417–419. [Google Scholar] [CrossRef]
- Fonin, A.V.; Sulatskaya, A.I.; Kuznetsova, I.M.; Turoverov, K.K. Fluorescence of Dyes in Solutions with High Absorbance. Inner Filter Effect Correction. PLoS ONE 2014, 9, e103878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.N.; Feng, M.; Nath, K.; Nelson, D.; Roman, J.; Zhao, H.; Lin, Z.; Glickson, J.; Li, L.Z. Optical Redox Imaging of Lonidamine Treatment Response of Melanoma Cells and Xenografts. Mol. Imaging Biol. 2018, 21, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, F.; Abramov, A.Y. Measurement of Mitochondrial NADH and FAD Autofluorescence in Live Cells. Polyamines 2015, 1264, 263–270. [Google Scholar] [CrossRef]
- Forkink, M.; Basit, F.; Teixeira, J.; Swarts, H.G.; Koopman, W.J.H.; Willems, P.H.G.M. Complex I and complex III inhibition specifically increase cytosolic hydrogen peroxide levels without inducing oxidative stress in HEK293 cells. Redox Biol. 2015, 6, 607–616. [Google Scholar] [CrossRef]
- Pye, K.; Chance, B. Sustained sinusoidal oscillations of reduced pyridine nucleotide in a cell-free extract of Saccharomyces carlsbergensis. Proc. Natl. Acad. Sci. USA 1966, 55, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Chance, B.; Baltscheffsky, H. Respiratory Enzymes in Oxidative Phosphorylation. J. Biol. Chem. 1958, 233, 736–739. [Google Scholar] [CrossRef]
- Moon, L.; Frederick, D.W.; Baur, J.A.; Li, L.Z. Imaging Redox State in Mouse Muscles of Different Ages. In Oxygen Transport to Tissue XXXIX; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2017; Volume 977, pp. 51–57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, H.N.; Li, S.; Davila, A., Jr.; Chellappa, K.; Davis, J.G.; Guan, Y.; Frederick, D.W.; Chu, W.; Zhao, H.; et al. Rapamycin maintains NAD+/NADH redox homeostasis in muscle cells. Aging 2020, 12, 17786–17799. [Google Scholar] [CrossRef]
- Podsednik, A.; Jiang, J.; Jacob, A.; Li, L.Z.; Xu, H.N. Optical Redox Imaging of Treatment Responses to Nampt Inhibition and Combination Therapy in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 5563. [Google Scholar] [CrossRef]
- Guirguis, M.; Bhandari, C.; Li, J.; Eroy, M.; Prajapati, S.; Margolis, R.; Shrivastava, N.; Hoyt, K.; Hasan, T.; Obaid, G. Membrane composition is a functional determinant of NIR-activable liposomes in orthotopic head and neck cancer. Nanophotonics 2021, 10, 3169–3185. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.R.; Wallrabe, H.; Christopher, K.G.; Siller, K.H.; Periasamy, A. Characterization of mitochondrial dysfunction due to laser damage by 2-photon FLIM microscopy. Sci. Rep. 2022, 12, 11938. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, J.; Orlova, N.; Grewe, B.F. Wide. Fast. Deep: Recent Advances in Multiphoton Microscopy of In Vivo Neuronal Activity. J. Neurosci. 2019, 39, 9042–9052. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Honma, M.; Nakabayashi, T.; Kinjo, M.; Ohta, N. pH Dependence of the Fluorescence Lifetime of FAD in Solution and in Cells. Int. J. Mol. Sci. 2013, 14, 1952–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, T.M.; Lagarto, J.L.; Dyer, B.T.; Garcia, E.; Kelly, D.J.; Peters, N.S.; Lyon, A.R.; French, P.M.W.; Dunsby, C. Characterization of NADH fluorescence properties under one-photon excitation with respect to temperature, pH, and binding to lactate dehydrogenase. OSA Contin. 2021, 4, 1610. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitan, B.; Inglut, C.; Kanniyappan, U.; Xu, H.N.; Conant, E.F.; Frankle, L.; Li, L.Z.; Chen, Y.; Huang, H.-C. Development of an Endoscopic Auto-Fluorescent Sensing Device to Aid in the Detection of Breast Cancer and Inform Photodynamic Therapy. Metabolites 2022, 12, 1097. https://doi.org/10.3390/metabo12111097
Gaitan B, Inglut C, Kanniyappan U, Xu HN, Conant EF, Frankle L, Li LZ, Chen Y, Huang H-C. Development of an Endoscopic Auto-Fluorescent Sensing Device to Aid in the Detection of Breast Cancer and Inform Photodynamic Therapy. Metabolites. 2022; 12(11):1097. https://doi.org/10.3390/metabo12111097
Chicago/Turabian StyleGaitan, Brandon, Collin Inglut, Udayakumar Kanniyappan, He N. Xu, Emily F. Conant, Lucas Frankle, Lin Z. Li, Yu Chen, and Huang-Chiao Huang. 2022. "Development of an Endoscopic Auto-Fluorescent Sensing Device to Aid in the Detection of Breast Cancer and Inform Photodynamic Therapy" Metabolites 12, no. 11: 1097. https://doi.org/10.3390/metabo12111097
APA StyleGaitan, B., Inglut, C., Kanniyappan, U., Xu, H. N., Conant, E. F., Frankle, L., Li, L. Z., Chen, Y., & Huang, H.-C. (2022). Development of an Endoscopic Auto-Fluorescent Sensing Device to Aid in the Detection of Breast Cancer and Inform Photodynamic Therapy. Metabolites, 12(11), 1097. https://doi.org/10.3390/metabo12111097





