The Influence of Energy Balance, Lipolysis and Ketogenesis on Metabolic Adaptation in Cows Milked Twice and Three Times Daily
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Management
2.2. Energy Balance
2.3. Models and Cows Included in Experiment
2.4. Blood Metabolic Analysis
2.5. Statistical Analysis
3. Results
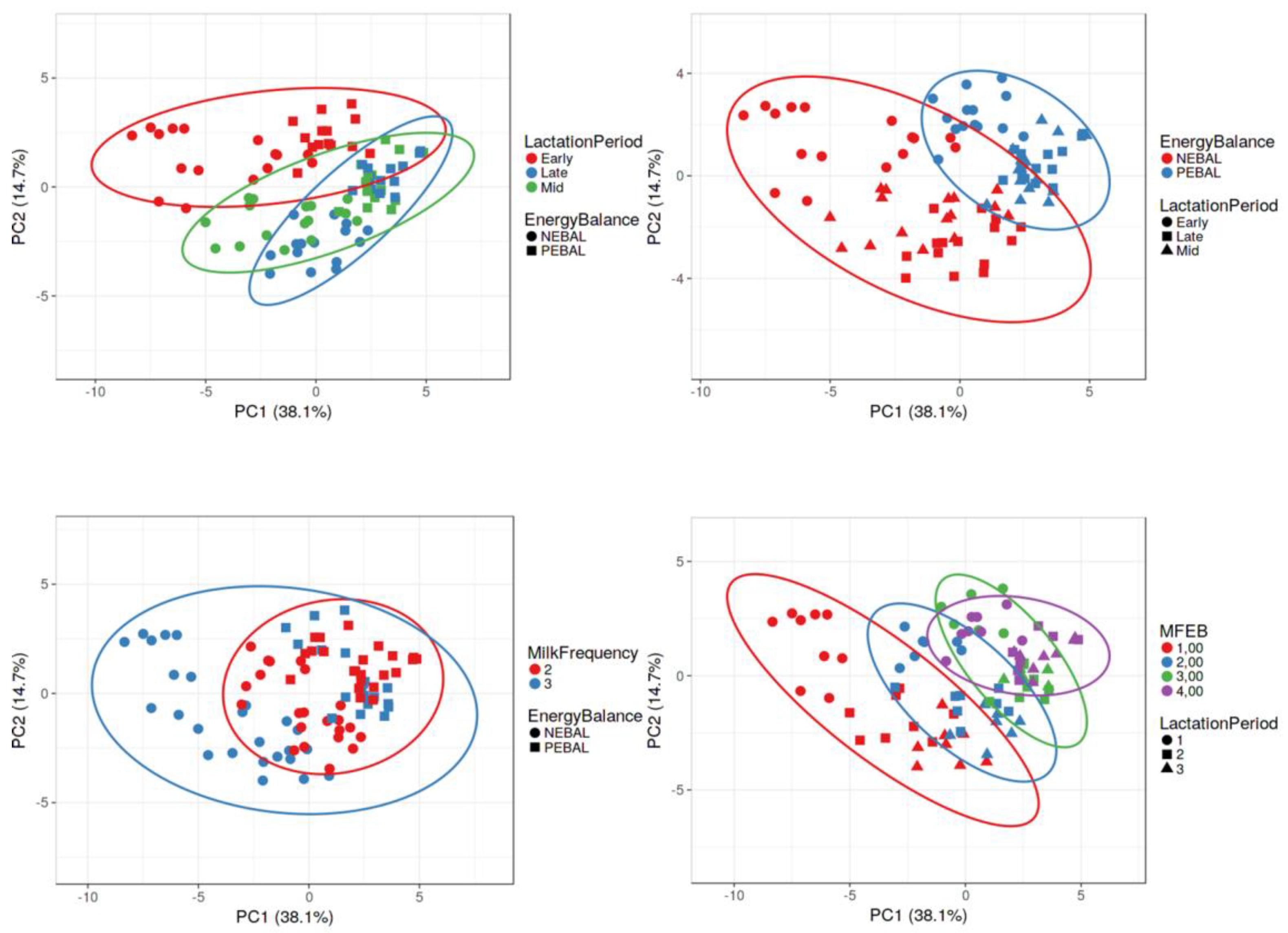
3.1. Clustering of Cows According to Lactation Period, Energy Balance, Milking Frequency, and Their Interactions Based on the Values of Blood Parameters
3.2. Value of Blood Parameters in Function of Lactation Period, Energy Balance, Milking Frequency, and Their Interactions
3.3. Correlation between Energy Balance, NEFA, BHB, and Other Blood Parameters in Double- and Triple-Milked Cows in Positive and Negative Energy Balance
4. Discussion
4.1. Milking Frequency and Energy Balance Adaptation
4.2. Milking Frequency, Energy Balance, and Metabolic Adaptation
4.3. Milking Frequency, Energy Balance, and Endocrine Adaptation
4.4. Limitation of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Everitt, G.C.; Phillips, D.S. Calf rearing by multiple suckling and the effects on the lactation performance of the cow. Proc. N. Z. Soc. Anim. Prod. 1971, 31, 22–40. [Google Scholar]
- Allen, D.B.; DePeters, E.J.; Laben, R.C. Three times a day milking: Effects on milk production, reproduction efficiency, and udder health. J. Dairy Sci. 1986, 69, 1441–1446. [Google Scholar] [CrossRef]
- Amos, H.; Kiser, T.; Loewenstein, M. Influence of milking frequency on productive and reproductive efficiencies of dairy cows. J. Dairy Sci. 1985, 68, 732–739. [Google Scholar] [CrossRef]
- DePeters, E.J.; Smith, N.E.; Acedo, R.J. Three or two times daily milking of older cows and first lactation cows for entire lactations. J. Dairy Sci. 1985, 68, 123–132. [Google Scholar] [CrossRef]
- Erdman, R.A.; Varner, M. Fixed yield response to increased milking frequency. J. Dairy Sci. 1995, 78, 1199–1203. [Google Scholar] [CrossRef]
- Bar-Pelled, U.; Maltz, E.; Bruckental, I.; Folman, Y.; Kali, Y.; Gacitua, H.; Lehrer, A.; Knight, C.; Robinson, B.; Voet, H.; et al. Relationship between frequent milking in early lactation and milk production of high producing dairy cows. J. Dairy Sci. 1995, 78, 2726–2736. [Google Scholar] [CrossRef]
- Hale, S.A.; Capuco, A.V.; Erdman, R.A. Milk yield and mammary growth effects due to increased milking frequency during early lactation. J. Dairy Sci. 2003, 86, 2061–2071. [Google Scholar] [CrossRef]
- Hristov, A.N.; Price, W.J.; Shafii, B. A meta-analysis on the relationship between intake of nutrients and body weight with milk volume and milk protein yield in dairy cows. J. Dairy Sci. 2005, 88, 2860–2869. [Google Scholar] [CrossRef]
- Barnes, M.A.; Pearson, R.E.; Lukes-Wilson, A.J. Effects of milking frequency and selection for milk yield on productive efficiency of Holstein cows. J. Dairy Sci. 1990, 73, 1603–1611. [Google Scholar] [CrossRef]
- Andersen, J.B.; Friggens, N.C.; Larse, T.; Vestergaard, M.; Ingvartsen, K.L. Effect of energy density in the diet and milking frequency on plasma metabolites and hormones in early lactation dairy cows. J. Vet. Med. A 2004, 51, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Soberon, F.; Lukas, J.L.; Van Amburgh, M.E.; Capuco, A.V.; Galton, D.M.; Overton, T.R. Effects of increased milking frequency on metabolism and mammary cell proliferation in Holstein dairy cows. J. Dairy Sci. 2010, 93, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Delamaire, E.; Guinard-Flamentm, J. Longer milking intervals alter mammary epithelial permeability and the udder’s ability to extract nutrients. J. Dairy Sci. 2006, 89, 2007–2016. [Google Scholar] [CrossRef]
- Veerkamp, R.; Koenen, E. Genetics of food intake, live weight, condition score and energy balance. BSAP Occas. Publ. 1999, 24, 63–73. [Google Scholar] [CrossRef]
- Hüttmann, H.; Stamer, E.; Junge, W.; Thaller, G.; Kalm, E. Analysis of feed intake and energy balance of high-yielding first lactating Holstein cows with fixed and random regression models. Animal 2009, 3, 181–188. [Google Scholar] [CrossRef]
- Li, B.; Fikse, W.; Løvendahl, P.; Lassen, J.; Lidauer, M.; Mäntysaari, P.; Berglund, B. Genetic heterogeneity of feed intake, energy-corrected milk, and body weight across lactation in primiparous Holstein, Nordic Red, and Jersey cows. J. Dairy Sci. 2018, 101, 10011–10021. [Google Scholar] [CrossRef]
- Patton, J.; Kenny, D.; Mee, J.; O’Mara, F.; Wathes, D.; Cook, M.; Murphy, J. Effect of milking frequency and diet on milk production, energy balance, and reproduction in dairy cows. J. Dairy Sci. 2006, 89, 1478–1487. [Google Scholar] [CrossRef]
- Stelwagen, K.; Phyn, C.; Davis, S.; Guinard-Flament, J.; Pomies, D.; Roche, J.; Kay, J. Invited review: Reduced milking frequency: Milk production and management implications. J. Dairy Sci. 2013, 96, 3401–3413. [Google Scholar] [CrossRef]
- Schlamberger, G.; Wiedemann, S.; Vitrro, E.; Meyer, H.H.D.; Kaske, M. Effects of continuous milking during the dry period or once daily milking in the first 4 weeks of lactation on metabolism and productivity of dairy cows. J. Dairy Sci. 2010, 93, 2471–2485. [Google Scholar] [CrossRef]
- McNamara, S.; Murphy, J.J.; O’Mara, F.P.; Rath, M.; Mee, J.F. Effect of milking frequency on energy metabolism, milk production and reproductive performance of dairy cows. Livestock Sci. 2008, 117, 7078. [Google Scholar] [CrossRef]
- Williamson, M.; Serrenho, R.C.; McBride, B.W.; LeBlanc, S.J.; DeVries, T.J.; Duffield, T.F. Reducing milking frequency from twice to once daily as an adjunct treatment for ketosis in lactating dairy cows—A randomized controlled trial. J. Dairy Sci. 2022, 105, 1402–1417. [Google Scholar] [CrossRef]
- Cincović, M.R.; Đoković, R.; Belić, B.; Lakić, I.; Stojanac, N.; Stevančević, O.; Staničkov, N. Insulin resistance in cows during the periparturient period. Acta Agric. Serb. 2018, 23, 233–245. [Google Scholar] [CrossRef]
- Cincović, M.R.; Belić, B.; Radojičić, B.; Hristov, S.; Đoković, R. Influence of lipolysis and ketogenesis to metabolic and hematological parameters in dairy cows during periparturient period. Acta Vet. 2012, 62, 429–444. [Google Scholar] [CrossRef]
- Belić, B.; Cincović, M.; Lakić, I.; Đoković, R.; Petrović, M.; Ježek, J.; Starič, J. Metabolic status of dairy cows grouped by anabolic and catabolic indicators of metabolic stress in early lactation. Acta Sci. Vet. 2018, 46, 9. [Google Scholar] [CrossRef]
- Djoković, R.; Cincović, M.; Belić, B.; Toholj, B.; Davidov, I.; Hristovska, T. Relationship between blood metabolic hormones, metabolites and energy balance in Simmental dairy cows during peripartum period and lactation. Pak. Vet. J. 2015, 35, 163–167. [Google Scholar]
- Djoković, R.; Kurćubić, V.; Ilić, Z.; Cincović, M.; Lalović, M.; Jašović, B.; Bojkovski, J. Correlation between blood biochemical metabolites milk yield, dry matter intake and energy balance in dairy cows during early and mid lactation. Adv. Diab. Metab. 2017, 5, 26–30. [Google Scholar] [CrossRef]
- Petrović, M.; Cincović, M.; Starič, J.; Djoković, R.; Belić, B.; Radinović, M.; Majkić, M.; Ilić, Z. The Correlation between Extracellular Heat Shock Protein 70 and Lipid Metabolism in a Ruminant Model. Metabolites 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- NRC-National Research Council. Nutrient Requirements of Dairy Cattle; National Academies Press: Washington, DC, USA, 2001.
- Grubić, G.; Đorđević, N.; Milenković, A. Ocena Ispunjenosti Jasala u Ishrani Muznih Krava. XX Savetovanje Agronoma, Veterinara i Tehnologa; Zbornik Naučnih Radova; Institut PKB Agroekonomik: Padinska Skela-Beograd, Serbia, 2006. [Google Scholar]
- Reist, M.; Erdin, D.; Von Euw, D.; Tschuemperlin, K.; Leuenberger, H.; Chilliard, Y.; Hammon, H.M.; Morel, C.; Zbinden, Y.; Kuenzi, J.; et al. Estimation of energy balance at the individual and herd level using blood and milk traits in high-yielding dairy cows. J. Dairy Sci. 2002, 85, 3314–3327. [Google Scholar] [CrossRef]
- Ilyas, M.; Adzim, M.; Simbak, N.; Atif, A. Sample size calculation for animal studies using degree of freedom (E); an easy and statistically defined approach for metabolomics and genetic research. ILAR J. 2017, 43, 207–213. [Google Scholar]
- Guyot, H.; Detilleux, J.; Lebreton, P.; Garnier, C.; Bonvoisin, M.; Rollin, F.; Sandersen, C. Comparison of Various Indices of Energy Metabolism in Recumbent and Healthy Dairy Cows. PLoS ONE 2017, 12, e0169716. [Google Scholar] [CrossRef]
- Sanchez-Duarte, J.I.; Garcia, A.; Rodríguez-Hernández, K.; Reta-Sánchez, D.G.; Salinas-Gonzalez, H.; Ochoa-Martínez, E.; Reyes-González, A. Production response in dairy cows milked two or three times a day: A meta-analysis. Vet. Mex. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Ayadi, M. Optimization of Milking Frequency in Dairy Ruminants. In Lactation in Farm Animals-Biology, Physiological Basis, Nutritional Requirements, and Modelization; M’Hamdi, N., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Leduc, A.; Souchet, S.; Gelé, M.; Le Provost, F.; Boutinaud, M. Effect of feed restriction on dairy cow milk production: A review. J. Anim. Sci. 2021, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.W.; Custodio, A.A. Feed efficiency: A composite trait of dairy cattle. J. Dairy Sci. 1984, 67, 2075–2083. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, K.; Liu, J. Effects of glucose availability on expression of the key genes involved in synthesis of milk fat, lactose and glucose metabolism in bovine mammary epithelial cells. PLoS ONE 2013, 8, e66092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q. Biology of glucose transport in the mammary gland. J. Mammary Gland. Biol. 2014, 19, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.; Sundrum, A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals 2020, 10, 1028. [Google Scholar] [CrossRef]
- Guinard-Flament, J.; Delamaire, E.; Lamberton, P.; Peyraud, J.L. Adaptions of mammary uptake and nutrient use to oncedaily milking and feed restriction in dairy cows. J. Dairy Sci. 2007, 90, 5062–5072. [Google Scholar] [CrossRef]
- Cincović, M.; Kirovski, D.; Vujanac, I.; Belić, B.; Djoković, R. Relationship between the indexes of insulin resistance and metabolic status in dairy cows during early lactation. Acta Vet. 2017, 67, 57–70. [Google Scholar] [CrossRef]
- Bjerre-Harpøth, V.; Friggens, N.; Thorup, V.; Larsen, T.; Damgaard, B.; Ingvartsen, K.; Moyes, K. Metabolic and production profiles of dairy cows in response to decreased nutrient density to increase physiological imbalance at different stages of lactation. J. Dairy Sci. 2012, 95, 2362–2380. [Google Scholar] [CrossRef]
- Wathes, D.C.; Cheng, Z.; Bourne, N.; Taylor, V.J.; Coffey, M.P.; Brotherstone, S. Differences between primiparous and multiparous dairy cows in the inter-relationships between metabolic traits, milk yield and body condition score in the periparturient period. Domest. Anim. Endocrinol. 2006, 33, 203–225. [Google Scholar] [CrossRef]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and β-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef]
- Overton, T.R.; McArt, J.A.A.; Nydam, D.V. A 100-Year Review: Metabolic health indicators and management of dairy cattle. J. Dairy Sci. 2017, 100, 10398–10417. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using nonesterified fatty acids and β-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Mann, S.; Nydam, D.V.; Overton, T.R.; McArt, J.A.A. Concentrations of nonesterified fatty acids and β-hydroxybutyrate in dairy cows are not well correlated during the transition period. J. Dairy Sci. 2015, 98, 6284–6290. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, F.; DiGiacomo, K.; Cameron, A.W.N.; Leury, B.J. Associations between nonesterified fatty acids, β-hydroxybutyrate, and glucose in periparturient dairy goats. J. Dairy Sci. 2020, 103, 6672–6678. [Google Scholar] [CrossRef] [PubMed]
- Botham, K.M.; Mayes, P.A. Oxidation of fatty acids: Ketogenesis. In Harper’s Illustrated Biochemistry; Rodwell, V.W., Bender, D.A., Botham, K.M., Kennelly, P.J., Weil P, Eds.; McGraw Hill: New York, NY, USA, 2016. [Google Scholar]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet. Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef]
- Hachenberg, S.; Weinkauf, C.; Hiss, S.; Sauerwein, H. Evaluation of classification modes potentially suitable to identify metabolic stress in healthy dairy cows during the peripartal period. J. Anim. Sci. 2007, 85, 1923–1932. [Google Scholar] [CrossRef][Green Version]
- Kessel, S.; Stroehl, M.; Meyer, H.H.D.; Hiss, S.; Sauerwein, H.; Schwarz, F.J.; Bruckmaier, R.M. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J. Anim. Sci. 2008, 86, 2903–2912. [Google Scholar] [CrossRef]
- Doepel, L.; Lapierre, H.; Kennelly, J.J. Peripartum Performance and Metabolism of Dairy Cows in Response to Prepartum Energy and Protein Intake. J. Dairy Sci. 2002, 85, 2315–2334. [Google Scholar] [CrossRef]
- Delić, B.; Belić, B.; Cincović, M.R.; Djokovic, R.; Lakić, I. Metabolic adaptation in first week after calving and early prediction of ketosis type I and II in dairy cows. Large Anim. Rev. 2020, 26, 51–55. [Google Scholar]
- Marinković, M.D.; Belić, B.; Cincović, M.R.; Đoković, R.; Lakić, I.; Stojanac, N.; Stevančević, O.; Devečerski, G. Relationship between insulin, glucose, non-esterified fatty acid and indices of insuliresistance in obese cows during the dry period and early lactation. Acta Vet Brno. 2019, 88, 143–155. [Google Scholar] [CrossRef]
- Mostafavi, M.; Seifi, H.A.; Mohri, M.; Jamshidi, A. Optimal thresholds of metabolic indicators of hepatic lipidosis in dairy cows. Rev. Méd. Vét. 2013, 164, 564–571. [Google Scholar]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited Review: Pathology, Etiology, Prevention, and Treatment of Fatty Liver in Dairy Cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Djoković, R.; Ilić, Z.; Kurćubić, V.; Petrović, M.; Dosković, V. Functional and morphological state of the liver in Simmental dairy cows during transitional period. Rev. Méd. Vét. 2011, 162, 574–579. [Google Scholar]
- Seal, C.J.; Reynolds, C.K. Nutritional implications of gastrointestinal and liver metabolism in ruminants. Nutr. Res. Rev. 1993, 6, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, M.; Markiewicz, H. Culling and fertility of cows with energy metabolic disturbances in the early postparturient period. Bull. Vet. Inst. Pulawy 2009, 53, 375–381. [Google Scholar]
- Rastani, R.R.; Lobos, N.E.; Aguerre, M.J.; Grummer, R.R.; Wattiaux, M.A. Relationships between blood urea nitrogen and energy balance or measures of tissue mobilization in Holstein cows during the periparturient period. Appl. Anim. Sci. 2006, 22, 382–385. [Google Scholar] [CrossRef]
- Šamanc, H.; Kirovski, D.; Stojic, V.; Stojanović, D.; Vujanac, I.; Prodanovic, R.; Bojkovic-Kovacevic, S. Application of the metabolic profile test in the prediction and diagnosis of fatty liver in Holstein cows. Acta Vet. 2011, 61, 543–553. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Liu, G.; Li, X.; Xie, G.; Xia, C.; Zhang, H.Y. Metabolic Characteristic of the Liver of Dairy Cows during Ketosis Based on Comparative Proteomics. Asian-Australas J. Anim. Sci. 2008, 21, 1003–1010. [Google Scholar] [CrossRef]
- Schutz, Y. Protein turnover, ureagenesis and gluconeogenesis. Int. J. Vitamin Nutr. Res. 2011, 81, 101–107. [Google Scholar] [CrossRef]
- Marczuk, J.; Brodzki, P.; Brodzki, A.; Kurek, Ł. The concentration of free amino acids in blood serum of dairy cows with primary ketosis. Pol. J. Vet. Sci. 2018, 21, 149–156. [Google Scholar] [CrossRef]
- Larsen, T.; Møller, G.; Bellio, R. Evaluation of Clinical and Clinical Chemical Parameters in Periparturient Cows. J. Dairy Sci. 2001, 84, 1749–1758. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of subclinical hypocalcemia in dairy herds. Vet. J. 2011, 188, 122–124. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic predictors of displaced abomasum in dairy cattle. J. Dairy Sci. 2005, 88, 159–170. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Djoković, R.; Šamanc, H.; Nikolić, Z.; Bošković-Bogosavljević, S. Changes in blood values of glucose, insulin and inorganic phosphorus in healthy and ketotic dairy cows after intravenous infusion of propionate solution. Acta Vet. Brno. 2007, 76, 533–539. [Google Scholar] [CrossRef]
- Cincović, M.R.; Djoković, R.; Belić, B.; Potkonjak, A.; Toholj, B.; Stojanac, N.; Stevančević, O.; Starič, J. Inorganic phosphorus decrease after intravenous glucose tolerance test is associated with insulin resistance in dairy cows. Vet. Arh. 2017, 87, 409–418. [Google Scholar] [CrossRef]
- Loiselle, M.; Ster, C.; Talbot, B.; Zhao, X.; Wagner, G.; Boisclair, Y.; Lacasse, P. Impact of postpartum milking frequency on the immune system and the blood metabolite concentration of dairy cows. J. Dairy Sci. 2009, 92, 1900–1912. [Google Scholar] [CrossRef]
- Rémond, B.; Pomiès, D. Once-daily milking of dairy cows: A review of recent French experiments. Anim. Res. 2005, 54, 427–442. [Google Scholar] [CrossRef][Green Version]
- Steinwidder, A.; Rohrer, H.; Pfister, R.; Gallnböck, M.; Podstatzky, L.; Gasteiner, J. Effects of concentrate supplementation strategies during the transition period and milking frequency in early lactation on seasonal winter-calving organic dairy cows. Livestock Sci. 2021, 250, 104595. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
- Oliveira, L.H.; Nascimento, A.B.; Monteiro, P.L.J., Jr.; Guardieiro, M.M.; Wiltbank, M.C.; Sartori, R. Development of insulin resistance in dairy cows by 150 days of lactation does not alter oocyte quality in smaller follicles. J. Dairy Sci 2016, 99, 9174–9183. [Google Scholar] [CrossRef]
- Hayirli, A. The role of exogenous insulin in the complex of hepatic lipidosis and ketosis associated with insulin resistance phenomenon in postpartum dairy cattle. Vet. Res. Commun. 2006, 30, 749–774. [Google Scholar] [CrossRef] [PubMed]
- Cincović, M.; Belić, B.; Djokovic, R.; Toholj, B.; Hristovska, T. Insulin resistance in cow during dry period and early lactation. Contemp. Agric. 2014, 63, 98–105. [Google Scholar]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Singh, S.; Bruckmaier, R.; Becker, F.; Kanitz, W.; et al. Variation in fat mobilization during early lactation differently affects feed intake, body condition, and lipid and glucose metabolism in high-yielding dairy cows. J. Dairy Sci. 2013, 96, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Huszenicza, G.; Kulcsar, M.; Rudas, P. Clinical endocrinology of thyroid gland function in ruminants: A review of literature. Vet. Med. Czech. 2002, 47, 191–202. [Google Scholar]
- Šamanc, H.; Stojić, V.; Kirovski, D.; Jovanović, M.; Cernescu, H.; Vujanac, I. Thyroid hormones concentrations during the mid-dry period: An early indicator of fatty liver in Holstein-Friesian dairy cows. J. Thyroid Res. 2010, 2010, 1–6. [Google Scholar] [CrossRef][Green Version]
- Steinhoff, L.; Jung, K.; Meyerholz, M.M.; Heidekorn-Dettmer, J.; Hoedemaker, M.; Schmicke, M. Thyroid hormone profiles and TSH evaluation during early pregnancy and the transition period in dairy cows. Theriogenology 2019, 129, 23–28. [Google Scholar] [CrossRef]
- Tiirats, T. Thyroxine, triiodothyronine and reverse-triiodothyronine concentrations in blood plasma in relation to lactational stage, milk yield, energy and dietary protein intake in Estonian dairy cows. Acta. Vet. Scand. 1997, 38, 339–348. [Google Scholar] [CrossRef]
- Mohebbi-Fani, M.; Omidi, A.; Mirzaei, A.; Nazifi, S.; Nowroozi, K. A field study on glucose, non-esterified fatty acids, beta-hydroxybutyrate and thyroid hormones in dairy cows during the breeding period in Fars province, Iran. Iran. J. Vet. Res. 2019, 20, 55–59. [Google Scholar]
- Moyes, K.M.; Drackley, J.K.; Salak-Johnson, J.L.; Morin, D.E.; Hope, J.C.; Loor, J.J. Dietary-induced negative energy balance has minimal effects on innate immunity during a Streptococcus uberis mastitis challenge in dairy cows during midlactation. J. Dairy Sci. 2009, 92, 4301–4316. [Google Scholar] [CrossRef]
- Beerda, B.; Kornalijnslijper, J.E.; Van der Werf, J.T.N.; Noordhuizen-Stassen, E.N.; Hopster, H. Effects of Milk Production Capacity and Metabolic Status on HPA Function in Early Postpartum Dairy Cows. J. Dairy Sci. 2004, 87, 2094–2102. [Google Scholar] [CrossRef]
- Forslund, B.K.; Ljungval, A.O.; Jones, V.B. Low cortisol in blood from dairy cows with ketosis: A field study. Acta. Vet. Scand. 2010, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R.; Drackley, J.K.; Douglas, G.N.; Emmert, L.S.; Clarck, J.H. Hepatic gluconeogenesis and whole-body protein metabolism of periparturient dairy cows as affected by source of energy and intake of the prepartum diet. J. Dairy Sci. 1998, 81, 295. [Google Scholar]
- Šamanc, H.A.; Kirovski, D. Adrenokortikalni Sistem Goveda; Naučni Institut za Veterinarstvo Srbije: Beograd, Serbia, 2008. [Google Scholar]
- Lucy, M.; Verkerk, G.; Whyte, B.; Macdonald, K.; Burton, L.; Cursons, R.; Roche, J.; Holmes, C. Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in pasture system. J. Dairy Sci. 2009, 92, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Abeni, F.; Calamari, L.; Calza, F.; Speroni, M.; Bertoni, G.; Pirlo, G. Welfare assessment based on metabolic and endocrine aspects in primiparous cows milked in a parlor or with an automatic milking system. J. Dairy Sci. 2005, 88, 3542–3552. [Google Scholar] [CrossRef]
- Gygax, L.; Neuffer, I.; Kaufmann, C.; Hauser, R.; Wechsler, B. Milk cortisol concentration in automatic milking systems compared with auto-tandem milking parlors. J. Dairy Sci. 2006, 89, 3447–3454. [Google Scholar] [CrossRef]
- Heuer, C.; Van Straalen, W.M.; Schukken, Y.H.; Dirkzwager, A.; Noordhuizen, J.P.T.M. Prediction of energy balance in high yielding dairy cows with test-day information. J. Dairy Sci. 2001, 84, 471–481. [Google Scholar] [CrossRef]
- Rumphorst, T.; Scheu, T.; Koch, C.; Sundrum, A. Balancing Trade-Offs in Milk Production by Making Use of Animal Individual Energy Balancing. Dairy 2022, 3, 345–363. [Google Scholar] [CrossRef]
- Nishiura, A.; Sasaki, O.; Tanigawa, T.; Kubota, A.; Takeda, H.; Saito, Y. Prediction of energy balance from milk traits of Holsteins in Japan. Anim. Sci. J. 2022, 93, e13757. [Google Scholar] [CrossRef]
- Rumphorst, T.; Scheu, T.; Koch, C.; Sundrum, A. Inter- and Intra-Individual Variation in the Behavior of Feed Intake on Nutrient Availability in Early Lactating Dairy Cows. Animals 2022, 12, 37. [Google Scholar] [CrossRef]

| Component * | Dry Matter, % | Crude Protein, % | Crude Fat, % | Raw Ash, % | NEL **, MJ/kg | NDF, % | ADF, % | Lignin,% | Ca, % | P, % | kg DM | CONCENTRATE, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43.6 | 7 | 3.3 | 3.1 | 6.73 | 32.2 | 17.5 | 1.4 | 0.28 | 0.26 | 8.72 | / |
| 2 | 33 | 17.3 | 2.5 | 10.4 | 5.43 | 44.1 | 33.4 | 6.6 | 1.39 | 0.36 | 1.65 | / |
| 3 | 84.3 | 19.6 | 1.4 | 11.5 | 5.8 | 40.9 | 29.9 | 5.5 | 1.37 | 0.3 | 1.94 | / |
| 4 | 26.9 | 10 | 7 | 4 | 7.34 | 48 | 23.1 | 5 | 0.91 | 0.09 | 1.08 | / |
| 5 | 21.8 | 28.4 | 5.2 | 4.9 | 7.15 | 47.1 | 23.1 | 4.7 | 0.35 | 0.59 | 0.96 | / |
| 6 | 88.1 | 9.4 | 4.3 | 1.5 | 8.41 | 9.5 | 3.4 | 0.9 | 0.04 | 0.3 | 5.38 | 50.33 |
| 7 | 91 | 12.4 | 2.2 | 2.9 | 7.78 | 20.8 | 7.2 | 1.9 | 0.06 | 0.39 | 0.61 | 5.52 |
| 8 | 90.3 | 37.8 | 5.4 | 7.4 | 7.36 | 29.8 | 20.5 | 9.5 | 0.75 | 1.1 | 0.92 | 8.40 |
| 9 | 89.1 | 49.9 | 1.6 | 6.6 | 8.91 | 14.9 | 10 | 0.7 | 0.4 | 0.71 | 0.87 | 8.07 |
| 10 | 92.2 | 37 | 1.4 | 7.7 | 5.78 | 40.3 | 30 | 9.5 | 0.48 | 1 | 1.44 | 12.85 |
| 11 | 90.3 | 32.6 | 1.7 | 6.5 | 6.57 | 36.1 | 22.1 | 8.3 | 0.4 | 0.83 | 0.55 | 5.02 |
| 12 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 39.4 | 0 | 0.13 | 1.07 |
| 13 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0.06 | 0.49 |
| 14 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0.06 | 0.49 |
| 15 | 98 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0.41 |
| 16 | 99 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 32.8 | 0 | 0.18 | 1.48 |
| 17 | 99 | 56 | 0 | 85.1 | 0 | 0 | 0 | 0 | 12 | 16 | 0.12 | 0.99 |
| 18 | 99 | 283 | 0 | 0.03 | 1.9 | 0 | 0 | 0 | 6.8 | 0 | 0.18 | 1.48 |
| 19 | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0.16 |
| 20 | 100 | 0 | 99 | 0 | 32.64 | 0 | 0 | 0 | 0 | 0 | 0.39 | 3.21 |
| 21 | 52.89 | 17.01 | 4.76 | 6.81 | 7.24 | 28.13 | 16.53 | 3.1 | 0.97 | 0.45 | 25.3 | 100.0 |
| Production Parameters | Energy Balance | Cows Milked 3X Daily | Cows Milked 2X Daily | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early | Mid | Late | Early | Mid | Late | ||||||||
| Mean | SD * | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Milk (kg/day) | Neg | 30.7 | 2.4 | 43.8 | 5.03 | 40.0 | 5.5 | 29.7 | 1.7 | 37.3 | 1.7 | 38.0 | 3.1 |
| Pos | 25.2 | 1.83 | 40.1 | 1.77 | 41.3 | 2.3 | 25.9 | 1.6 | 34.0 | 2.6 | 35.1 | 2.5 | |
| Milk Fat (%) | Neg | 4.61 | 0.92 | 4.46 | 0.43 | 4.35 | 0.44 | 4.32 | 0.71 | 4.48 | 0.4 | 4.7 | 0.51 |
| Pos | 3.57 | 0.28 | 3.47 | 0.26 | 3.53 | 0.31 | 3.70 | 0.4 | 3.8 | 0.2 | 3.63 | 0.2 | |
| Milk Protein (%) | Neg | 3.65 | 0.35 | 3.89 | 0.49 | 3.95 | 0.50 | 3.40 | 0.1 | 3.57 | 0.8 | 3.96 | 0.6 |
| Pos | 3.48 | 0.45 | 3.95 | 0.5 | 2.47 | 0.65 | 3.73 | 0.3 | 3.57 | 0.7 | 3.58 | 0.2 | |
| Lactose (%) | Neg | 4.93 | 0.1 | 5.03 | 0.2 | 4.95 | 0.25 | 4.94 | 0.15 | 5.06 | 0.2 | 4.91 | 0.15 |
| Pos | 4.82 | 0.12 | 4.85 | 0.11 | 4.78 | 0.11 | 4.81 | 0.2 | 4.8 | 0.2 | 4.73 | 0.07 | |
| Energy-corrected Milk (kg/day) | Neg | 34.3 | 6.03 | 48.8 | 5.4 | 43.9 | 5.14 | 31.3 | 2.97 | 40.72 | 2.07 | 43.32 | 3.09 |
| Pos | 23.9 | 1.76 | 36.47 | 1.04 | 37.6 | 0.96 | 25.4 | 2 | 33.87 | 1.92 | 33.97 | 2.64 | |
| DMI Predicted (kg/day) | Neg | 19.8 | 2.28 | 26.8 | 1.94 | 25.51 | 1.73 | 19.6 | 1.1 | 24.1 | 0.8 | 25.3 | 1.04 |
| Pos | 17.3 | 1.02 | 23 | 0.35 | 23.41 | 0.31 | 18.04 | 0.75 | 21.9 | 0.77 | 22.19 | 0.88 | |
| Body Weight (kg) | Neg | 582.2 | 15.5 | 611.2 | 19.9 | 619.7 | 15.5 | 592.6 | 17.5 | 620.2 | 10.4 | 629.5 | 12.6 |
| Pos | 588.3 | 22.3 | 605.6 | 21.5 | 625.8 | 16.8 | 589.5 | 18.9 | 611.4 | 15.2 | 631.7 | 14.3 | |
| Energy Balance (MJ NEL/day) | Neg | −13.68 | 4.95 | −11.27 | 4.09 | −5.01 | 2.37 | −5.88 | 2.37 | −4.73 | 1.84 | −4.51 | 2.55 |
| Pos | 1.44 | 0.92 | 0.98 | 0.86 | 0.9 | 0.31 | 1.97 | 1.5 | 1.94 | 1.5 | 3.31 | 2.21 | |
| Number of Cows | Neg | 9 | 7 | 8 | 7 | 8 | 7 | ||||||
| Pos | 6 | 8 | 7 | 8 | 7 | 8 | |||||||
| MF | EB | LP | MF×EB | MF×LP | EB×LP | MF×EB×LP | |
|---|---|---|---|---|---|---|---|
| Milk_LperDay | 0.000 | 0.000 | 0.000 | 0.131 | 0.033 | 0.214 | 0.334 |
| MilkFat | 0.925 | 0.000 | 0.984 | 0.077 | 0.373 | 0.724 | 0.030 |
| MilkProtein | 0.593 | 0.000 | 0.448 | 0.244 | 0.004 | 0.000 | 0.066 |
| Lactose | 0.118 | 0.000 | 0.075 | 0.153 | 0.450 | 0.141 | 0.438 |
| NEFA | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.050 | 0.144 |
| AST | 0.001 | 0.000 | 0.000 | 0.001 | 0.071 | 0.041 | 0.179 |
| GGT | 0.001 | 0.000 | 0.000 | 0.001 | 0.072 | 0.041 | 0.179 |
| Ca | 0.000 | 0.188 | 0.527 | 0.109 | 0.928 | 0.183 | 0.170 |
| P | 0.325 | 0.009 | 0.431 | 0.073 | 0.075 | 0.389 | 0.941 |
| TPROT | 0.114 | 0.012 | 0.269 | 0.924 | 0.972 | 0.198 | 0.841 |
| ALB | 0.815 | 0.000 | 0.008 | 0.001 | 0.944 | 0.540 | 0.103 |
| UREA | 0.048 | 0.000 | 0.000 | 0.164 | 0.280 | 0.667 | 0.052 |
| INS | 0.265 | 0.000 | 0.431 | 0.034 | 0.746 | 0.869 | 0.001 |
| RQUICKIBHB | 0.108 | 0.000 | 0.001 | 0.397 | 0.158 | 0.001 | 0.984 |
| T3 | 0.012 | 0.000 | 0.000 | 0.000 | 0.801 | 0.299 | 0.019 |
| T4 | 0.647 | 0.002 | 0.000 | 0.000 | 0.079 | 0.012 | 0.118 |
| CORT | 0.142 | 0.000 | 0.002 | 0.010 | 0.953 | 0.150 | 0.223 |
| Parameter | Energy Balance | 3X Milking | 2X Milking | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early | Mid | Late | Early | Mid | Late | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| NEFA (mmol/L) | Neg | 1.29 a | 0.32 | 0.68 b | 0.17 | 0.43 c | 0.20 | 0.60 b | 0.19 | 0.34 c | 0.16 | 0.38 c | 0.19 |
| Pos | 0.74 b | 0.17 | 0.35 c | 0.11 | 0.32 c | 0.15 | 0.59 b | 0.21 | 0.37 c | 0.10 | 0.42 c | 0.15 | |
| BHB (mmol/L) | Neg | 2.15 a | 0.29 | 1.46 c | 0.41 | 1.04 e | 0.47 | 0.67 d | 0.21 | 0.36 f | 0.24 | 0.50 d | 0.17 |
| Pos | 0.89 b | 0.30 | 0.64 d | 0.16 | 0.41 f | 0.11 | 0.51 d | 0.26 | 0.30 f | 0.15 | 0.50 d | 0.15 | |
| GLU (mmol/L) | Neg | 1.94 a | 0.19 | 2.20 c | 0.21 | 2.56 d | 0.14 | 2.27 c | 0.08 | 2.73 f | 0.29 | 2.88 f | 0.22 |
| Pos | 2.41 b | 0.11 | 2.54 d | 0.17 | 2.78 e | 0.11 | 2.33 c | 0.11 | 2.74 f | 0.36 | 3.00 f | 0.29 | |
| CHOL (mmol/L) | Neg | 2.58 a | 0.30 | 2.74 a | 0.53 | 3.63 c | 0.65 | 3.48 c | 0.38 | 4.08 b | 0.58 | 4.51 b | 0.63 |
| Pos | 4.08 b | 0.99 | 4.50 b | 0.78 | 4.49 b | 1.17 | 3.13 c | 1.08 | 4.20 b | 1.50 | 4.49 b | 1.07 | |
| TGC (mmol/L) | Neg | 0.09 a | 0.01 | 0.09 a | 0.02 | 0.12 c | 0.02 | 0.12 c | 0.01 | 0.14 d | 0.02 | 0.15 d | 0.02 |
| Pos | 0.16 b | 0.04 | 0.17 b | 0.03 | 0.17 b | 0.04 | 0.12 c | 0.04 | 0.16 b | 0.06 | 0.17 b | 0.04 | |
| TBIL (μmol/L) | Neg | 18.6 a | 4.79 | 11.3 c | 5.34 | 6.73 e | 2.52 | 8.86 f | 3.40 | 8.36 f | 5.31 | 5.05 e | 1.94 |
| Pos | 9.52 b | 3.59 | 4.11 d | 1.42 | 4.47 d | 1.48 | 8.08 f | 1.94 | 5.63 e | 1.72 | 4.82 d | 1.86 | |
| AST (U/L) | Neg | 96.8 a | 24.9 | 58.8 b | 27.8 | 35.0 d | 13.1 | 46.1 b | 17.7 | 43.5 b | 27.6 | 26.3 e | 10.1 |
| Pos | 46.6 b | 17.6 | 20.1 c | 6.94 | 21.9 c | 7.25 | 39.6 b | 9.48 | 27.6 e | 8.42 | 23.6 c | 9.11 | |
| GGT (U/L) | Neg | 32.3 a | 8.30 | 19.6 b | 9.25 | 11.7 d | 4.37 | 15.6 b | 5.90 | 14.5 b | 9.20 | 8.75 c | 3.36 |
| Pos | 15.6 b | 5.86 | 6.71 c | 2.31 | 7.30 c | 2.42 | 13.2 b | 3.16 | 9.19 d | 2.81 | 7.87 c | 3.04 | |
| Ca (mmol/L) | Neg | 2.22 a | 0.46 | 2.00 a | 0.40 | 1.86 b | 0.44 | 2.73 d | 0.20 | 2.53 d | 0.22 | 2.66 d | 0.29 |
| Pos | 2.18 a | 0.25 | 2.13 a | 0.15 | 2.40 c | 0.23 | 2.60 d | 0.33 | 2.67 d | 0.52 | 2.59 d | 0.20 | |
| P (mmol/L) | Neg | 1.89 a | 0.19 | 1.95 a | 0.22 | 1.72 a | 0.49 | 1.77 a | 0.60 | 1.93 a | 0.22 | 2.04 b | 0.18 |
| Pos | 2.22 b | 0.47 | 2.13 c | 0.06 | 2.21 b | 0.18 | 1.82 a | 0.41 | 1.91 a | 0.37 | 2.21 b | 0.42 | |
| TPROT (g/L) | Neg | 57.4 a | 5.78 | 63.5 c | 5.98 | 63.4 c | 6.99 | 65.4 c | 6.25 | 63.8 c | 5.48 | 69.7 c | 7.61 |
| Pos | 75.1 b | 10.8 | 77.6 b | 6.32 | 79.9 b | 3.92 | 68.9 c | 7.69 | 76.9 b | 4.49 | 73.9 b | 3.29 | |
| ALB (g/L) | Neg | 23.1 a | 3.31 | 26.9 b | 3.55 | 30.5 b | 3.63 | 25.4 a | 4.13 | 32.3 d | 2.94 | 32.9 d | 3.58 |
| Pos | 26.6 b | 6.29 | 38.4 c | 4.28 | 36.0 c | 6.29 | 32.7 d | 6.98 | 33.9 d | 4.30 | 36.2 c | 5.19 | |
| UREA (mmol/L) | Neg | 7.53 a | 1.63 | 4.99 b | 1.31 | 4.42 b | 2.02 | 6.57 a | 1.32 | 5.33 b | 1.29 | 4.32 c | 1.25 |
| Pos | 5.35 b | 1.22 | 4.04 b | 1.73 | 4.99 b | 1.25 | 3.74 c | 1.19 | 4.00 c | 1.47 | 4.38 c | 1.22 | |
| INS (mU/L) | Neg | 2.49 a | 0.97 | 4.45 b | 0.90 | 4.01 b | 0.70 | 3.04 c | 0.61 | 3.38 c | 1.31 | 3.14 c | 0.69 |
| Pos | 4.42 b | 0.67 | 4.90 b | 1.32 | 5.11 b | 0.58 | 5.14 b | 1.25 | 6.86 d | 2.21 | 7.68 d | 1.70 | |
| RQUICKIBHB | Neg | 0.40 a | 0.14 | 0.46 c | 0.04 | 0.62 b | 0.14 | 0.58 b | 0.09 | 0.65 b | 0.08 | 0.63 b | 0.16 |
| Pos | 0.58 b | 0.09 | 0.83 d | 0.13 | 0.71 d | 0.10 | 0.52 b | 0.09 | 0.62 b | 0.16 | 0.54 b | 0.06 | |
| T3 (nmol/L) | Neg | 0.48 a | 0.19 | 0.98 b | 0.22 | 0.95 b | 0.42 | 0.55 a | 0.18 | 0.83 c | 0.28 | 0.81 c | 0.42 |
| Pos | 1.05 b | 0.32 | 1.07 b | 0.18 | 0.99 b | 0.30 | 1.14 b | 0.26 | 1.38 b | 0.34 | 1.37 b | 0.38 | |
| T4 (nmol/L) | Neg | 12.1 a | 6.49 | 28.4 c | 10.2 | 29.7 c | 11.9 | 23.2 d | 4.04 | 29.3 c | 7.69 | 38.3 b | 15.7 |
| Pos | 37.1 b | 9.10 | 42.9 b | 21.6 | 27.6 c | 10.9 | 36.5 b | 9.04 | 43.7 b | 12.9 | 56.5 d | 18.5 | |
| CORT (nmol/L) | Neg | 27.2 a | 4.88 | 20.8 c | 8.73 | 18.9 c | 4.68 | 16.9 c | 2.06 | 12.9 d | 4.89 | 15.4 b | 2.14 |
| Pos | 15.4 b | 3.15 | 12.0 d | 3.40 | 12.2 d | 2.99 | 11.6 d | 3.90 | 8.90 e | 3.90 | 11.3 d | 4.79 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krnjaić, S.; Cincović, M.; Djoković, R.; Belić, B.; Ježek, J.; Starič, J. The Influence of Energy Balance, Lipolysis and Ketogenesis on Metabolic Adaptation in Cows Milked Twice and Three Times Daily. Metabolites 2022, 12, 1090. https://doi.org/10.3390/metabo12111090
Krnjaić S, Cincović M, Djoković R, Belić B, Ježek J, Starič J. The Influence of Energy Balance, Lipolysis and Ketogenesis on Metabolic Adaptation in Cows Milked Twice and Three Times Daily. Metabolites. 2022; 12(11):1090. https://doi.org/10.3390/metabo12111090
Chicago/Turabian StyleKrnjaić, Srđan, Marko Cincović, Radojica Djoković, Branislava Belić, Jožica Ježek, and Jože Starič. 2022. "The Influence of Energy Balance, Lipolysis and Ketogenesis on Metabolic Adaptation in Cows Milked Twice and Three Times Daily" Metabolites 12, no. 11: 1090. https://doi.org/10.3390/metabo12111090
APA StyleKrnjaić, S., Cincović, M., Djoković, R., Belić, B., Ježek, J., & Starič, J. (2022). The Influence of Energy Balance, Lipolysis and Ketogenesis on Metabolic Adaptation in Cows Milked Twice and Three Times Daily. Metabolites, 12(11), 1090. https://doi.org/10.3390/metabo12111090










