Quantification of the Tissue Oxygenation Delay Induced by Breath-Holding in Patients with Carotid Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
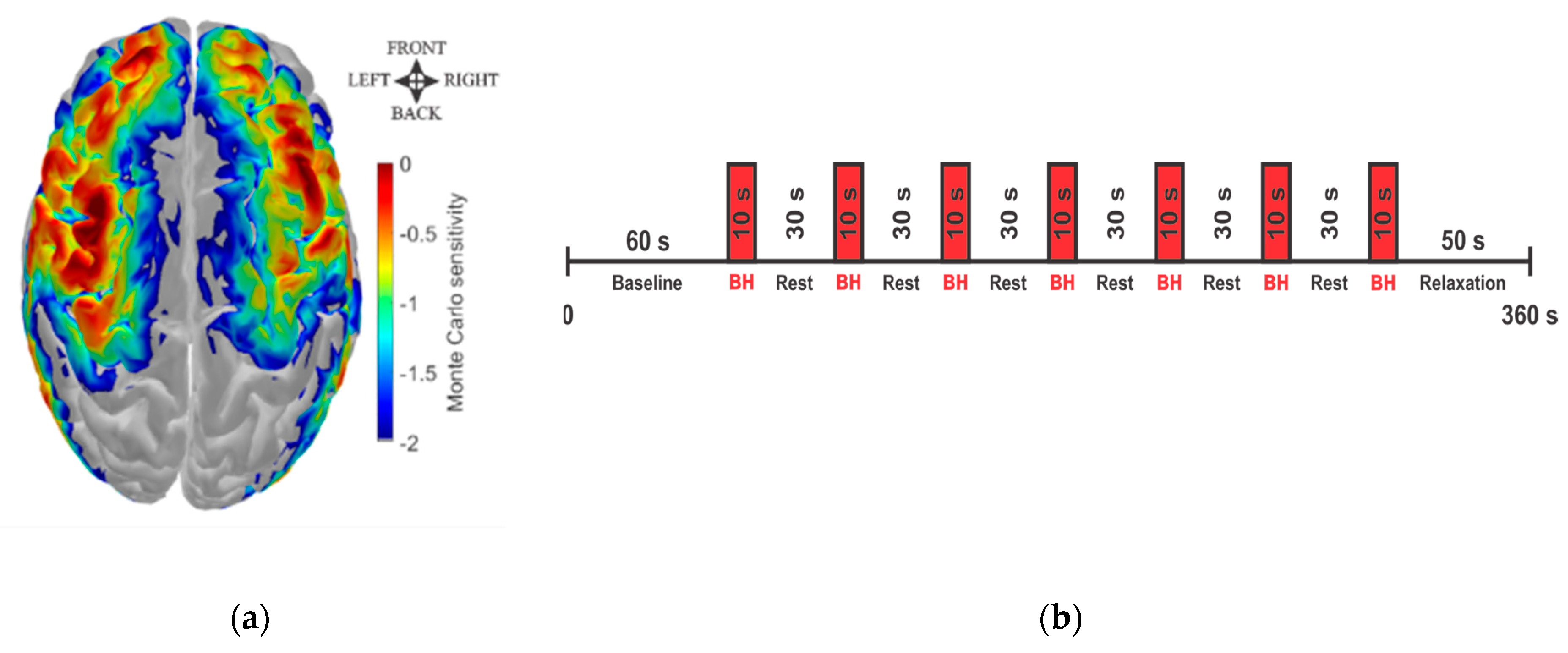
2.2. fNIRS Data Acquisition
2.3. fNIRS Data Processing
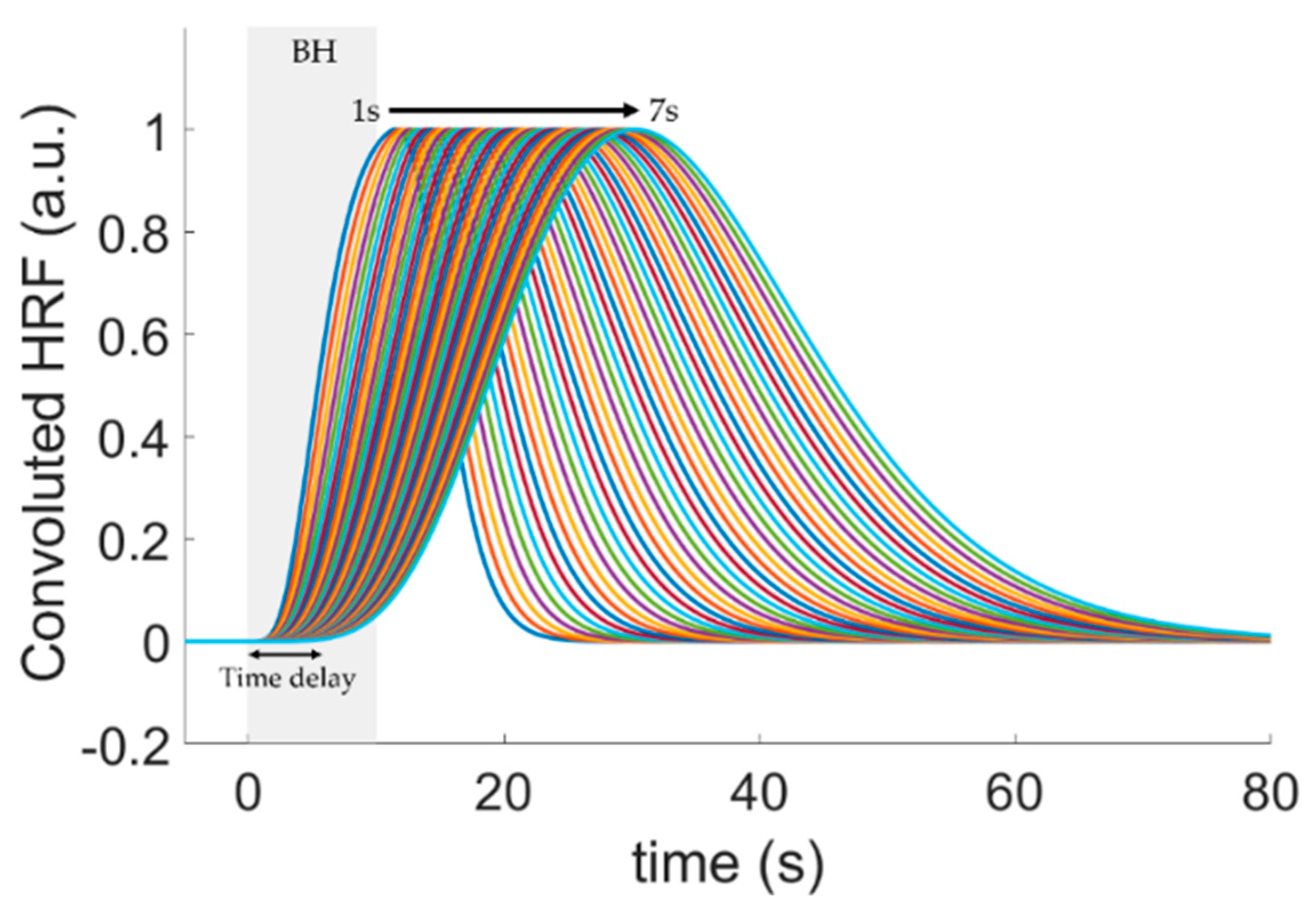
2.4. Quantification of the Hemodynamic Delay
2.5. Statistical Analysis
3. Results
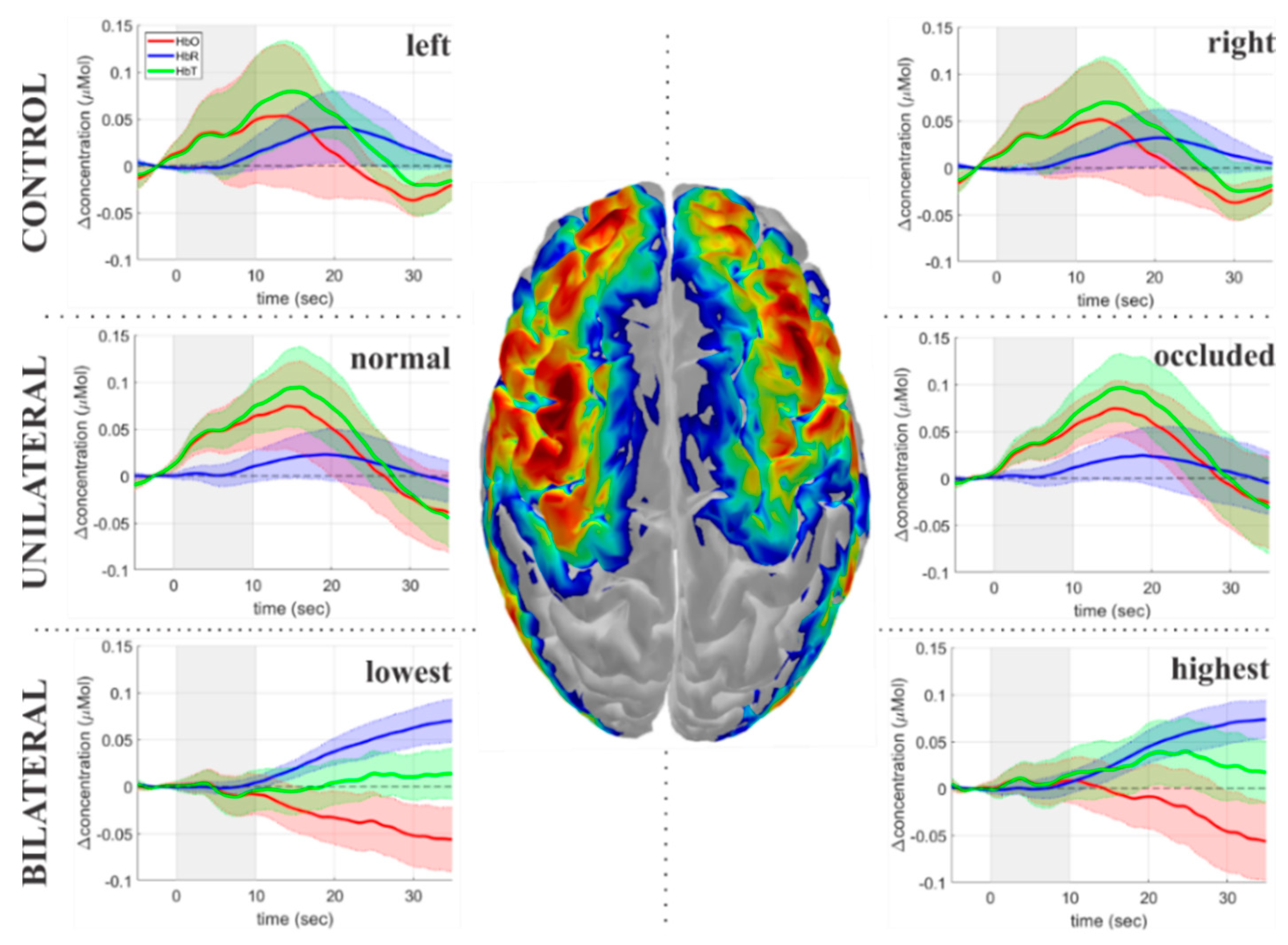
3.1. Temporal Dynamics of the Hemodynamic Response to Breath-Holding
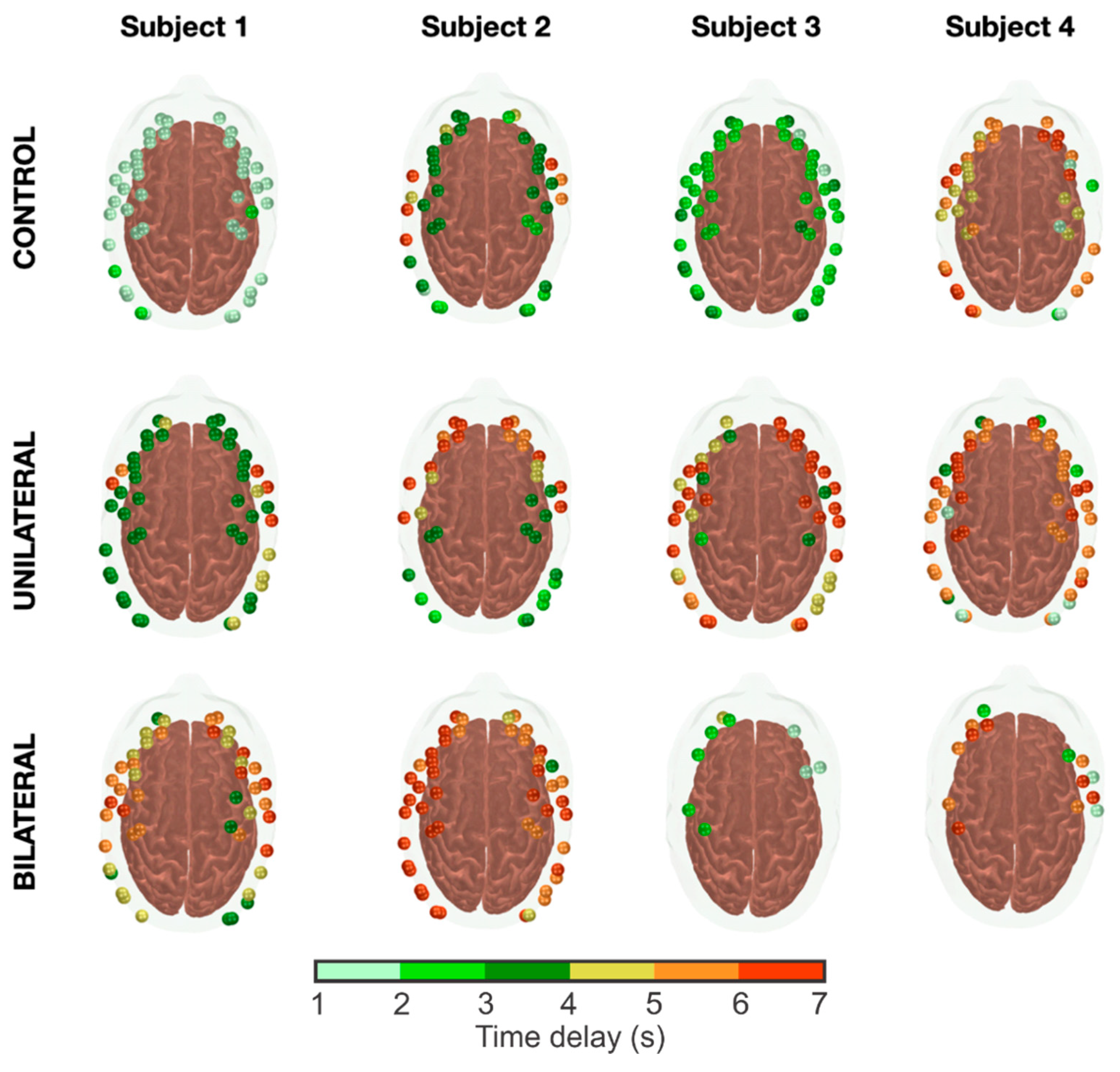
3.2. Characterization of the Hemodynamic Response Delays across Groups
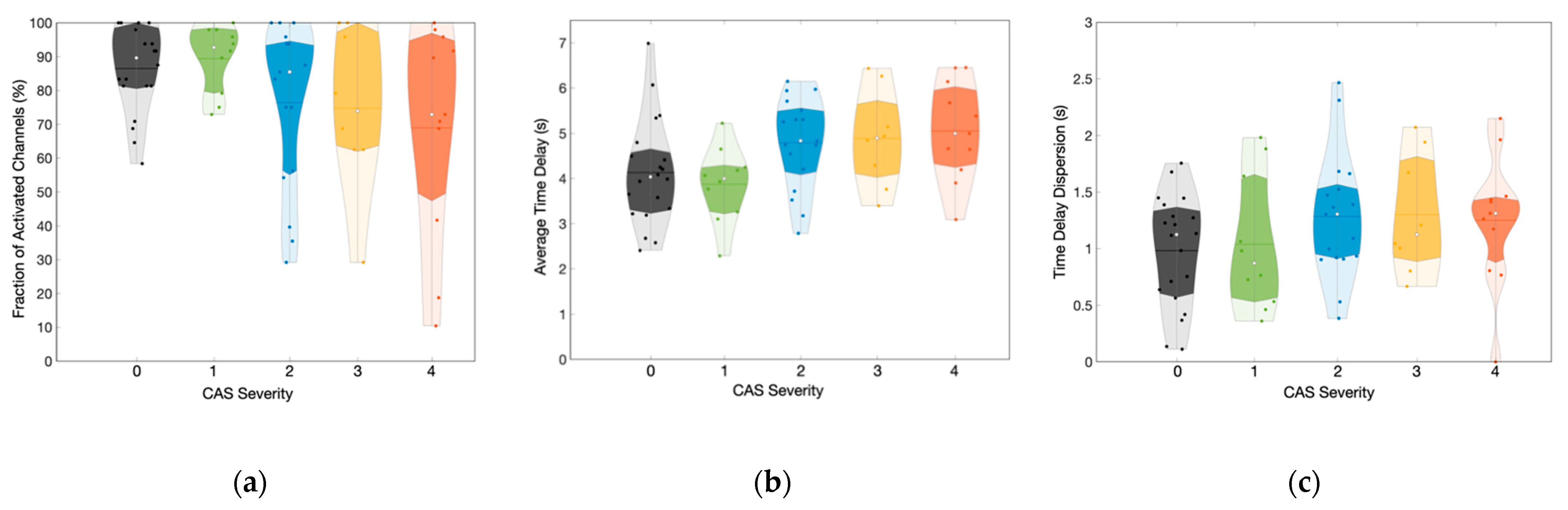
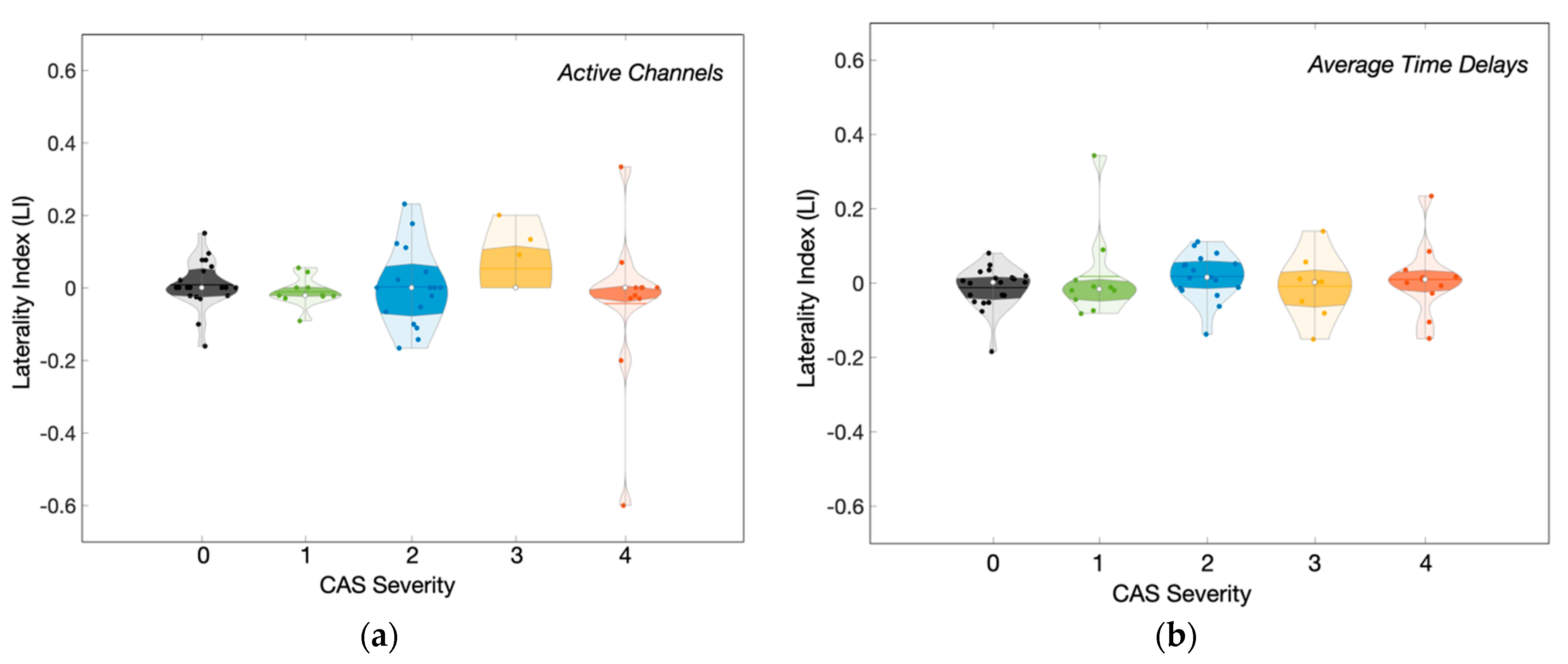
3.3. Influence of CAS Severity on the Hemodynamic Response Delays across Groups
3.4. Modeling of the Hemodynamic Response Delays as a Function of the CAS Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; Fowkes, F.J.I.; Rudan, I. Global and Regional Prevalence, Burden, and Risk Factors for Carotid Atherosclerosis: A Systematic Review, Meta-Analysis, and Modelling Study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef]
- Porcu, M.; Mannelli, L.; Melis, M.; Suri, J.S.; Gerosa, C.; Cerrone, G.; Defazio, G.; Faa, G.; Saba, L. Carotid Plaque Imaging Profiling in Subjects with Risk Factors (Diabetes and Hypertension). Cardiovasc. Diagn. Ther. 2020, 10, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Gupta, A.; Baradaran, H.; Schweitzer, A.D. Carotid Plaque MRI and Stroke Risk: A Systematic Review and Meta-Analysis. J. Vasc. Surg. 2014, 59, 1175. [Google Scholar] [CrossRef]
- Flaherty, M.L.; Kissela, B.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Ferioli, S.; Adeoye, O.; Broderick, J.P.; et al. Carotid Artery Stenosis as a Cause of Stroke. Neuroepidemiology 2013, 40, 36–41. [Google Scholar] [CrossRef]
- Marshall, R.S.; Pavol, M.A.; Cheung, Y.K.; Strom, I.; Slane, K.; Asllani, I.; Lazar, R.M. Dissociation among Hemodynamic Measures in Asymptomatic High Grade Carotid Artery Stenosis. J. Neurol. Sci. 2016, 367, 143–147. [Google Scholar] [CrossRef]
- Balestrini, S.; Perozzi, C.; Altamura, C.; Vernieri, F.; Luzzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Severe Carotid Stenosis and Impaired Cerebral Hemodynamics Can Influence Cognitive Deterioration. Neurology 2013, 80, 2145–2150. [Google Scholar] [CrossRef]
- Buratti, L.; Viticchi, G.; Falsetti, L.; Balucani, C.; Altamura, C.; Petrelli, C.; Provinciali, L.; Vernieri, F.; Silvestrini, M. Thresholds of Impaired Cerebral Hemodynamics That Predict Short-Term Cognitive Decline in Asymptomatic Carotid Stenosis. J. Cereb. Blood Flow Metab. 2016, 36, 1804–1812. [Google Scholar] [CrossRef]
- Shakur, S.F.; Hrbac, T.; Alaraj, A.; Du, X.; Aletich, V.A.; Charbel, F.T.; Amin-Hanjani, S. Effects of Extracranial Carotid Stenosis on Intracranial Blood Flow. Stroke 2014, 45, 3427–3429. [Google Scholar] [CrossRef]
- Derdeyn, C.P.; Grubb, R.L.; Powers, W.J. Cerebral Hemodynamic Impairment: Methods of Measurement and Association with Stroke Risk. Neurology 1999, 53, 251. [Google Scholar] [CrossRef]
- Cigada, M.; Marzorati, S.; Tredici, S.; Iapichino, G. Cerebral CO2 Vasoreactivity Evaluation by Transcranial Doppler Ultrasound Technique: A Standardized Methodology. Intensive Care Med. 2000, 26, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.; Russell, D.; Nyberg-Hansen, R.; Rootwelt, K.; Bakke, S.J. Cerebral Vasoreactivity in Unilateral Carotid Artery Disease. A Comparison of Blood Flow Velocity and Regional Cerebral Blood Flow Measurements. Stroke 1994, 25, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Baradaran, H.; Schweitzer, A.D.; Kamel, H.; Pandya, A.; Delgado, D.; Wright, D.; Hurtado-Rua, S.; Wang, Y.; Sanelli, P.C. Oxygen Extraction Fraction and Stroke Risk in Patients with Carotid Stenosis or Occlusion: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2014, 35, 250–255. [Google Scholar] [CrossRef]
- Grubb, J.; Robert, L.; Derdeyn, C.P.; Fritsch, S.M.; Carpenter, D.A.; Yundt, K.D.; Videen, T.O.; Spitznagel, E.L.; Powers, W.J. Importance of Hemodynamic Factors in the Prognosis of Symptomatic Carotid Occlusion. JAMA 1998, 280, 1055–1060. [Google Scholar] [CrossRef]
- Markus, H.S.; Harrison, M.J. Estimation of Cerebrovascular Reactivity Using Transcranial Doppler, Including the Use of Breath-Holding as the Vasodilatory Stimulus. Stroke 1992, 23, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Voges, M.; Piepgras, U.; Schimrigk, K. Assessment of Cerebral Vasomotor Reactivity by Transcranial Doppler Ultrasound and Breath-Holding. Stroke 1995, 26, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. A Mini-Review on Functional Near-Infrared Spectroscopy (FNIRS): Where Do We Stand, and Where Should We Go? Photonics 2019, 6, 87. [Google Scholar] [CrossRef]
- Yücel, M.A.; Selb, J.J.; Huppert, T.J.; Franceschini, M.A.; Boas, D.A. Functional Near Infrared Spectroscopy: Enabling Routine Functional Brain Imaging. Curr. Opin. Biomed. Eng. 2017, 4, 78–86. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (FNIRS) Development and Fields of Application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Mesquita, R.C.; Yodh, A.G. Diffuse Optics: Fundamentals and Tissue Applications. Nano Opt. At. Transp. Light Matter Waves 2011, 173, 51–74. [Google Scholar] [CrossRef]
- Durduran, T.; Yu, G.; Burnett, M.G.; Detre, J.A.; Greenberg, J.H.; Wang, J.; Zhou, C.; Yodh, A.G. Diffuse Optical Measurement of Blood Flow, Blood Oxygenation, and Metabolism in a Human Brain during Sensorimotor Cortex Activation. Opt. Lett. 2004, 29, 1766. [Google Scholar] [CrossRef] [PubMed]
- Forti, R.M.; Katsurayama, M.; Valler, L.; Quiroga, A.; Simioni, L.; Menko, J.; Falcão, A.L.E.; Li, L.M.; Mesquita, R.C. Real-Time Monitoring of Neurocritical Patients with Diffuse Optical Spectroscopies. JoVE J. Vis. Exp. 2020, 165, e61608. [Google Scholar] [CrossRef]
- Forti, R.M.; Katsurayama, M.; Menko, J.; Valler, L.; Quiroga, A.; Falcão, A.L.E.; Li, L.M.; Mesquita, R.C. Real-Time Non-Invasive Assessment of Cerebral Hemodynamics With Diffuse Optical Spectroscopies in a Neuro Intensive Care Unit: An Observational Case Study. Front. Med. 2020, 7, 147. [Google Scholar] [CrossRef]
- Novi, S.L.; Forero, E.J.; Rubianes Silva, J.A.I.; de Souza, N.G.S.R.; Martins, G.G.; Quiroga, A.; Wu, S.-T.; Mesquita, R.C. Integration of Spatial Information Increases Reproducibility in Functional Near-Infrared Spectroscopy. Front. Neurosci. 2020, 14, 337–348. [Google Scholar] [CrossRef]
- Nakagawa, I.; Yokoyama, S.; Wajima, D.; Nishimura, F.; Yamada, S.; Yokota, H.; Motoyama, Y.; Park, Y.S.; Wada, T.; Kichikawa, K.; et al. Hyperventilation and Breath-Holding Test with Indocyanine Green Kinetics Predicts Cerebral Hyperperfusion after Carotid Artery Stenting. J. Cereb. Blood Flow Metab. 2019, 39, 901–912. [Google Scholar] [CrossRef]
- Forero, E.; Novi, S.; Avelar, W.; Anjos, C.; Menko, J.; Forti, R.; Oliveira, V.; Cendes, F.; Covolan, R.; Mesquita, R. Use of Near-Infrared Spectroscopy to Probe Occlusion Severity in Patients Diagnosed with Carotid Atherosclerotic Disease. Med. Res. Arch. 2017, 5, 1–22. [Google Scholar] [CrossRef]
- Zirak, P.; Delgado-Mederos, R.; Dinia, L.; Martí-Fàbregas, J.; Durduran, T. Microvascular versus Macrovascular Cerebral Vasomotor Reactivity in Patients with Severe Internal Carotid Artery Stenosis or Occlusion. Acad. Radiol. 2014, 21, 168–174. [Google Scholar] [CrossRef]
- Vasdekis, S.N.; Tsivgoulis, G.; Athanasiadis, D.; Andrikopoulou, A.; Voumvourakis, K.; Lazaris, A.M.; Stamboulis, E. Cerebrovascular Reacivity Assessment in Patients with Carotid Artery Disease: A Combined TCD and NIRS Study. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2012, 22, 261–265. [Google Scholar] [CrossRef]
- Vernieri, F.; Tibuzzi, F.; Pasqualetti, P.; Rosato, N.; Passarelli, F.; Rossini, P.M.; Silvestrini, M. Transcranial Doppler and Near-Infrared Spectroscopy Can Evaluate the Hemodynamic Effect of Carotid Artery Occlusion. Stroke 2004, 35, 64–70. [Google Scholar] [CrossRef]
- Smielewski, P.; Czosnyka, M.; Pickard, J.D.; Kirkpatrick, P. Clinical Evaluation of Near-Infrared Spectroscopy for Testing Cerebrovascular Reactivity in Patients With Carotid Artery Disease. Stroke 1997, 28, 331–338. [Google Scholar] [CrossRef]
- Aasted, C.M.; Yücel, M.A.; Cooper, R.J.; Dubb, J.; Tsuzuki, D.; Becerra, L.; Petkov, M.P.; Borsook, D.; Dan, I.; Boas, D.A. Anatomical Guidance for Functional Near-Infrared Spectroscopy: AtlasViewer Tutorial. Neurophotonics 2015, 2, 020801. [Google Scholar] [CrossRef] [PubMed]
- Novi, S.L.; Roberts, E.; Spagnuolo, D.; Spilsbury, B.M.; Price, D.C.; Imbalzano, C.A.; Forero, E.; Yodh, A.G.; Tellis, G.M.; Tellis, C.M.; et al. Functional Near-Infrared Spectroscopy for Speech Protocols: Characterization of Motion Artifacts and Guidelines for Improving Data Analysis. Neurophotonics 2020, 7, 015001. [Google Scholar] [CrossRef] [PubMed]
- Santosa, H.; Fishburn, F.; Zhai, X.; Huppert, T.J. Investigation of the Sensitivity-Specificity of Canonical- and Deconvolution-Based Linear Models in Evoked Functional near-Infrared Spectroscopy. Neurophotonics 2019, 6, 025009. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Yu, C.-H.; Sun, C.-W. Altered Near-Infrared Spectroscopy Response to Breath-Holding in Patients with Fibromyalgia. J. Biophotonics 2019, 12, e201800142. [Google Scholar] [CrossRef]
- Emir, U.E.; Ozturk, C.; Akin, A. Multimodal Investigation of FMRI and FNIRS Derived Breath Hold BOLD Signals with an Expanded Balloon Model. Physiol. Meas. 2007, 29, 49–63. [Google Scholar] [CrossRef]
- Vogt, W.C.; Romero, E.; LaConte, S.M.; Rylander, C.G. Mechanical Indentation Improves Cerebral Blood Oxygenation Signal Quality of Functional Near-Infrared Spectroscopy (FNIRS) during Breath Holding. In Proceedings of the Optical Tomography and Spectroscopy of Tissue X, San Francisco, CA, USA, 25 March 2013; Volume 8578, pp. 468–474. [Google Scholar] [CrossRef]
- Molinari, F.; Liboni, W.; Grippi, G.; Negri, E. Relationship between Oxygen Supply and Cerebral Blood Flow Assessed by Transcranial Doppler and near—Infrared Spectroscopy in Healthy Subjects during Breath—Holding. J. NeuroEngineering Rehabil. 2006, 3, 16. [Google Scholar] [CrossRef][Green Version]
- MacIntosh, B.J.; Klassen, L.M.; Menon, R.S. Transient Hemodynamics during a Breath Hold Challenge in a Two Part Functional Imaging Study with Simultaneous Near-Infrared Spectroscopy in Adult Humans. NeuroImage 2003, 20, 1246–1252. [Google Scholar] [CrossRef]
- Uga, M.; Dan, I.; Sano, T.; Dan, H.; Watanabe, E. Optimizing the General Linear Model for Functional Near-Infrared Spectroscopy: An Adaptive Hemodynamic Response Function Approach. Neurophotonics 2014, 1, 015004. [Google Scholar] [CrossRef]
- Bouten, J.; Bourgois, J.G.; Boone, J. Hold Your Breath: Peripheral and Cerebral Oxygenation during Dry Static Apnea. Eur. J. Appl. Physiol. 2020, 120, 2213–2222. [Google Scholar] [CrossRef]
- Shin, J.; Kwon, J.; Choi, J.; Im, C.-H. Ternary Near-Infrared Spectroscopy Brain-Computer Interface With Increased Information Transfer Rate Using Prefrontal Hemodynamic Changes During Mental Arithmetic, Breath-Holding, and Idle State. IEEE Access 2018, 6, 19491–19498. [Google Scholar] [CrossRef]
- Viola, S.; Viola, P.; Litterio, P.; Buongarzone, M.P.; Fiorelli, L. Correlation between the Arterial Pulse Wave of the Cerebral Microcirculation and CBF during Breath Holding and Hyperventilation in Human. Clin. Neurophysiol. 2012, 123, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Norrving, B.; Nilsson, B.; Risberg, J. RCBF in Patients with Carotid Occlusion. Resting and Hypercapnic Flow Related to Collateral Pattern. Stroke 1982, 13, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Sharma, V.K.; Lao, A.Y.; Malkoff, M.D.; Alexandrov, A.V. Validation of Transcranial Doppler With Computed Tomography Angiography in Acute Cerebral Ischemia. Stroke 2007, 38, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Demchuk, A.M.; Christou, I.; Wein, T.H.; Felberg, R.A.; Malkoff, M.; Grotta, J.C.; Alexandrov, A.V. Specific Transcranial Doppler Flow Findings Related to the Presence and Site of Arterial Occlusion. Stroke 2000, 31, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Badacz, R.; Przewłocki, T.; Karch, I.; Pieniążek, P.; Rosławiecka, A.; Mleczko, S.; Brzychczy, A.; Trystuła, M.; Żmudka, K.; Kabłak-Ziembicka, A. Low Prevalence of Collateral Cerebral Circulation in the Circle of Willis in Patients with Severe Carotid Artery Stenosis and Recent Ischemic Stroke. Postępy W Kardiol. Interwencyjnej Adv. Interv. Cardiol. 2015, 11, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yuan, Q.; Yang, J.; Yeo, J.H. The Role of the Circle of Willis in Internal Carotid Artery Stenosis and Anatomical Variations: A Computational Study Based on a Patient-Specific Three-Dimensional Model. Biomed. Eng. OnLine 2015, 14, 107. [Google Scholar] [CrossRef]
- Fang, H.; Song, B.; Cheng, B.; Wong, K.S.; Xu, Y.M.; Ho, S.S.Y.; Chen, X.Y. Compensatory Patterns of Collateral Flow in Stroke Patients with Unilateral and Bilateral Carotid Stenosis. BMC Neurol. 2016, 16, 39. [Google Scholar] [CrossRef]

| Parameter | Patients | Controls | |
|---|---|---|---|
| Unilateral | Bilateral | ||
| Number of subjects | 31 | 19 | 20 |
| Mean age (years, SD) | 67 (8) | 68 (7) | 63 (8) |
| Female | 7 (22.5%) | 5 (26.3%) | 6 (30%) |
| Location of stenosis | |||
| left ICA | 9 (29%) | 18 (94.7%) | - |
| right ICA | 19 (61.2%) | 18 (94.7%) | - |
| left CCA | 2 (6.5%) | 1 (5.3%) | - |
| right CCA | 1 (3.2%) | 1 (5.3%) | - |
| Degree of stenosis | |||
| 0–49% | 1(3.2%) | 0(0%) | - |
| 50–69% | 9 (29%) | 0(0%) | - |
| 70–90% | 20 (64.5%) | 13 (68.4%) | - |
| Occluded | 1 (3.2%) | 6 (31.6%) | - |
| Presence of collateral circulation | |||
| Anterior communicating arteries | 6 (40%) | 11 (69%) | - |
| Right posterior comm. arteries | 4 (27%) | 6 (38%) | - |
| Left posterior comm. arteries | 3 (20%) | 4 (25%) | - |
| Asymptomatic | 9 (29%) | 4 (21%) | - |
| Symptomatic | |||
| Transient ischemic attack | 2 (6.5%) | 3 (15.7%) | - |
| Ischemic stroke | 20 (64.5%) | 12 (63.1%) | - |
| Other conditions | |||
| Hypertension | 27 (87.1%) | 16 (84.2%) | 10 (50%) |
| Diabetes | 21 (67.7%) | 7 (36.8%) | 7 (35%) |
| Smoking | 18 (58%) | 10 (52.6%) | 5 (25%) |
| Dyslipidemia | 19 (61.2%) | 13 (68.4%) | 4 (20%) |
| Heart failure | 3 (9.6%) | 2 (10%) | 0 (0%) |
| Coronary artery disease | 2 (6.5%) | 4 (21%) | 0 (0%) |
| Chronic kidney insufficiency | 2 (6.5%) | 3 (15%) | 2 (10%) |
| Etilism | 11 (35.5%) | 6 (31.5%) | 0 (0%) |
| Obesity | 0 (0%) | 1 (5.2%) | 0 (0%) |
| Group | Stenosis Severity | Number of Subjects | Hemodynamic Features | ||
|---|---|---|---|---|---|
| Fraction Activated Channels | Time Delay | Laterality Index (LI) | |||
| Control | 0 | 20 | 90 (81; 99) % | 4.0 (3.3; 4.6) s | 0.00 (−0.04; 0.02) |
| Unilateral | 1 | 10 | 93 (79; 98) % | 4.0 (3.3; 4.3) s | −0.02 (−0.05; 0.01) |
| 2 | 17 | 85 (56; 94) % | 4.8 (4.1; 5.6) s | 0.02 (−0.01; 0.06) | |
| Bilateral | 3 | 9 | 74 (63; 98) % | 4.9 (4.0; 5.7) s | 0.00 (−0.07; 0.03) |
| 4 | 11 | 73 (48; 95) % | 5.0 (4.3; 6.0) s | 0.01 (−0.02; 0.03) | |
| Group | Stenosis Severity | Number of Subjects | Total Collateral Circulation | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Unilateral | 1 | 7 | 2 (29%) | 4 (57%) | 1 (14%) | 0 (0%) |
| 2 | 14 | 5 (36%) | 6 (43%) | 3 (21%) | 0 (0%) | |
| Bilateral | 3 | 8 | 0 (0%) | 5 (63%) | 2 (25%) | 1 (13%) |
| 4 | 9 | 3 (33%) | 4 (44%) | 0 (0%) | 2 (22%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga, A.; Novi, S.; Martins, G.; Bortoletto, L.F.; Avelar, W.; Guillaumon, A.T.; Li, L.M.; Cendes, F.; Mesquita, R.C. Quantification of the Tissue Oxygenation Delay Induced by Breath-Holding in Patients with Carotid Atherosclerosis. Metabolites 2022, 12, 1156. https://doi.org/10.3390/metabo12111156
Quiroga A, Novi S, Martins G, Bortoletto LF, Avelar W, Guillaumon AT, Li LM, Cendes F, Mesquita RC. Quantification of the Tissue Oxygenation Delay Induced by Breath-Holding in Patients with Carotid Atherosclerosis. Metabolites. 2022; 12(11):1156. https://doi.org/10.3390/metabo12111156
Chicago/Turabian StyleQuiroga, Andrés, Sergio Novi, Giovani Martins, Luis Felipe Bortoletto, Wagner Avelar, Ana Terezinha Guillaumon, Li Min Li, Fernando Cendes, and Rickson C. Mesquita. 2022. "Quantification of the Tissue Oxygenation Delay Induced by Breath-Holding in Patients with Carotid Atherosclerosis" Metabolites 12, no. 11: 1156. https://doi.org/10.3390/metabo12111156
APA StyleQuiroga, A., Novi, S., Martins, G., Bortoletto, L. F., Avelar, W., Guillaumon, A. T., Li, L. M., Cendes, F., & Mesquita, R. C. (2022). Quantification of the Tissue Oxygenation Delay Induced by Breath-Holding in Patients with Carotid Atherosclerosis. Metabolites, 12(11), 1156. https://doi.org/10.3390/metabo12111156








