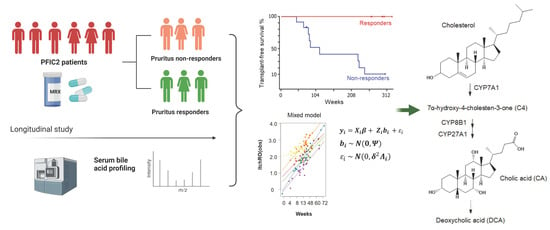
Serum Bile Acid Profiling and Mixed Model Analysis Reveal Biomarkers Associated with Pruritus Reduction in Maralixibat-Treated Patients with BSEP Deficiency
Abstract
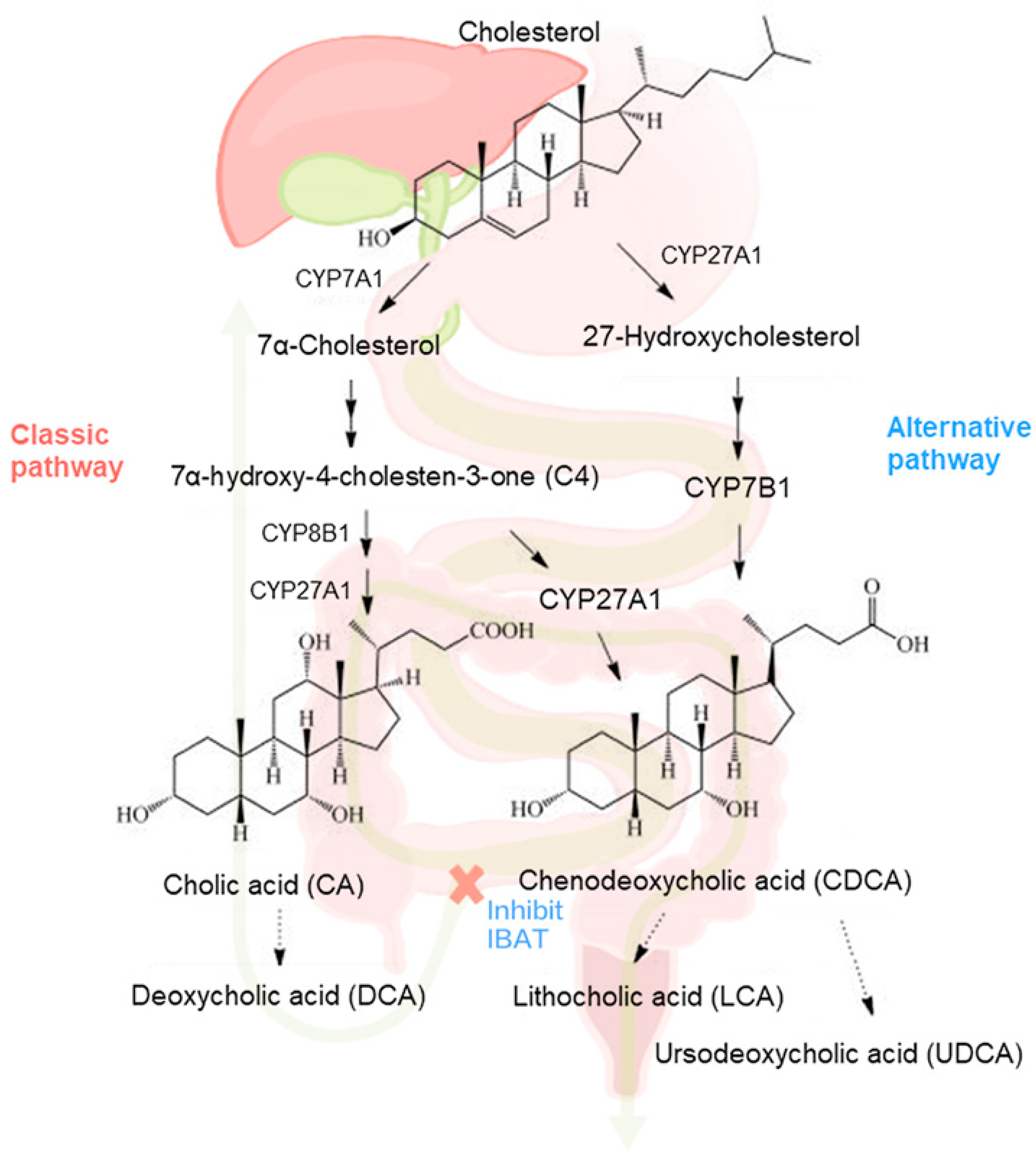
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analysis of Serum Bile Acids by Stable Isotope Diluted UHPLC-MS/MS
2.3. Quantification of Serum 7α-Hydroxy-4-cholesten-3-one
2.4. Statistical Analysis
3. Results
3.1. Longitudinal Profiling of Serum BA and 7α-C4 by UHPLC-MS/MS
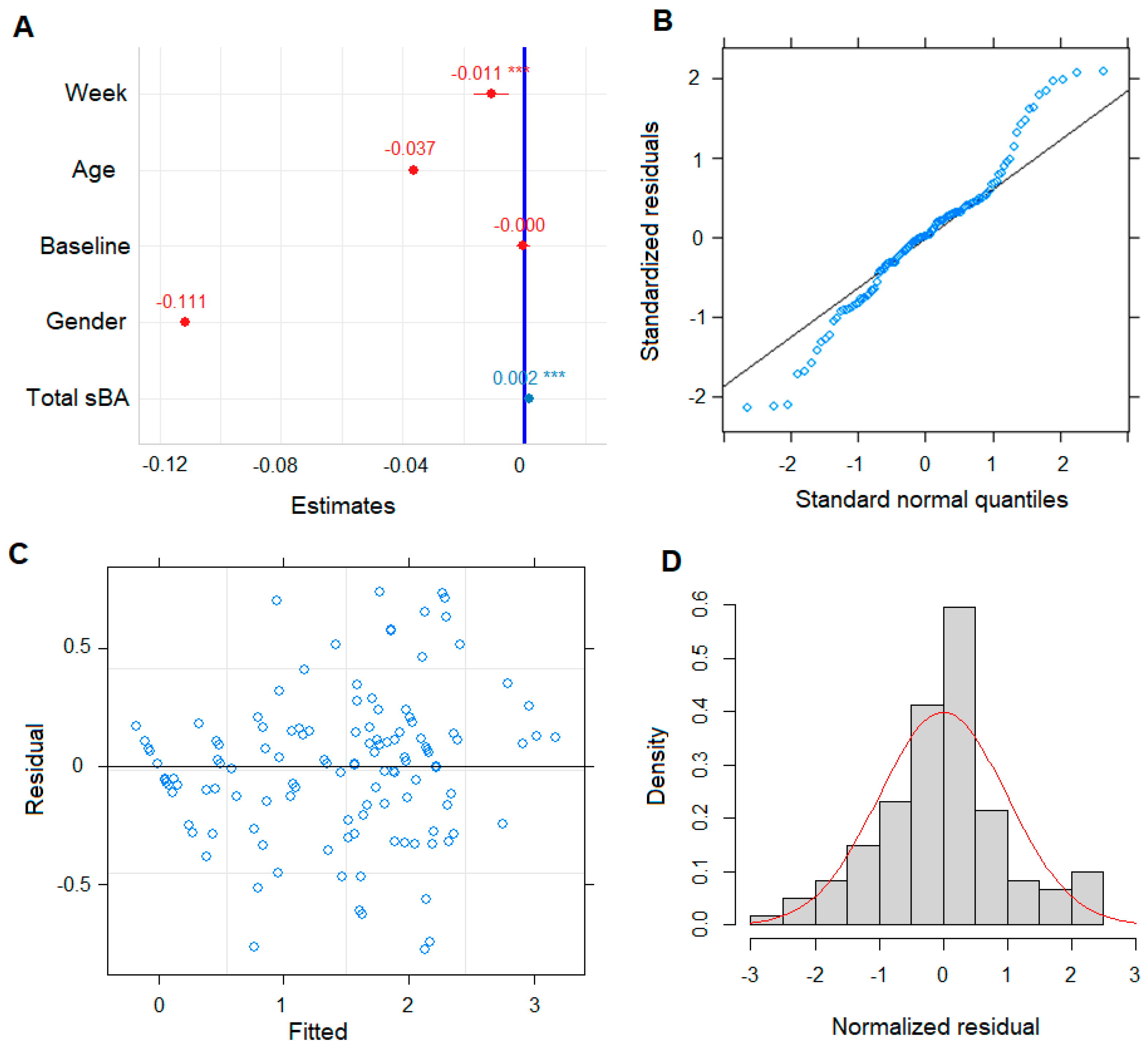
3.2. Pruritus ItchRO Score as Continuous Variable in Building LMM Model
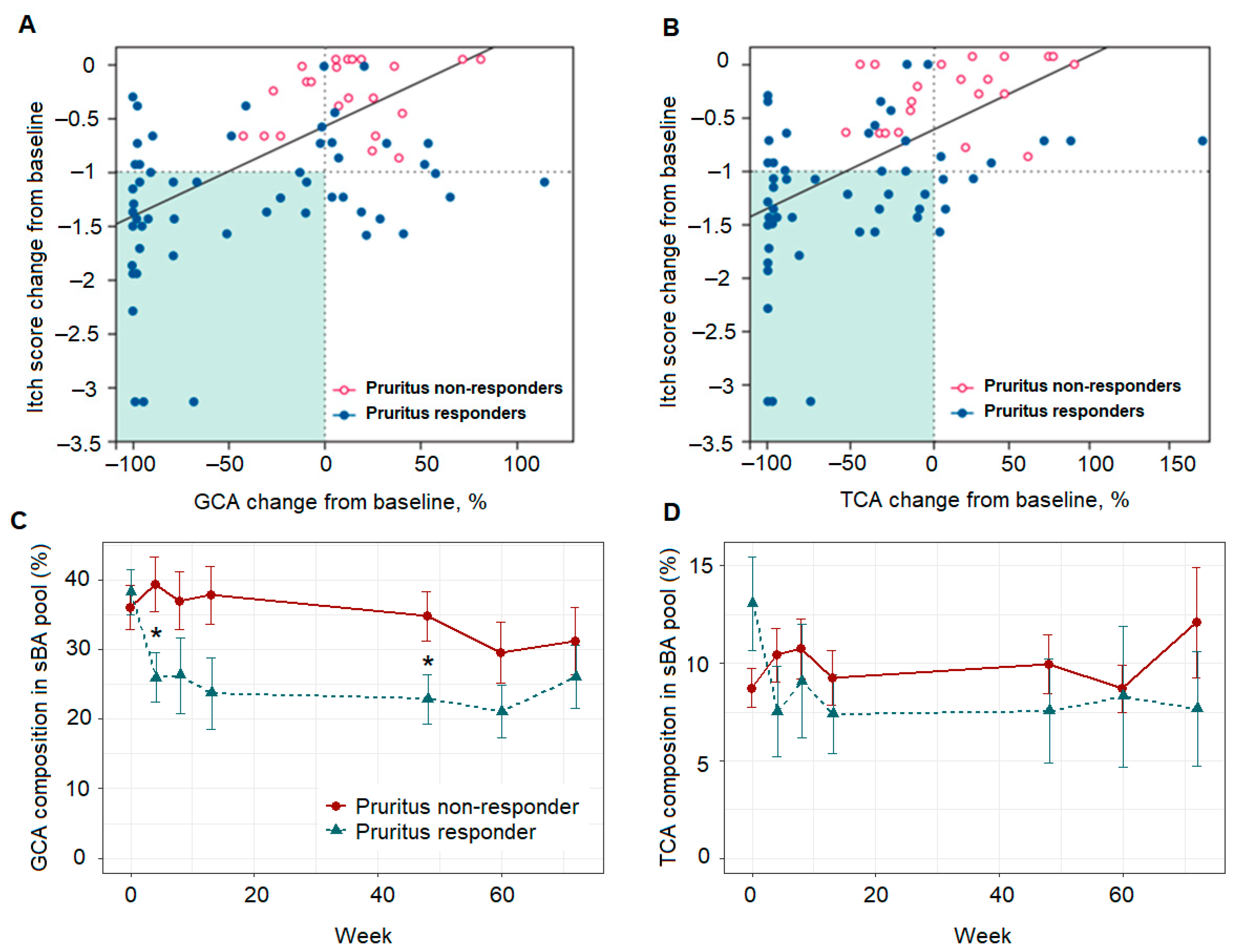
3.3. LMM Revealed sBA Pool Associates with Patient Pruritus Response
3.4. Pruritus Response Class Variable as Binary Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malatack, J.J.; Doyle, D. A Drug Regimen for Progressive Familial Cholestasis Type 2. Pediatrics 2018, 141, e20163877. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Kerkar, N.; Todorova, L.; Kamath, B.M.; Houwen, R.H.J. Systematic review of progressive familial intrahepatic cholestasis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Jones-Hughes, T.; Campbell, J.; Crathorne, L. Epidemiology and burden of progressive familial intrahepatic cholestasis: A systematic review. Orphanet J. Rare Dis. 2021, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A. Progressive familial intrahepatic cholestasis. J. Clin. Exp. Hepatol. 2014, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, J.; Beuers, U.H.; Oude Elferink, R.P.J. Cholestasis-Associated Pruritus and Its Pruritogens. Front. Med. 2021, 8, 639674. [Google Scholar] [CrossRef] [PubMed]
- Hegade, V.S.; Krawczyk, M.; Kremer, A.E.; Kuczka, J.; Gaouar, F.; Kuiper, E.M.; van Buuren, H.R.; Lammert, F.; Corpechot, C.; Jones, D.E. The safety and efficacy of nasobiliary drainage in the treatment of refractory cholestatic pruritus: A multicentre European study. Aliment. Pharmacol. Ther. 2016, 43, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, E.; Hardikar, W.; Stormon, M.; Baker, A.; Hierro, L.; Gliwicz, D.; Lacaille, F.; Lachaux, A.; Sturm, E.; Setchell, K.D.R.; et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): A randomised phase 2 study. Lancet 2021, 398, 1581–1592. [Google Scholar] [CrossRef]
- Mirum Pharmaceuticals. Livmarli™ (Maralixibat) Oral Solution; USPI (Package insert): Addison, TX, USA, 2021. [Google Scholar]
- Loomes, K.M.; Squires, R.H.; Kelly, D.; Rajwal, S.; Soufi, N.; Lachaux, A.; Jankowska, I.; Mack, C.; Setchell, K.D.R.; Karthikeyan, P.; et al. Maralixibat for the treatment of PFIC: Long-term, IBAT inhibition in an open-label, Phase 2 study. Hepatol. Commun. 2022, 6, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Javitt, N.B. Bile acid synthesis from cholesterol: Regulatory and auxiliary pathways. FASEB J. 1994, 8, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, E.M.; van Erpecum, K.J.; Beuers, U.; Hansen, B.E.; Thio, H.B.; de Man, R.A.; Janssen, H.L.; van Buuren, H.R. The potent bile acid sequestrant colesevelam is not effective in cholestatic pruritus: Results of a double-blind, randomized, placebo-controlled trial. Hepatology 2010, 52, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Ghent, C.N.; Bloomer, J.R.; Klatskin, G. Elevations in skin tissue levels of bile acids in human cholestasis: Relation to serum levels and topruritus. Gastroenterology 1977, 73, 1125–1130. [Google Scholar] [CrossRef]
- Wanichthanarak, K.; Jeamsripong, S.; Pornputtapong, N.; Khoomrung, S. Accounting for biological variation with linear mixed-effects modelling improves the quality of clinical metabolomics data. Comput. Struct. Biotechnol. J. 2019, 17, 611–618. [Google Scholar] [CrossRef] [PubMed]
- van Wessel, D.B.E.; Thompson, R.J.; Gonzales, E.; Jankowska, I.; Sokal, E.; Grammatikopoulos, T.; Kadaristiana, A.; Jacquemin, E.; Spraul, A.; Lipinski, P.; et al. Genotype correlates with the natural history of severe bile salt export pump deficiency. J. Hepatol. 2020, 73, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Setchell, K.D.R.; Huang, R.; Mallawaarachchi, I.; Ehsan, L.; Dobrzykowski Iii, E.; Zhao, J.; Syed, S.; Ma, J.Z.; Iqbal, N.T.; et al. Bile Acid Profiling Reveals Distinct Signatures in Undernourished Children with Environmental Enteric Dysfunction. J. Nutr. 2021, 151, 3689–3700. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Recent advances in understanding bile acid homeostasis. F1000Research 2017, 6, 2029. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.B.; Samuel, R.; Hegade, V.S.; Jones, D.E.; Reynolds, N.J. Pruritus secondary to primary biliary cholangitis: A review of the pathophysiology and management with phototherapy. Br. J. Dermatol. 2019, 181, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Heaton, K.W.; Burton, J.L. Pruritic effect of bile salts. Br. Med. J. 1974, 4, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Harrison, D.L.; Gilbert, J.M.; Mupthy, G.M. Serum unconjugated bile acids: Qualitative and quantitative profiles in ileal resection and bacterial overgrowth. Clin. Chim. Acta 1985, 152, 297–306. [Google Scholar] [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]



| Characteristics | All | Pruritus Responder | Pruritus Non-Responder |
|---|---|---|---|
| Number of participants | 19 | 11 | 8 |
| Age (at enrollment), y, median (IQR) | 3 (1.5, 4.5) | 3.0 (1, 4.5) | 3.5 (2.8, 5.5) |
| Female No. (%) | 68 | 73 | 63 |
| Pruritus score, median (IQR) | 1.9 (1.9, 2.1) | 2.5 (1.9, 3.0) | 1.9 (1.5,2.0) |
| Mutation | Mild/Moderate (7/12) | Mild/Moderate (4/7) | Mild/Moderate (3/5) |
| Total BA (µM), median (IQR) | 423 (286, 478) | 423 (238, 440) | 437 (303, 501) |
| 7α-C4 (ng/mL), median (IQR) | 2.3 (1.1, 3.8) | 1.9 (1.1, 4.8) | 2.5 (2.0,3.0) |
| FGF 19 (pg/mL), median (IQR) | 181 (107, 810) | 116 (84,568) | 687 (171,993) |
| Urso supplement (n) | 19 | 11 | 8 |
| ItchRO Weekly Average Score | |||
|---|---|---|---|
| Predictors | Estimates | CI | p-Value |
| (Intercept) | 1.49 | 0.47–2.51 | 0.005 * |
| Week | −0.01 | −0.02–−0.01 | <0.001 |
| Age | −0.04 | −0.14–0.07 | 0.479 |
| Pre-dose baseline (sBA) | −0.0003 | −0.00–0.00 | 0.779 |
| Gender | −0.06 | −0.42–0.31 | 0.761 |
| Total sBA | 0.002 | 0.00–0.00 | <0.001 |
| N Subject | 19 | ||
| Observations | 121 | ||
| ItchRO Weekly Average Score | Pruritus Responder | |||||
|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p-Value | Odds Ratios | CI | p-Value |
| (Intercept) | 2.17 | 1.80–2.54 | <0.001 | 0.00 | 0.00–0.00 | 0.008 |
| Week | −0.01 | −0.02–−0.00 | 0.001 | 0.99 | 0.78–1.26 | 0.940 |
| GCDCA | −0.02 | −0.02–−0.01 | <0.001 | 1.21 | 1.00–1.46 | 0.046 |
| N | 19 Subject_ID | 19 Subject_ID | ||||
| Observations | 121 | 121 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, W.; Vig, P.; Kostrub, C.; Setchell, K.D.R. Serum Bile Acid Profiling and Mixed Model Analysis Reveal Biomarkers Associated with Pruritus Reduction in Maralixibat-Treated Patients with BSEP Deficiency. Metabolites 2022, 12, 952. https://doi.org/10.3390/metabo12100952
Zhao X, Zhang W, Vig P, Kostrub C, Setchell KDR. Serum Bile Acid Profiling and Mixed Model Analysis Reveal Biomarkers Associated with Pruritus Reduction in Maralixibat-Treated Patients with BSEP Deficiency. Metabolites. 2022; 12(10):952. https://doi.org/10.3390/metabo12100952
Chicago/Turabian StyleZhao, Xueheng, Wujuan Zhang, Pamela Vig, Cory Kostrub, and Kenneth D. R. Setchell. 2022. "Serum Bile Acid Profiling and Mixed Model Analysis Reveal Biomarkers Associated with Pruritus Reduction in Maralixibat-Treated Patients with BSEP Deficiency" Metabolites 12, no. 10: 952. https://doi.org/10.3390/metabo12100952
APA StyleZhao, X., Zhang, W., Vig, P., Kostrub, C., & Setchell, K. D. R. (2022). Serum Bile Acid Profiling and Mixed Model Analysis Reveal Biomarkers Associated with Pruritus Reduction in Maralixibat-Treated Patients with BSEP Deficiency. Metabolites, 12(10), 952. https://doi.org/10.3390/metabo12100952