Assessing the Sensitivity of Multi-Distance Hyperspectral NIRS to Changes in the Oxidation State of Cytochrome C Oxidase in the Brain
Abstract
1. Introduction
2. Methods
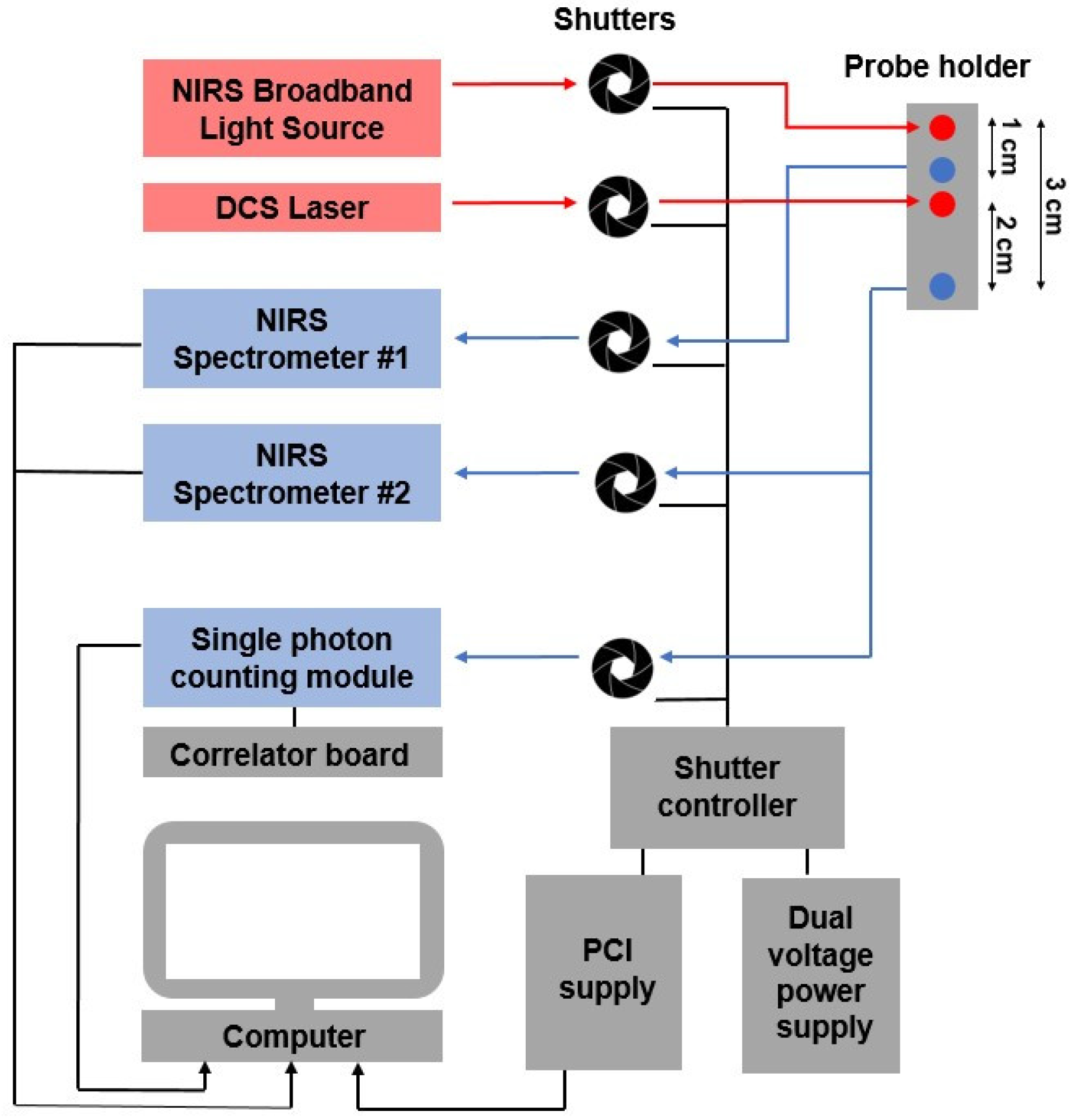
2.1. Instrumentation
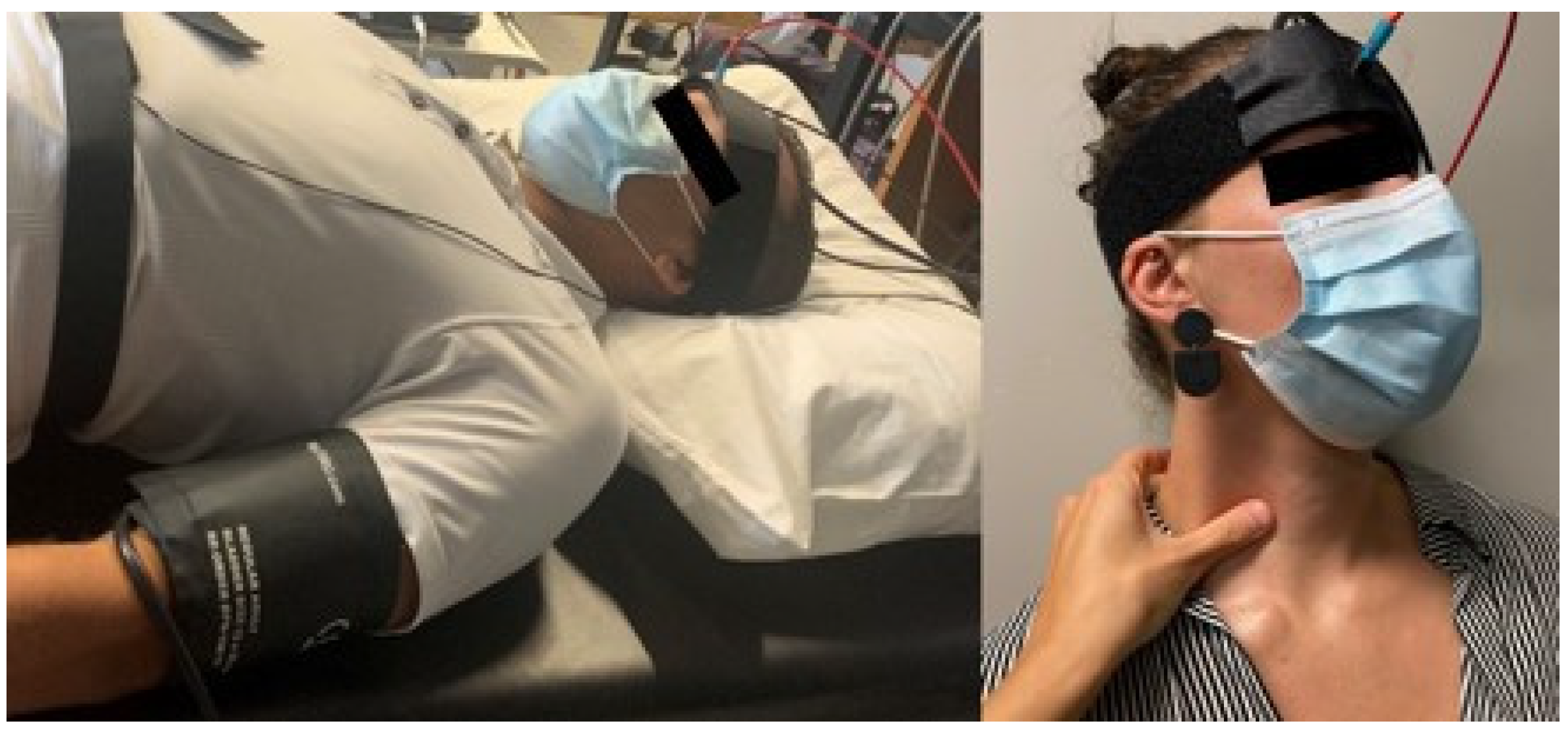
2.2. Experimental Protocol
2.2.1. Carotid Compressions (CC)
2.2.2. Hypercapnia
2.3. Data Analysis
2.3.1. Hyperspectral NIRS
2.3.2. DCS
2.3.3. Regression Analysis
2.3.4. Cerebrovascular Reactivity
2.3.5. Statistical Analysis
3. Results
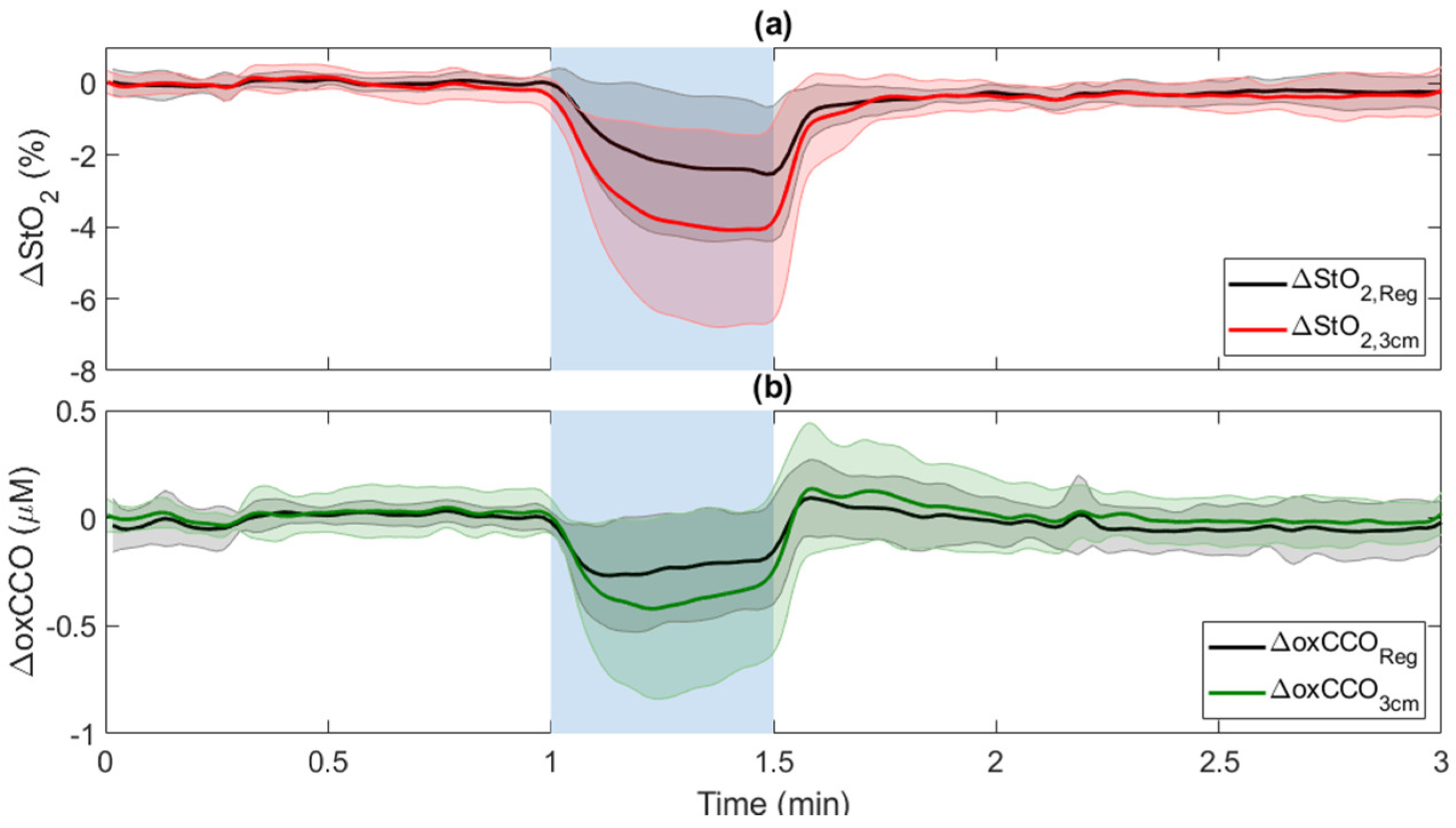
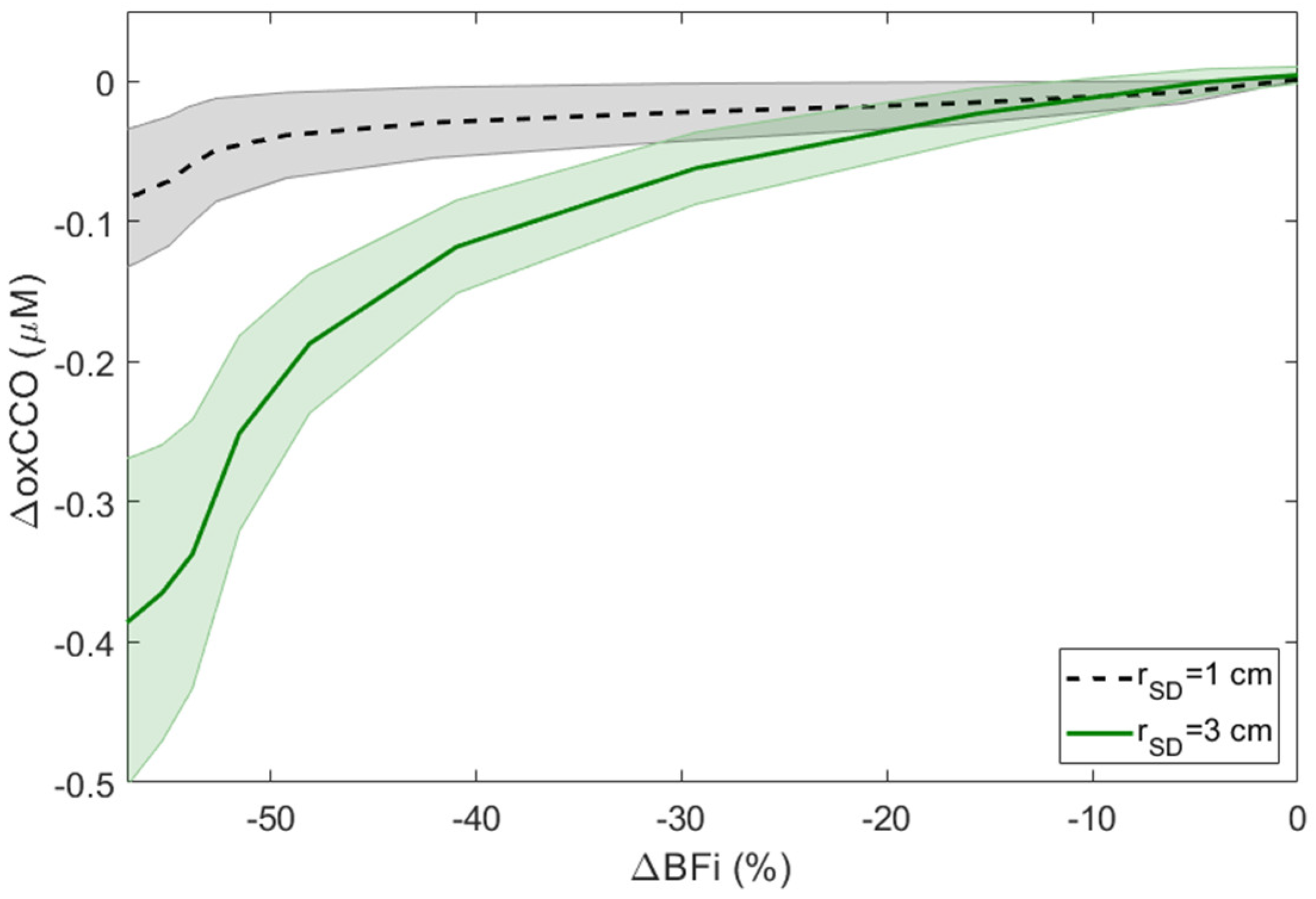
3.1. Carotid Compressions (CC)
3.2. Hypercapnia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sultan, I.; Bianco, V.; Kilic, A.; Jovin, T.; Jadhav, A.; Jankowitz, B.; Aranda-Michel, E.; D’angelo, M.P.; Navid, F.; Wang, Y.; et al. Predictors and Outcomes of Ischemic Stroke after Cardiac Surgery. Ann. Thorac. Surg. 2020, 110, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Bucerius Stroke after cardiac surgery: A risk factor analysis of 16,184 consecutive adult patients: Invited commentary. Ann. Thorac. Surg. 2003, 75, 478. [CrossRef]
- Khattar, N.K.; Friedlander, R.M.; Chaer, R.A.; Avgerinos, E.D.; Kretz, E.S.; Balzer, J.R.; Crammond, D.J.; Habeych, M.H.; Thirumala, P.D. Perioperative stroke after carotid endarterectomy: Etiology and implications. Acta Neurochir. 2016, 158, 2377–2383. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, E.J.; Jung, S.H.; Lee, J.W.; Kim, J.S.; Kim, J.B.; Kwon, S.U. Cerebral atherosclerosis and early ischemic stroke after left-sided valve replacement surgery. J. Thorac. Cardiovasc. Surg. 2022, 163, 967–976.e6. [Google Scholar] [CrossRef]
- Ottens, T.H.; Hendrikse, J.; Slooter, A.J.C.; Van Herwerden, L.A.; Dieleman, J.M.; Dijk, D. Van Low Incidence of Early Postoperative Cerebral Edema after Coronary Artery Bypass Grafting. J. Cardiothorac. Vasc. Anesth. 2015, 29, 632–636. [Google Scholar] [CrossRef]
- Aceto, P.; Lai, C.; de Crescenzo, F.; Crea, M.A.; Di Franco, V.; Pellicano, G.R.; Perilli, V.; Lai, S.; Papanice, D.; Sollazzi, L. Cognitive decline after carotid endarterectomy: Systematic review and meta-analysis. Eur. J. Anaesthesiol. 2020, 37, 1066–1074. [Google Scholar] [CrossRef]
- Kertai, M.D.; Whitlock, E.L.; Avidan, M.S. Brain monitoring with electroencephalography and the electroencephalogram- derived bispectral index during cardiac surgery. Anesth. Analg. 2012, 114, 533–543. [Google Scholar] [CrossRef]
- Sultan, I.; Brown, J.A.; Serna-gallegos, D.; Thirumala, P.D.; Balzer, J.R.; Paras, S.; Fleseriu, C.; Crammond, D.J.; Anetakis, K.M.; Kilic, A.; et al. Intraoperative neurophysiologic monitoring during aortic arch surgery. J. Thorac. Cardiovasc. Surg. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Murkin, J.M.; Kamar, M.; Silman, Z.; Balberg, M.; Adams, S.J. Intraoperative Cerebral Autoregulation Assessment Using Ultrasound-Tagged Near-Infrared-Based Cerebral Blood Flow in Comparison to Transcranial Doppler Cerebral Flow Velocity: A Pilot Study. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1187–1193. [Google Scholar] [CrossRef]
- Lewis, C.; Parulkar, S.D.; Bebawy, J.; Sherwani, S.; Hogue, C.W. Cerebral Neuromonitoring During Cardiac Surgery: A Critical Appraisal with an Emphasis on Near-Infrared Spectroscopy. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2313–2322. [Google Scholar] [CrossRef]
- Florence, G.; Guerit, J.M.; Gueguen, B. Electroencephalography (EEG) and somatosensory evoked potentials (SEP) to prevent cerebral ischaemia in the operating room. Neurophysiol. Clin. 2004, 34, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, H.L.; Rodriguez, R.A.; Audenaert, S.M.; Austin, E.H.; Pollock, S.B.; Ganzel, B.L. The role of neuromonitoring in cardiovascular surgery. J. Cardiothorac. Vasc. Anesth. 1996, 10, 15–23. [Google Scholar] [CrossRef]
- Cho, J.W.; Jang, J.S. Near-infrared spectroscopy versus transcranial doppler-based monitoring in carotid endarterectomy. Korean J. Thorac. Cardiovasc. Surg. 2017, 50, 448–452. [Google Scholar] [CrossRef]
- Milej, D.; Abdalmalak, A.; Rajaram, A.; Lawrence, K.S. Direct assessment of extracerebral signal contamination on optical measurements of cerebral blood flow, oxygenation, and metabolism. Neurophotonics 2020, 7, 045002. [Google Scholar] [CrossRef] [PubMed]
- Kovacsova, Z.; Bale, G.; Mitra, S.; Lange, F.; Tachtsidis, I. Absolute quantification of cerebral tissue oxygen saturation with multidistance broadband NIRS in newborn brain. Biomed. Opt. Express 2021, 12, 907. [Google Scholar] [CrossRef]
- Dix, L.M.L.; Van Bel, F.; Baerts, W.; Lemmers, P.M.A. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr. Res. 2013, 74, 557–563. [Google Scholar] [CrossRef]
- Kondov, S.; Beyersdorf, F.; Schöllhorn, J.; Benk, C.; Rylski, B.; Czerny, M.; Harloff, A.; Siepe, M. Outcome of Near-Infrared Spectroscopy–Guided Selective Shunting during Carotid Endarterectomy in General Anesthesia. Ann. Vasc. Surg. 2019, 61, 170–177. [Google Scholar] [CrossRef]
- Bale, G.; Elwell, C.E.; Tachtsidis, I. From Jöbsis to the present day: A review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J. Biomed. Opt. 2016, 21, 091307. [Google Scholar] [CrossRef]
- Cooper, C.E.; Cope, M.; Springett, R.; Amess, P.N.; Penrice, J.; Tyszczuk, L.; Punwani, S.; Ordidge, R.; Wyatt, J.; Delpy, D.T. Use of mitochondrial inhibitors to demonstrate that cytochrome oxidase near-infrared spectroscopy can measure mitochondrial dysfunction noninvasively in the brain. J. Cereb. Blood Flow Metab. 1999, 19, 27–38. [Google Scholar] [CrossRef]
- Cooper, C.E. The steady-state kinetics of cytochrome c oxidation by cytochrome oxidase. Biochim. Biophys. Acta (BBA)-Bioenerg. 1990, 1017, 187–203. [Google Scholar] [CrossRef]
- Milej, D.; Rajaram, A.; Suwalski, M.; Morrison, L.B.; Shoemaker, L.N.; Lawrence, K.S. Assessing the relationship between the cerebral metabolic rate of oxygen and the oxidation state of cytochrome-c-oxidase. Neurophotonics 2022, 9, 035001–035002. [Google Scholar] [CrossRef] [PubMed]
- Bale, G.; Rajaram, A.; Kewin, M.; Morrison, L.; Bainbridge, A.; Diop, M.; Lawrence, K.S.; Tachtsidis, I. Broadband NIRS cerebral cytochrome-C-oxidase response to anoxia before and after hypoxic-ischaemic injury in piglets. Adv. Exp. Med. Biol. 2018, 1072, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Vezyroglou, A.; Hebden, P.; De Roever, I.; Thornton, R.; Mitra, S.; Worley, A.; Alves, M.; Dean, E.; Cross, J.H.; Tachtsidis, I. Broadband-NIRS System Identifies Epileptic Focus in a Child with Focal Cortical Dysplasia—A Case Study. Metabolites 2022, 12, 260. [Google Scholar] [CrossRef]
- Bale, G.; Mitra, S.; de Roever, I.; Chan, M.; Caicedo-Dorado, A.; Meek, J.; Robertson, N.; Tachtsidis, I. Interrelationship Between Broadband NIRS Measurements of Cerebral Cytochrome C Oxidase and Systemic Changes Indicates Injury Severity in Neonatal Encephalopathy. Adv. Exp. Med. Biol. 2016, 923, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, K.; Diop, M.; Lee, A.; Morrison, L.B.; Lee, T.-Y.; Lawrence, K.S. Assessment of a multi-layered diffuse correlation spectroscopy method for monitoring cerebral blood flow in adults. Biomed. Opt. Express 2016, 7, 3659. [Google Scholar] [CrossRef] [PubMed]
- Phan, P.; Highton, D.; Lai, J.; Smith, M.; Elwell, C.; Tachtsidis, I. Multi-channel multi-distance broadband near-infrared spectroscopy system to measure the spatial response of cellular oxygen metabolism and tissue oxygenation. Biomed. Opt. Express 2016, 7, 4424. [Google Scholar] [CrossRef]
- Rajaram, A.; Milej, D.; Suwalski, M.; Yip, L.C.M.; Guo, L.R.; Chu, M.W.A.; Chui, J.; Diop, M.; Murkin, J.M.; Lawrence, K.S. Optical monitoring of cerebral perfusion and metabolism in adults during cardiac surgery with cardiopulmonary bypass. Biomed. Opt. Express 2020, 11, 5967. [Google Scholar] [CrossRef]
- Kolyva, C.; Ghosh, A.; Tachtsidis, I.; Highton, D.; Cooper, C.E.; Smith, M.; Elwell, C.E. Cytochrome c oxidase response to changes in cerebral oxygen delivery in the adult brain shows higher brain-specificity than haemoglobin. Neuroimage 2014, 85, 234–244. [Google Scholar] [CrossRef]
- Ferrari, M.; Zanette, E.; Sideri, G.; Giannini, I.; Fieschi, C.; Carpi, A. Effects of carotid compression, as assessed by near infrared spectroscopy, upon cerebral blood volume and haemoglobin oxygen saturation. J. R. Soc. Med. 1987, 80, 83–87. [Google Scholar] [CrossRef]
- Sorteberg, A.; Sorteberg, W.; Bakke, S.J.; Lindegaard, K.F.; Boysen, M.; Nornes, H. Varying impact of common carotid artery digital compression and internal carotid artery balloon test occlusion on cerebral hemodynamics. Head Neck 1998, 20, 687–694. [Google Scholar] [CrossRef]
- Naraynsingh, V.; Harnarayan, P.; Maharaj, R.; Dan, D.; Hariharan, S. Preoperative Digital Carotid Compression as a Predictor of the Need for Shunting During Carotid Endarterectomy. Open Cardiovasc. Med. J. 2013, 7, 110–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolyva, C.; Ghosh, A.; Tachtsidis, I.; Highton, D.; Smith, M.; Elwell, C.E. Dependence on NIRS source-detector spacing of cytochrome c oxidase response to hypoxia and hypercapnia in the adult brain. Adv. Exp. Med. Biol. 2013, 789, 353–359. [Google Scholar] [CrossRef]
- Milej, D.; Shahid, M.; Abdalmalak, A.; Rajaram, A.; Diop, M.; Lawrence, K.S. Characterizing dynamic cerebral vascular reactivity using a hybrid system combining time-resolved near-infrared and diffuse correlation spectroscopy. Biomed. Opt. Express 2020, 11, 4571. [Google Scholar] [CrossRef] [PubMed]
- Saager, R.B.; Berger, A.J. Direct characterization and removal of interfering absorption trends in two-layer turbid media. J. Opt. Soc. Am. A 2005, 22, 1874. [Google Scholar] [CrossRef] [PubMed]
- Milej, D.; Abdalmalak, A.; Rajaram, A.; Jhajj, A.; Owen, A.M.; Lawrence, K.S. Incorporating early and late-arriving photons to improve the reconstruction of cerebral hemodynamic responses acquired by time-resolved near-infrared spectroscopy. J. Biomed. Opt. 2021, 26, 056003. [Google Scholar] [CrossRef]
- Rajaram, A.; Yip, L.C.M.; Milej, D.; Suwalski, M.; Kewin, M.; Lo, M.; Carson, J.J.L.; Han, V.; Bhattacharya, S.; Diop, M.; et al. Perfusion and Metabolic Neuromonitoring during Ventricular Taps in Infants with Post-Hemorrhagic Ventricular Dilatation. Brain Sci. 2020, 10, 452. [Google Scholar] [CrossRef]
- Rajaram, A.; Milej, D.; Suwalski, M.; Kebaya, L.; Kewin, M.; Yip, L.; de Ribaupierre, S.; Han, V.; Diop, M.; Bhattacharya, S.; et al. Assessing cerebral blood flow, oxygenation and cytochrome c oxidase stability in preterm infants during the first 3 days after birth. Sci. Rep. 2022, 12, 181. [Google Scholar] [CrossRef]
- Khalid, M.; Milej, D.; Rajaram, A.; Abdalmalak, A.; Morrison, L.; Diop, M.; Lawrence, K.S. Development of a stand-alone DCS system for monitoring absolute cerebral blood flow. Biomed. Opt. Express 2019, 10, 4607. [Google Scholar] [CrossRef]
- Shoemaker, L.; Suwalski, M.; Milej, D.; Shoemaker, J.K.; Chui, J.; Lawrence, K.S. Assessment by Multi-Distance Hyperspectral NIRS of Changes in the Oxidation State of Cytochrome C Oxidase (oxCCO) to Carotid Artery Compressions. FASEB J. 2022, 36, R3459. [Google Scholar] [CrossRef]
- Rajaram, A.; Bale, G.; Kewin, M.; Morrison, L.B.; Tachtsidis, I.; Lawrence, K.S. Diop, M. Simultaneous monitoring of cerebral perfusion and cytochrome c oxidase by combining broadband near-infrared spectroscopy and diffuse correlation spectroscopy. Biomed. Opt. Express 2018, 9, 2588. [Google Scholar] [CrossRef]
- Kewin, M.; Rajaram, A.; Milej, D.; Abdalmalak, A.; Morrison, L.; Diop, M.; Lawrence, K.S. Evaluation of hyperspectral NIRS for quantitative measurements of tissue oxygen saturation by comparison to time-resolved NIRS. Biomed. Opt. Express 2019, 10, 4789. [Google Scholar] [CrossRef] [PubMed]
- Matcher, S.J.; Cooper, C.E. Absolute quantification of deoxyhaemoglobin concentration in tissue near infrared spectroscopy. Phys. Med. Biol. 1994, 39, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Matcher, S.J.; Cope, M.; Delpy, D.T. Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Phys. Med. Biol. 1994, 39, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Essenpreis, M.; Cope, M.; Elwell, C.E.; Arridge, S.R.; van der Zee, P.; Delpy, D.T. Wavelength dependence of the differential pathlength factor and the log slope in time-resolved tissue spectroscopy. Adv. Exp. Med. Biol. 1993, 333, 9–20. [Google Scholar] [CrossRef]
- Verdecchia, K.; Diop, M.; Morrison, L.B.; Lee, T.-Y.; Lawrence, K.S. Assessment of the best flow model to characterize diffuse correlation spectroscopy data acquired directly on the brain. Biomed. Opt. Express 2015, 6, 4288. [Google Scholar] [CrossRef]
- Milej, D.; He, L.; Abdalmalak, A.; Baker, W.B.; Anazodo, U.C.; Diop, M.; Dolui, S.; Kavuri, V.C.; Pavlosky, W.; Wang, L.; et al. Quantification of cerebral blood flow in adults by contrast-enhanced near-infrared spectroscopy: Validation against MRI. J. Cereb. Blood Flow Metab. 2020, 40, 1672–1684. [Google Scholar] [CrossRef]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85 Pt 1, 51–63. [Google Scholar] [CrossRef]
- Poublanc, J.; Crawley, A.P.; Sobczyk, O.; Montandon, G.; Sam, K.; Mandell, D.M.; Dufort, P.; Venkatraghavan, L.; Duffin, J.; Mikulis, D.J.; et al. Measuring cerebrovascular reactivity: The dynamic response to a step hypercapnic stimulus. J. Cereb. Blood Flow Metab. 2015, 35, 1746–1756. [Google Scholar] [CrossRef]
- Guerouah, Z.; Lin, S.; Toronov, V. Measurement of Adult Human Brain Responses to Breath-Holding by Multi-Distance Hyperspectral Near-Infrared Spectroscopy. Appl. Sci. 2022, 12, 371. [Google Scholar] [CrossRef]
- Hoshi, Y.; Hazeki, O.; Kakihana, Y.; Tamura, M. Redox behavior of cytochrome oxidase in the rat brain measured by near- infrared spectroscopy. J. Appl. Physiol. 1997, 83, 1842–1848. [Google Scholar] [CrossRef]
- Cooper, C. The cytochrome oxidase redox state in vivo. Adv. Exp. Med. Biol. 1997, 428, 449–456. [Google Scholar] [CrossRef] [PubMed]

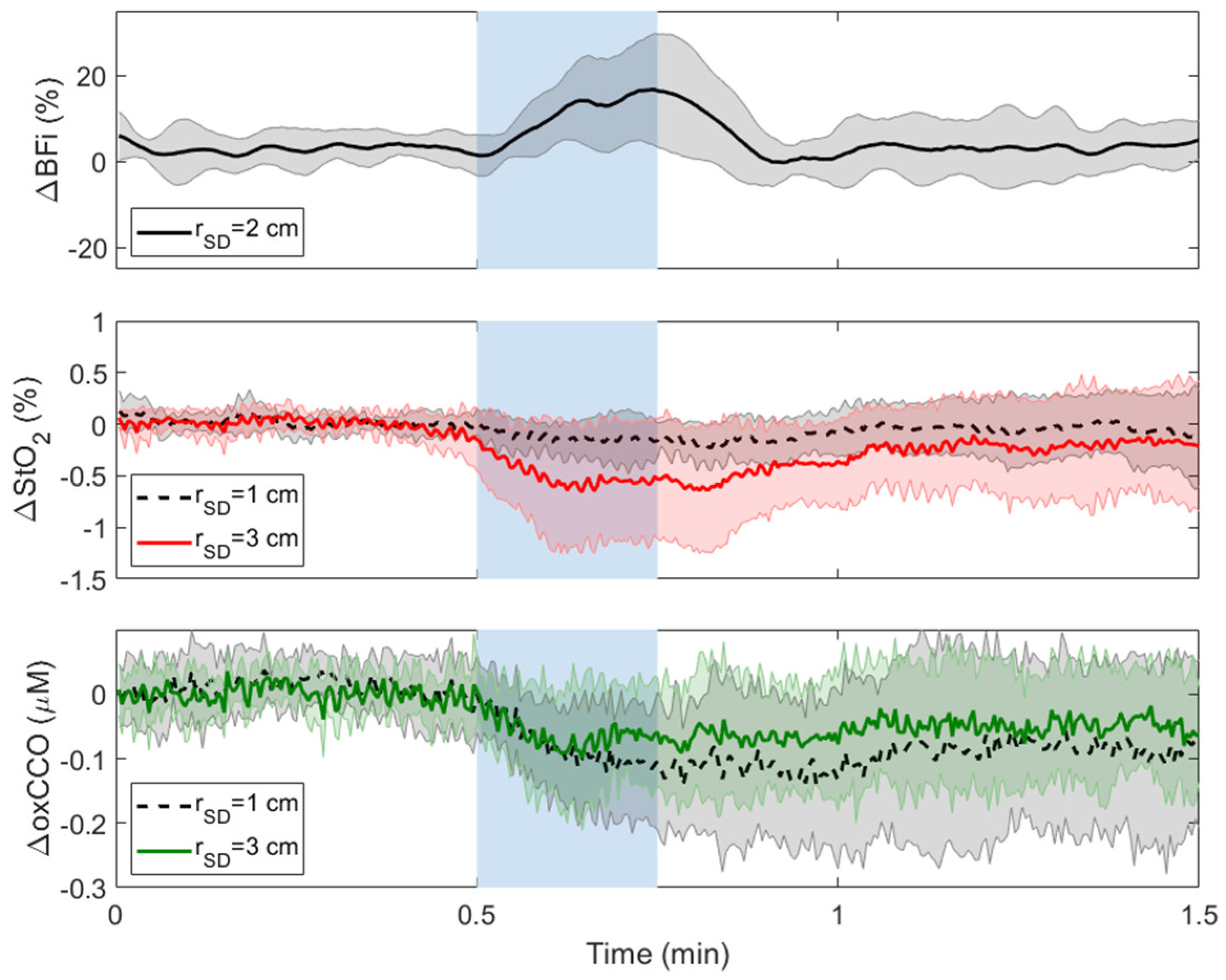
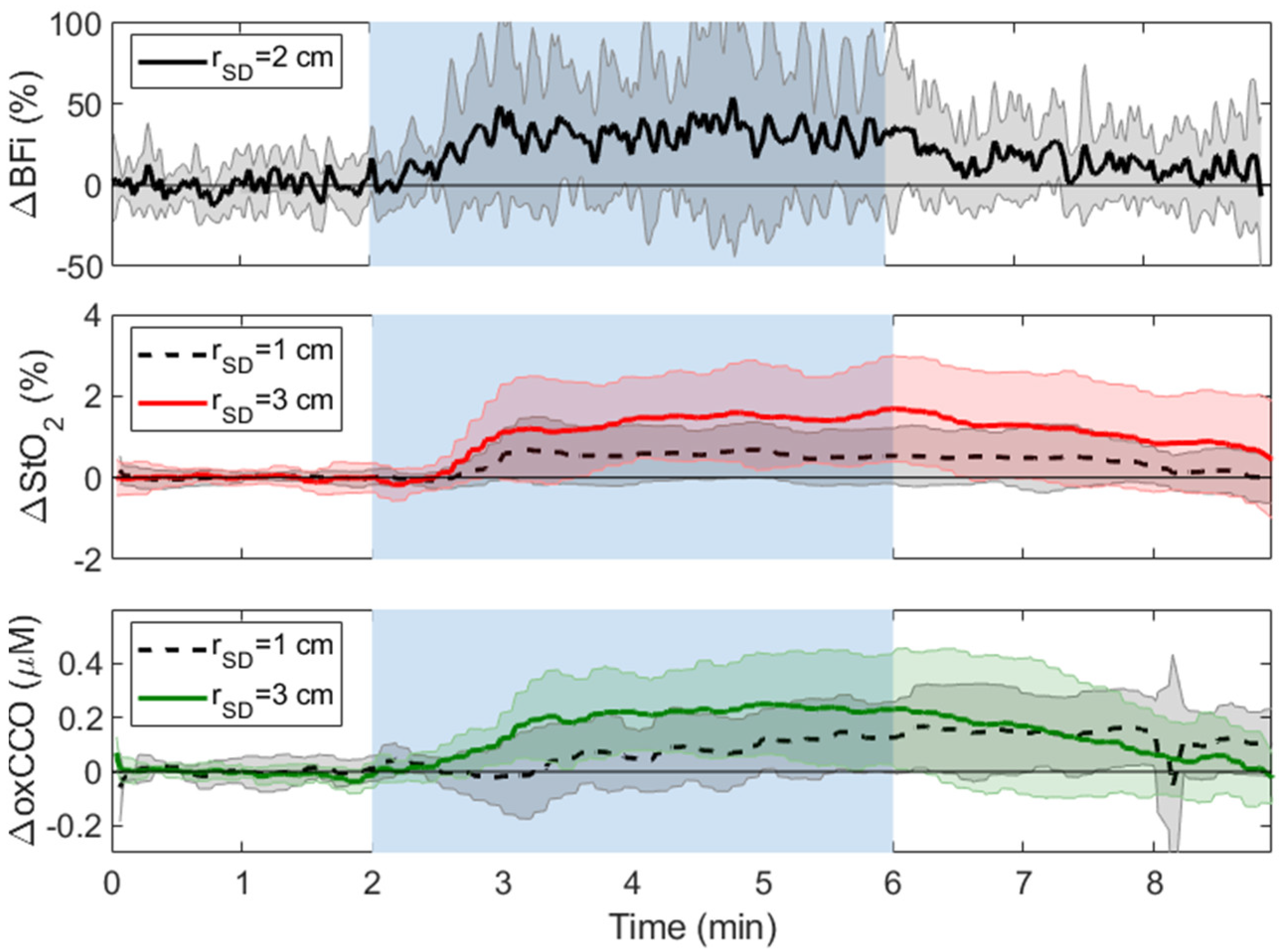

| CC | Ipsilateral | Contralateral | |||||
|---|---|---|---|---|---|---|---|
| Duration of CC (s) | 30 | Regression | 15 | 15 | |||
| rSD (cm) | 1 | 3 | – | 1 | 3 | 1 | 3 |
| ΔStO2(%) | −1.2 ± 0.7 ▽ | −4 ± 2.2 ▽,* | −2.4 ± 1.9 ▽ | −1 ± 0.5 ▽ | −3.1 ± 1.1 ▽,* | −0.2 ± 0.2 | −0.6 ± 0.6 |
| ΔoxCCO (μM) | −0.06 ± 0.1 | −0.4 ± 0.3 ▽,* | −0.21 ± 0.24 ▽ | −0.07 ± 0.2 | −0.3 ± 0.2 ▽,* | −0.12 ± 0.08 | −0.1 ± 0.1 |
| Hypercapnia (min 4–6) | Hypercapnia Regression | Post Hypercapnia (min 7–9) | Post Hypercapnia Regression | |||
|---|---|---|---|---|---|---|
| rSD (cm) | 1 | 3 | - | 1 | 3 | - |
| ΔStO2 (%) | 0.6 ± 0.6 | 1.5 ± 1.1 ▽,* | 0.82 ± 0.75 ▽ | 0.3 ± 0.6 | 0.9 ± 1.3 ▽ | 0.03 ± 0.1 ⯁ |
| ΔoxCCO (µM) | 0.1 ± 0.1 | 0.22 ± 0.19 ▽,* | 0.15 ± 0.11 ▽ | 0.14 ± 0.1 ▽ | 0.1 ± 0.1 ● | 0.03 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwalski, M.; Shoemaker, L.N.; Shoemaker, J.K.; Diop, M.; Murkin, J.M.; Chui, J.; St. Lawrence, K.; Milej, D. Assessing the Sensitivity of Multi-Distance Hyperspectral NIRS to Changes in the Oxidation State of Cytochrome C Oxidase in the Brain. Metabolites 2022, 12, 817. https://doi.org/10.3390/metabo12090817
Suwalski M, Shoemaker LN, Shoemaker JK, Diop M, Murkin JM, Chui J, St. Lawrence K, Milej D. Assessing the Sensitivity of Multi-Distance Hyperspectral NIRS to Changes in the Oxidation State of Cytochrome C Oxidase in the Brain. Metabolites. 2022; 12(9):817. https://doi.org/10.3390/metabo12090817
Chicago/Turabian StyleSuwalski, Marianne, Leena N. Shoemaker, J. Kevin Shoemaker, Mamadou Diop, John M. Murkin, Jason Chui, Keith St. Lawrence, and Daniel Milej. 2022. "Assessing the Sensitivity of Multi-Distance Hyperspectral NIRS to Changes in the Oxidation State of Cytochrome C Oxidase in the Brain" Metabolites 12, no. 9: 817. https://doi.org/10.3390/metabo12090817
APA StyleSuwalski, M., Shoemaker, L. N., Shoemaker, J. K., Diop, M., Murkin, J. M., Chui, J., St. Lawrence, K., & Milej, D. (2022). Assessing the Sensitivity of Multi-Distance Hyperspectral NIRS to Changes in the Oxidation State of Cytochrome C Oxidase in the Brain. Metabolites, 12(9), 817. https://doi.org/10.3390/metabo12090817







