Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Diet and Management
2.2. Sample Collection and Preparation
2.3. Plasma Analysis
2.4. Sample Metabolite Extraction
2.5. Ultra-High-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry Analysis and Data Processing
2.6. Statistical Analysis
3. Results
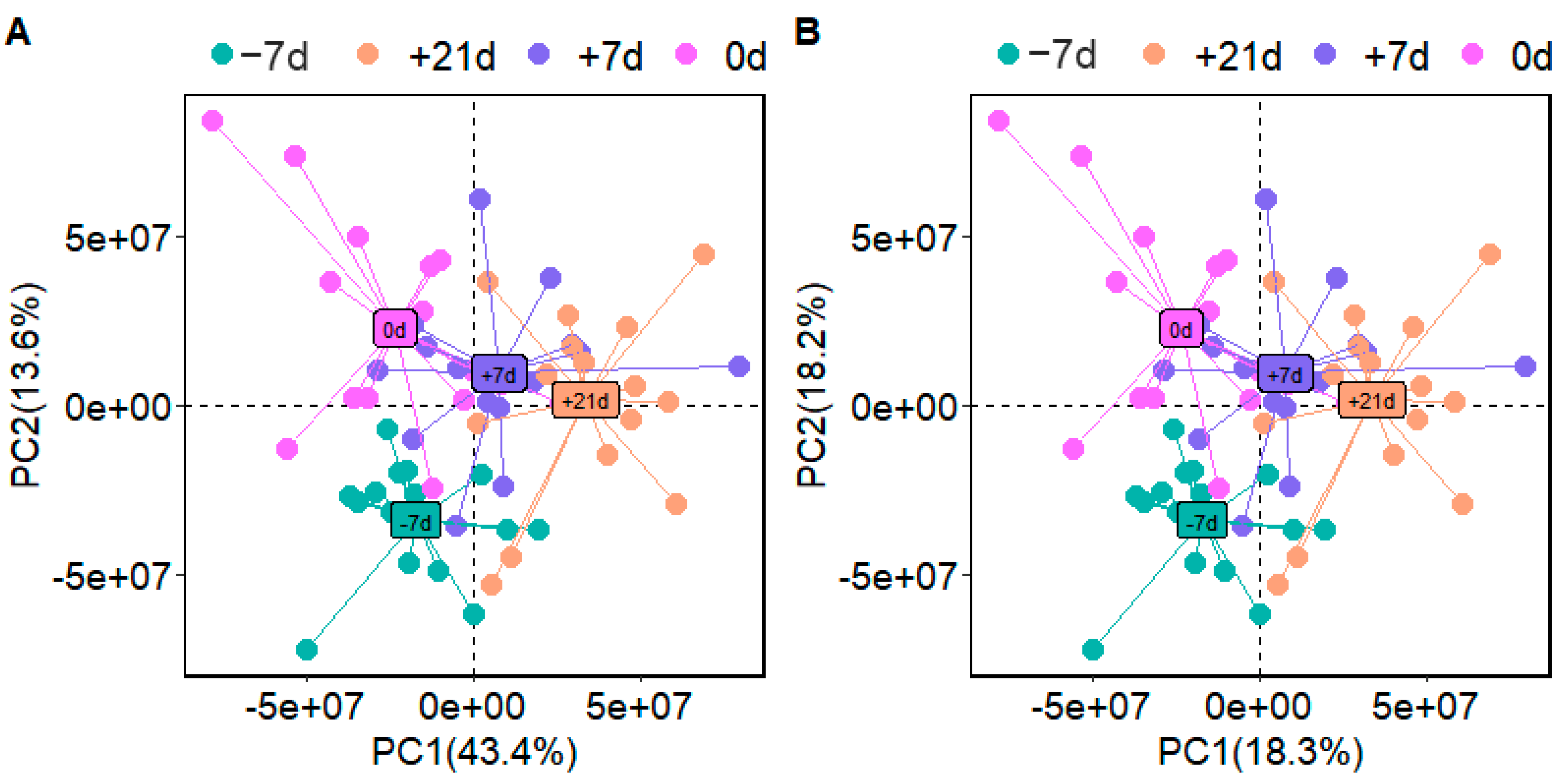
3.1. Plasma Analyses
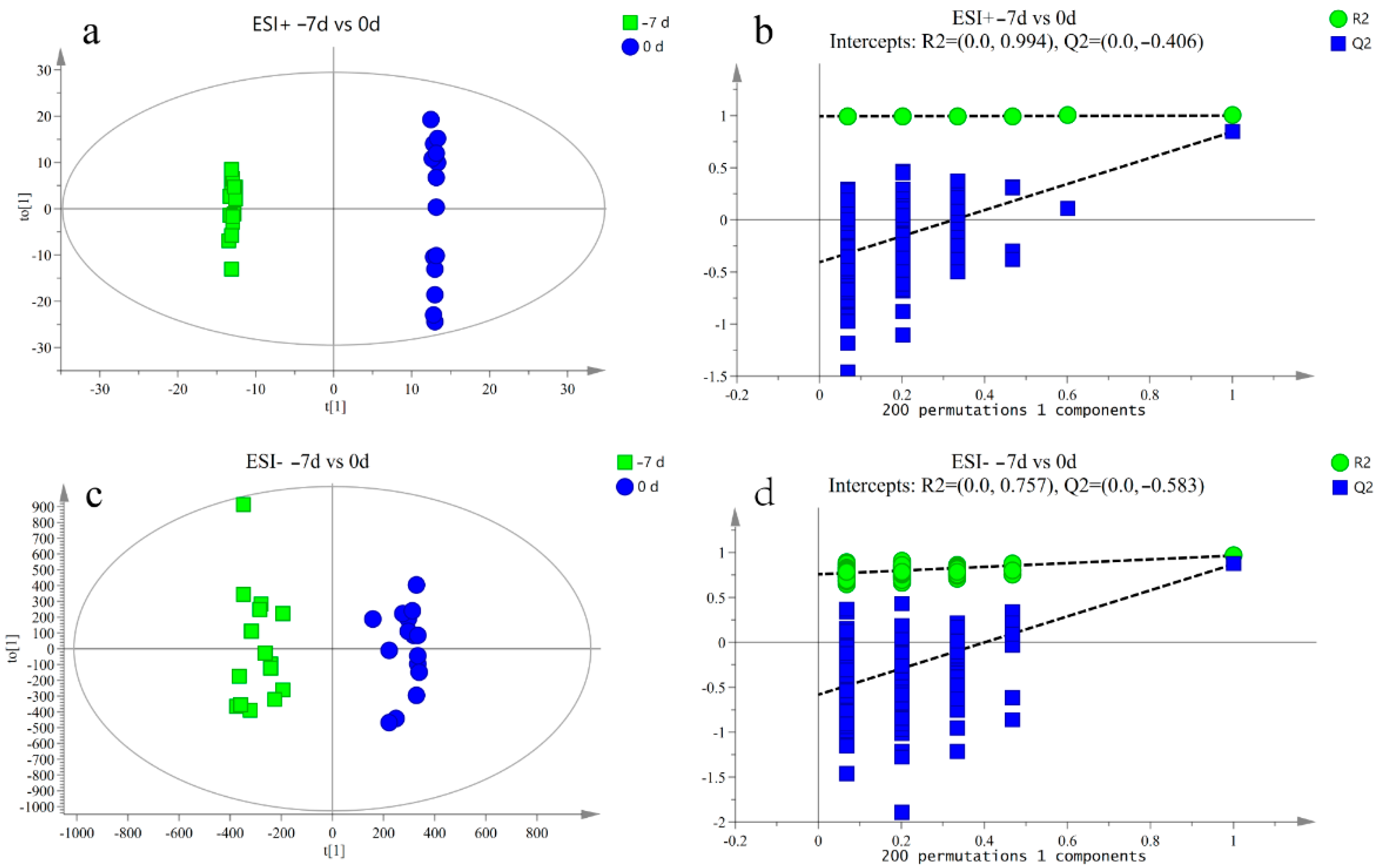
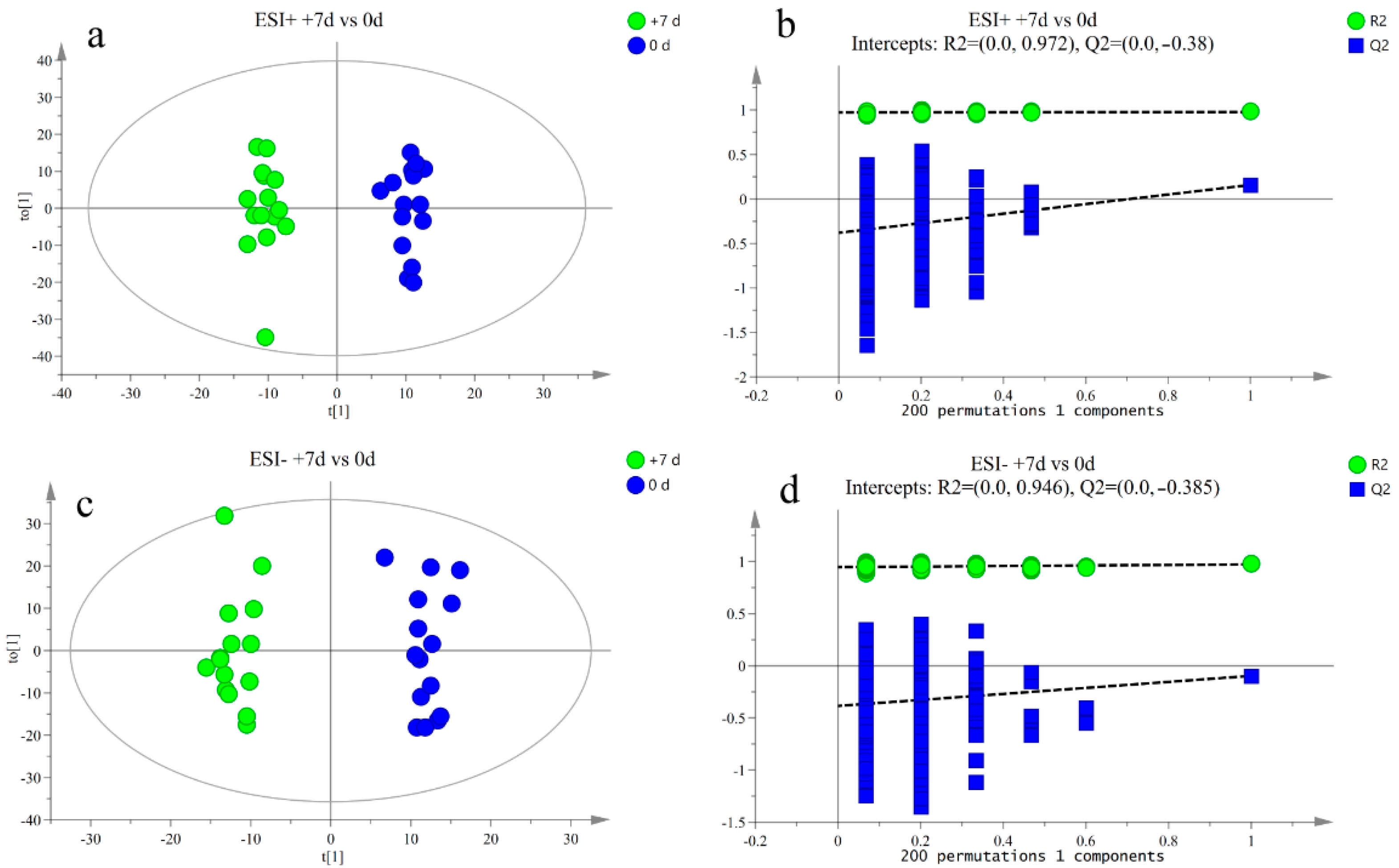
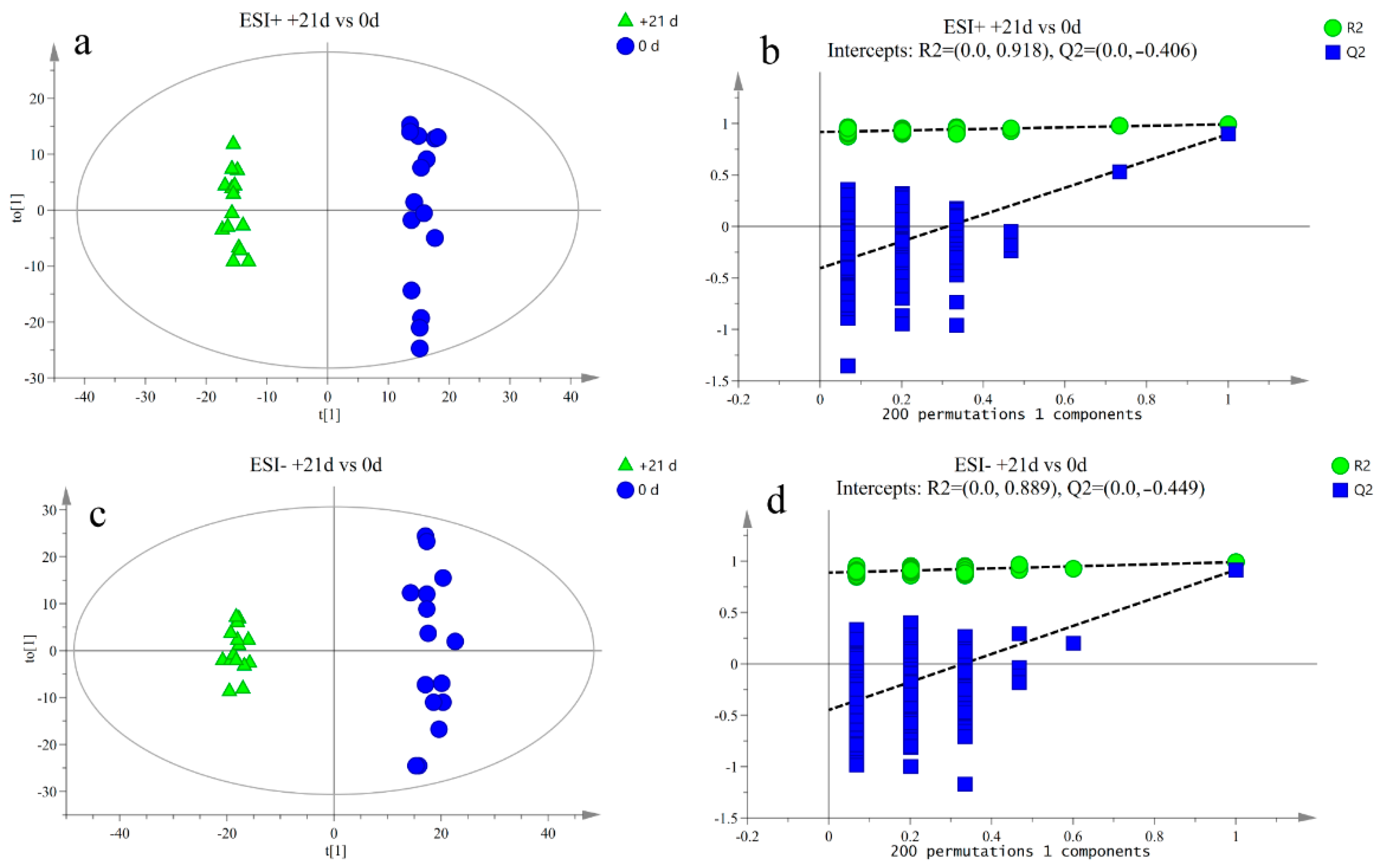
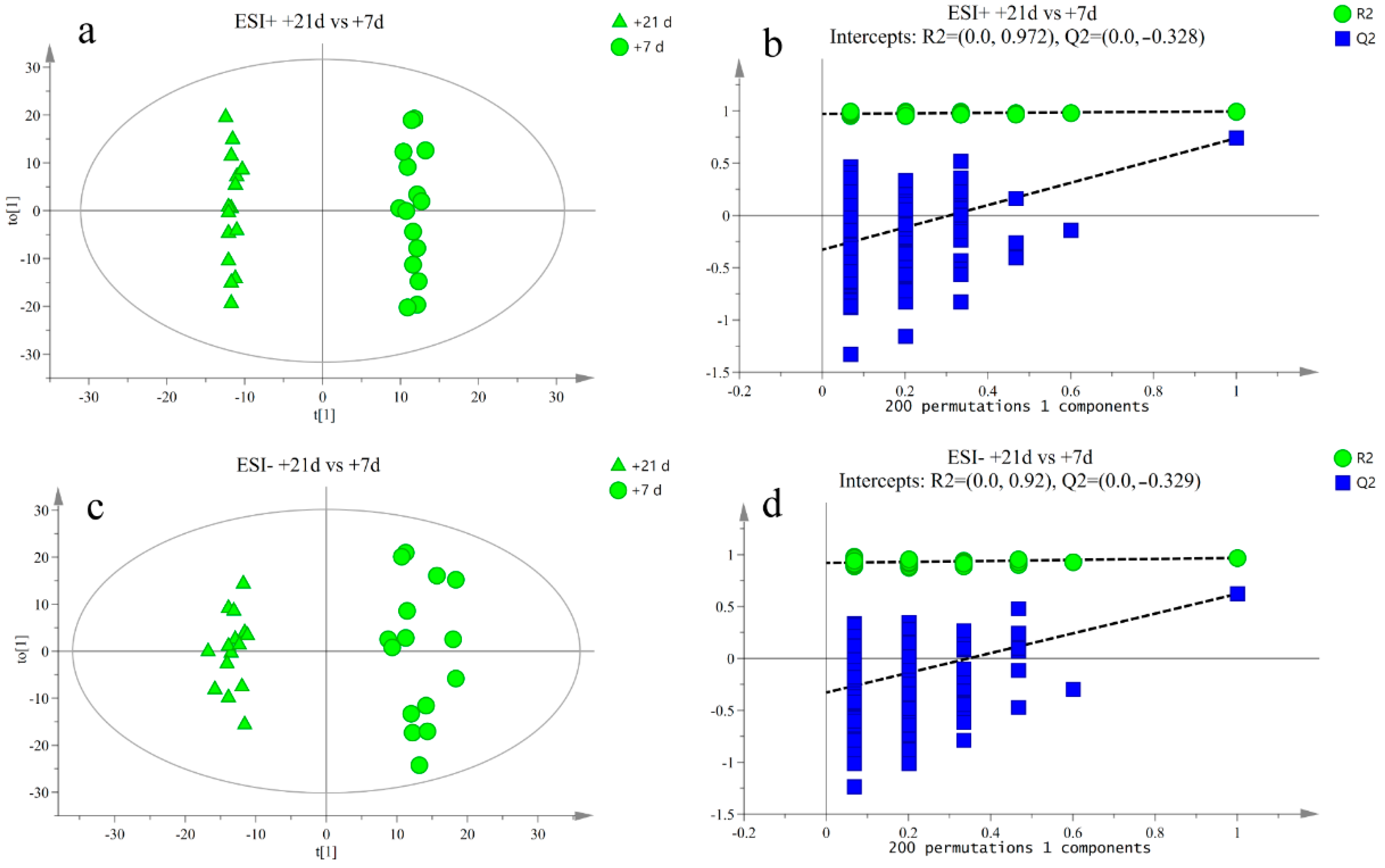
3.2. Metabolite Profile Analysis
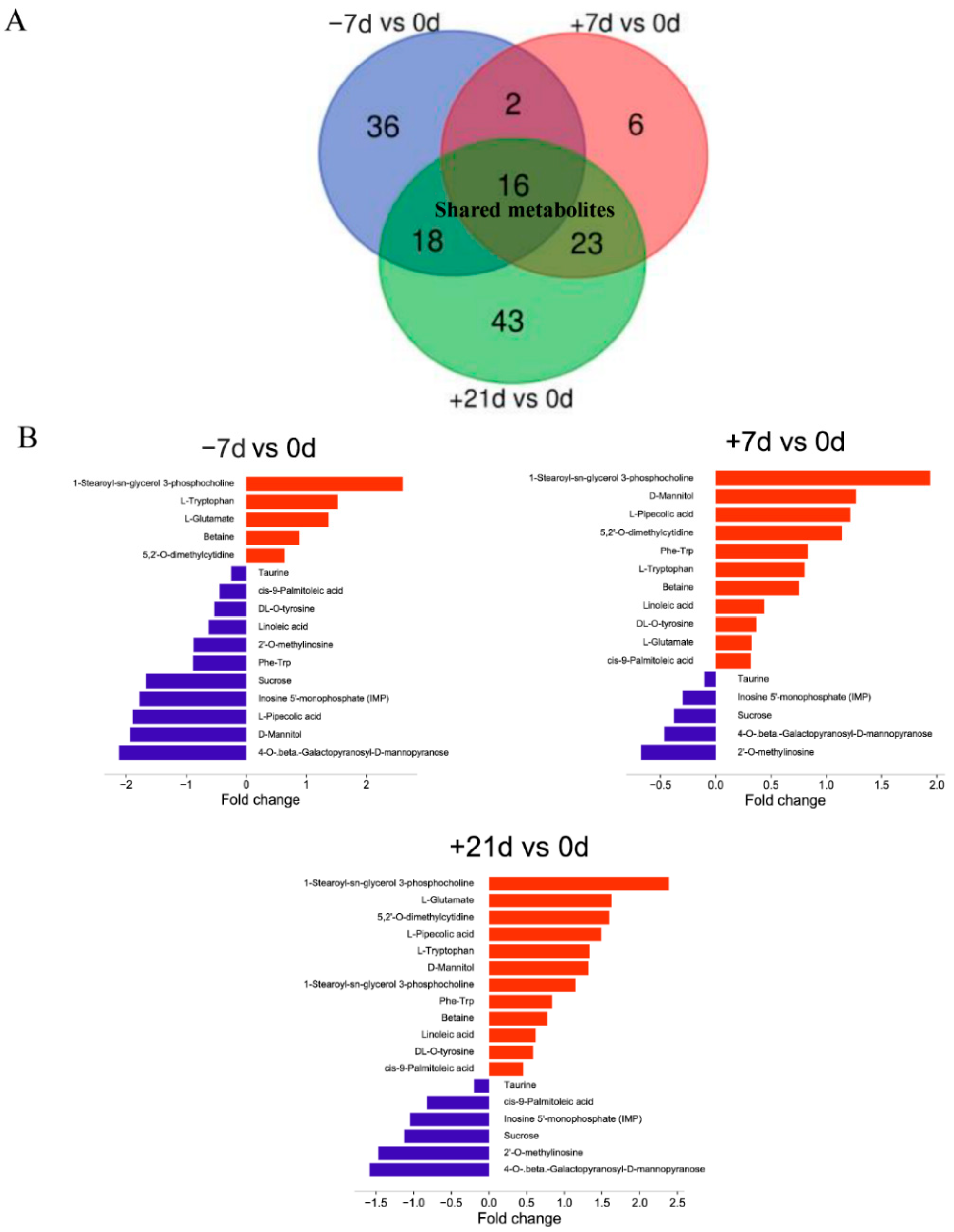
3.3. Differential Metabolite Analysis
3.4. Metabolic Pathway Enrichment Analysis
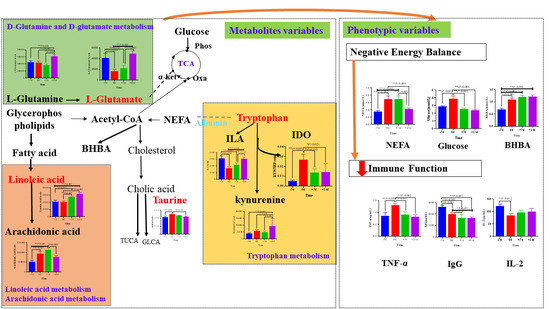
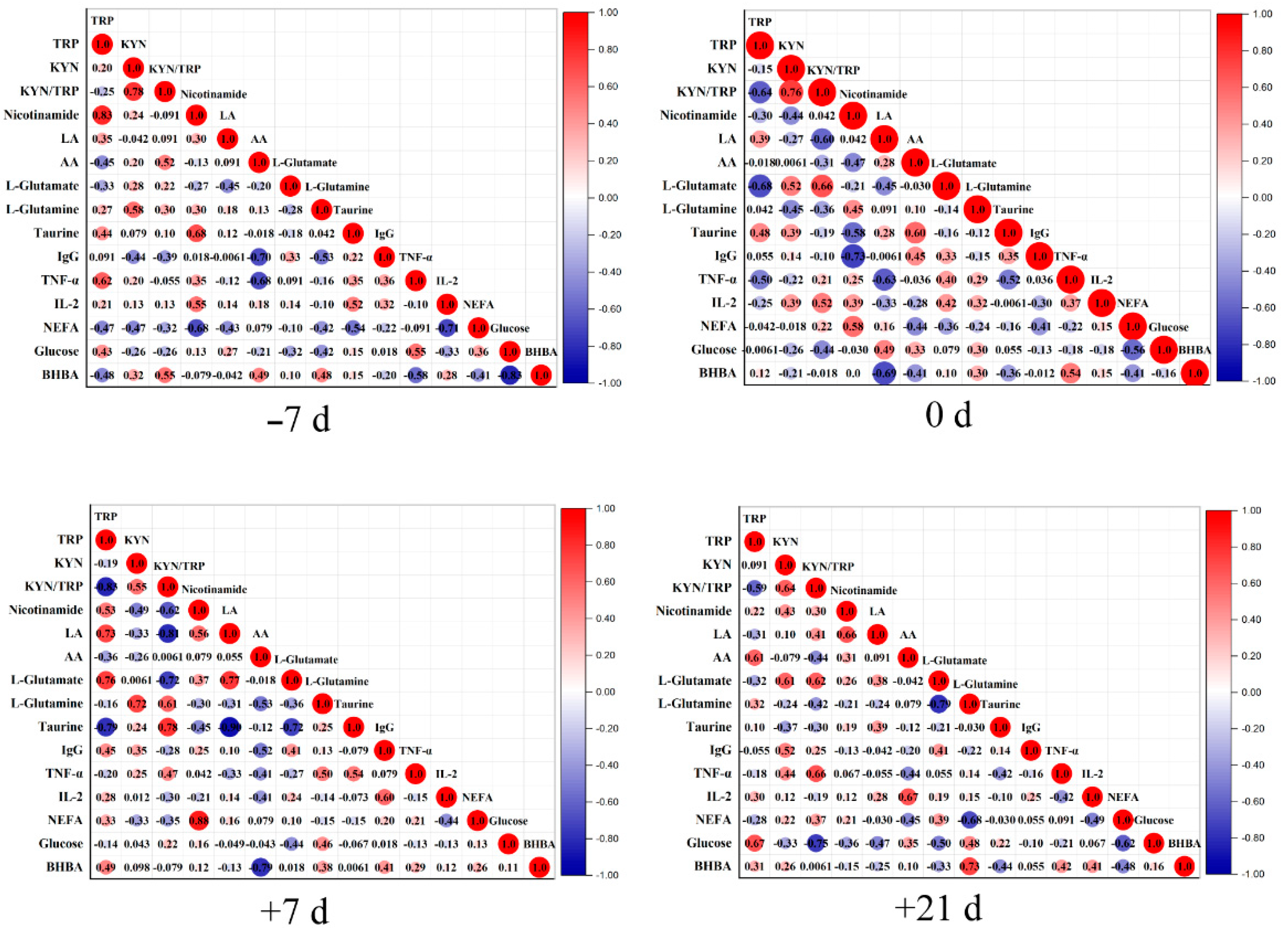
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifi, H.A.; Gorji-Dooz, M.; Mohri, M.; Dalir-Naghadeh, B.; Farzaneh, N. Variations of energy-related biochemical metabolites during transition period in dairy cows. Comp. Clin. Pathol. 2007, 16, 253–258. [Google Scholar] [CrossRef]
- Trevisi, E.; Minuti, A. Assessment of the innate immune response in the periparturient cow. Res. Vet. Sci. 2017, 116, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Major advances in our understanding of nutritional influences on bovine health. J. Dairy Sci. 2006, 89, 1292–1301. [Google Scholar] [CrossRef]
- Klaus, L.I.; Kasey, M.M. Factors contributing to immunosuppression in the dairy cow during the periparturient period. Jpn. J. Vet. Res. 2015, 63, S15–S24. [Google Scholar]
- Grummer, R.R.; Mashek, D.G.; Hayirli, A. Dry matter intake and energy balance in the transition period. Vet. Clin. Food Anim. 2004, 20, 447–470. [Google Scholar] [CrossRef]
- Marquardt, J.P.; Horst, R.L.; Jorgensen, N.A. Effect of parity on dry matter intake at parturition in dairy cattle. J. Dairy Sci. 1977, 60, 929–934. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The importance of energy balance. Eur. Endocrinol. 2013, 9, 111–115. [Google Scholar] [CrossRef]
- Kadokawa, H.; Martin, G.B. A new perspective on management of reproduction in dairy cows: The need for detailed metabolic information, an improved selection index and extended lactation. J. Reprod. Dev. 2006, 52, 161–168. [Google Scholar] [CrossRef]
- Karimian, M.; Khorvash, M.; Forouzmand, M.A.; Alikhani, M.; Rahmani, H.R.; Ghaffari, M.H. Effect of prepartal and postpartal dietary fat level on performance and plasma concentration of metabolites in transition dairy cows. J. Dairy Sci. 2015, 98, 330–337. [Google Scholar] [CrossRef]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yao, J. Regulation of nutritional metabolism in transition dairy cows: Energy homeostasis and health in response to post-ruminal choline and methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.K.; Aikman, P.C.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 2003, 86, 1201–1217. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Dervishi, E.; Dunn, S.M.; Mandal, R.; Liu, P.; Han, B. Metabotyping reveals distinct metabolic alterations in ketotic cows and identifies early predictive serum biomarkers for the risk of disease. Metabolomics 2017, 13, 43. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef]
- Zhang, G.S.; Deng, Q.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. DI/LC-MS/MS-based metabolic profiling for identification of early predictive serum biomarkers of metritis in transition dairy cows. J. Agric. Food Chem. 2017, 65, 8510–8521. [Google Scholar] [CrossRef]
- Sun, H.Z.; Shi, K.; Wu, X.H.; Xue, M.Y.; Wei, Z.H.; Liu, J.X.; Liu, H.Y. Lactation-related metabolic mechanism investigated based on mammary gland metabolomics and 4 biofluids’ metabolomics relationships in dairy cows. BMC Genom. 2017, 18, 936. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Dunn, S.M.; Mandal, R.; Wishart, D.S.; Ametaj, B.N. GC-MS metabolomics identifies metabolite alterations that precede subclinical mastitis in the blood of transition dairy cows. J. Proteome Res. 2016, 16, 433–446. [Google Scholar] [CrossRef]
- Kenéz, Á.; Dänicke, S.; Rolle-Kampczyk, U.; Bergen, M.V.; Huber, K. A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics 2016, 12, 165. [Google Scholar] [CrossRef]
- Naegeli, A.; Bratanis, E.; Karlsson, C.; Shannon, O.; Collin, M. Streptococcus pyogenes evades adaptive immunity through specific IgG glycan hydrolysis. J. Exp. Med. 2019, 216, 1615–1629. [Google Scholar] [CrossRef]
- Prisco, A.R.; Prisco, M.R.; Carlson, B.E.; Greene, A.S. TNF-α increases endothelial progenitor cell adhesion to the endothelium by increasing bond expression and affinity. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1368–H1381. [Google Scholar] [CrossRef] [PubMed]
- Overwijk, W.W.; Tagliaferri, M.A.; Zalevsky, J. Engineering IL-2 to give new life to t cell immunotherapy. Annu. Rev. Med. 2021, 72, 281–311. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R.; Mcart, J.A.A.; Nydam, D.V. A 100-year review: Metabolic health indicators and management of dairy cattle. J. Dairy Sci. 2017, 100, 10398–10417. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. Food. Anim. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 6071. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Franci, O.; Scalia, D.; Bernabucci, U.; Ronchi, B.; Nardone, A. Effects of nonesterified fatty acids and beta-hydroxybutyrate on functions of mononuclear cells obtained from ewes. Am. J. Vet. Res. 2002, 63, 414–418. [Google Scholar] [CrossRef]
- Banos, G.; Wall, E.; Coffey, M.P.; Bagnall, A.; Gillespie, S.; Russell, G.C.; McNeilly, T.N. Identification of immune traits correlated with dairy cow health, reproduction and productivity. PLoS ONE 2013, 8, e65766. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Koiwa, M.; Hatsugaya, A.; Kudo, K.; Hoshi, F.; Itoh, N.; Yokota, H.; Okada, H.; Kawamura, S. Relationship between serum TNF activity and insulin resistance in dairy cows affected with naturally occurring fatty liver. J. Vet. Med. Sci. 2001, 63, 1021. [Google Scholar] [CrossRef]
- Schoenberg, K.M.; Perfield, K.L.; Farney, J.K.; Bradford, B.J.; Boisclair, Y.R.; Overton, T.R. Effects of prepartum 2,4-thiazolidinedione on insulin sensitivity, plasma concentrations of tumor necrosis factor-α and leptin, and adipose tissue gene expression. J. Dairy Sci. 2011, 94, 5523–5532. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.C.; Zhang, Z.H.; Zhang, C.Z.; Su, H.W.; Li, S.L. Effect of prepartum maternal energy density on the growth performance, immunity, and antioxidation capability of neonatal calve. J. Dairy Sci. 2012, 95, 4510–4518. [Google Scholar] [CrossRef]
- Franklin, S.T.; Newman, M.C.; Newman, K.E.; Meek, K.I. Immune parameters of dry cows fed mannan oligosaccharide and subsequent transfer of immunity to calves. J. Dairy Sci. 2005, 88, 766–775. [Google Scholar] [CrossRef]
- Shi, X.; Li, D.; Deng, Q.; Li, Y.; Sun, G.; Yuan, X.; Song, Y.; Wang, Z.; Li, X. NEFAs activate the oxidative stress-mediated NF-κB signaling pathway to induce inflammatory response in calf hepatocytes. FASEB J. 2015, 145, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ai-Salem, H.S.; Ai-Yousef, H.M.; Ashour, A.E.; Ahmed, A.F.; Amina, M.; Issa, I.M.; Bhat, R.S. Antioxidant and hepatorenal protective effects of bee pollen fractions agains propionic acid-induced autistic feature in rats. Food Sci. Nutr. 2020, 8, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Trevisi, E.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Wu, T.Y.; Zhang, H.; Loor, J.J.; Wang, M.Z.; Peng, A.L.; Wang, H.R. Rapid communication: Jugular infusion of arginine has a positive effect on antioxidant mechanisms in lactating dairy cows challenged intravenously with lipopolysaccharide. J. Anim. Sci. 2018, 96, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Young, K.D.; Drevets, W.C.; Dantzer, R.; Teague, T.K.; Bodurka, J.; Savitz, J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav. Immun. 2016, 56, 335–342. [Google Scholar] [CrossRef]
- Liu, H.Z.; Liu, L.; Fletcher, B.S.; Visner, G.A. Novel action of indoleamine 2,3-dioxygenase attenuating acute lung allograft injury. Am. J. Respir. Crit. Care Med. 2012, 173, 566–572. [Google Scholar] [CrossRef]
- Proietti, E.; Rossini, S.; Grohmann, U.; Mondanelli, G. Polyamines and Kynurenines at the intersection of immune modulation. Trends Immunol. 2020, 41, 1037–1050. [Google Scholar] [CrossRef]
- Bubnoff, D.V.; Fimmers, R.; Bogdanow, M.; Matz, H.; Koch, S.; Bieber, T. Asymptomatic atopy is associated with increased indoleamine 2,3-dioxygenase activity and interleukin-10 production during seasonal allergen exposure. Clin. Exp. Allergy 2004, 34, 1056–1063. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease—ScienceDirect. Cell Host Microbe 2018, 23, 716. [Google Scholar] [CrossRef]
- Mateus, L.; Costa, L.L.D.; Bernardo, F.; Silva, J.R. Influence of puerperal uterine infection on uterine involution and postpartum ovarian activity in dairy cows. Reprod. Domest. Anim. 2010, 37, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Meade, C.J.; Mertin, J. The mechanism of immunoinhibition by arachidonic and linoleic acid: Effects on the lymphoid and reticulo-endothelial systems. Int. Archs Allergy Appl. Immun. 1976, 51, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation and immunity: Pouring oil on troubled waters or another fishy tale? Nutr. Res. 2001, 21, 309–341. [Google Scholar] [CrossRef]
- Raju, K.; Doulias, P.T.; Evans, P.; Krizman, E.N.; Jackson, J.G.; Horyn, O.; Daikhin, Y.; Nissim, I.; Yudkoff, M. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci. Signal. 2015, 8, ra68. [Google Scholar] [CrossRef]
- Sugiyama, K.; Ebinuma, H.; Nakamoto, N.; Sakasegawa, N.; Murakami, Y.; Chu, P.S.; Usui, S.; Ishibashi, Y.; Wakayama, Y.; Taniki, N.; et al. Prominent steatosis with hypermetabolism of the cell line permissive for years of infection with hepatitis C virus. PLoS ONE 2014, 9, e94440. [Google Scholar] [CrossRef] [PubMed]
- Doepel, L.; Lobley, G.E.; Bernier, J.F.; Dubreuil, P.; Lapierre, H. Effect of glutamine supplementation on splanchnic metabolism in lactating dairy cows. J. Dairy Sci. 2007, 90, 4325–4333. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Meng, Q.; Yue, C.; Xu, C. Identification and expression of cysteine sulfinate decarboxylase, possible regulation of taurine biosynthesis in Crassostrea gigas in response to low salinity. Sci. Rep. 2017, 7, 5505. [Google Scholar] [CrossRef]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2012, 42, 2223–2232. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Strus, M.; Walczewska, M.; Machul, A.; Mikoajczyk, D. Influence of taurine haloamines (TauCl and TauBr) on the development of pseudomonas aeruginosa biofilm: A preliminary study. Adv. Exp. Med. Biol. 2013, 775, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, T.; Han, S.Y.; Hu, Y.; Nagao, K.; Kitajima, H.; Murakami, S. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipids Health Dis. 2008, 7, 38. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Luo, F.; Liu, G.; Luo, Z.; Ma, S.; Gao, H.; He, H.; Tao, J. Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows. Metabolites 2022, 12, 953. https://doi.org/10.3390/metabo12100953
Yang Z, Luo F, Liu G, Luo Z, Ma S, Gao H, He H, Tao J. Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows. Metabolites. 2022; 12(10):953. https://doi.org/10.3390/metabo12100953
Chicago/Turabian StyleYang, Zhuo, Fang Luo, Guolin Liu, Zhengzhong Luo, Sijia Ma, Hang Gao, Hailong He, and Jinzhong Tao. 2022. "Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows" Metabolites 12, no. 10: 953. https://doi.org/10.3390/metabo12100953
APA StyleYang, Z., Luo, F., Liu, G., Luo, Z., Ma, S., Gao, H., He, H., & Tao, J. (2022). Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows. Metabolites, 12(10), 953. https://doi.org/10.3390/metabo12100953