Metabolic Disorders in Menopause
Abstract
1. Introduction
2. Definition of Menopause and Metabolic Disorders
2.1. Menopause
2.2. Change of Metabolism in Menopausal Women
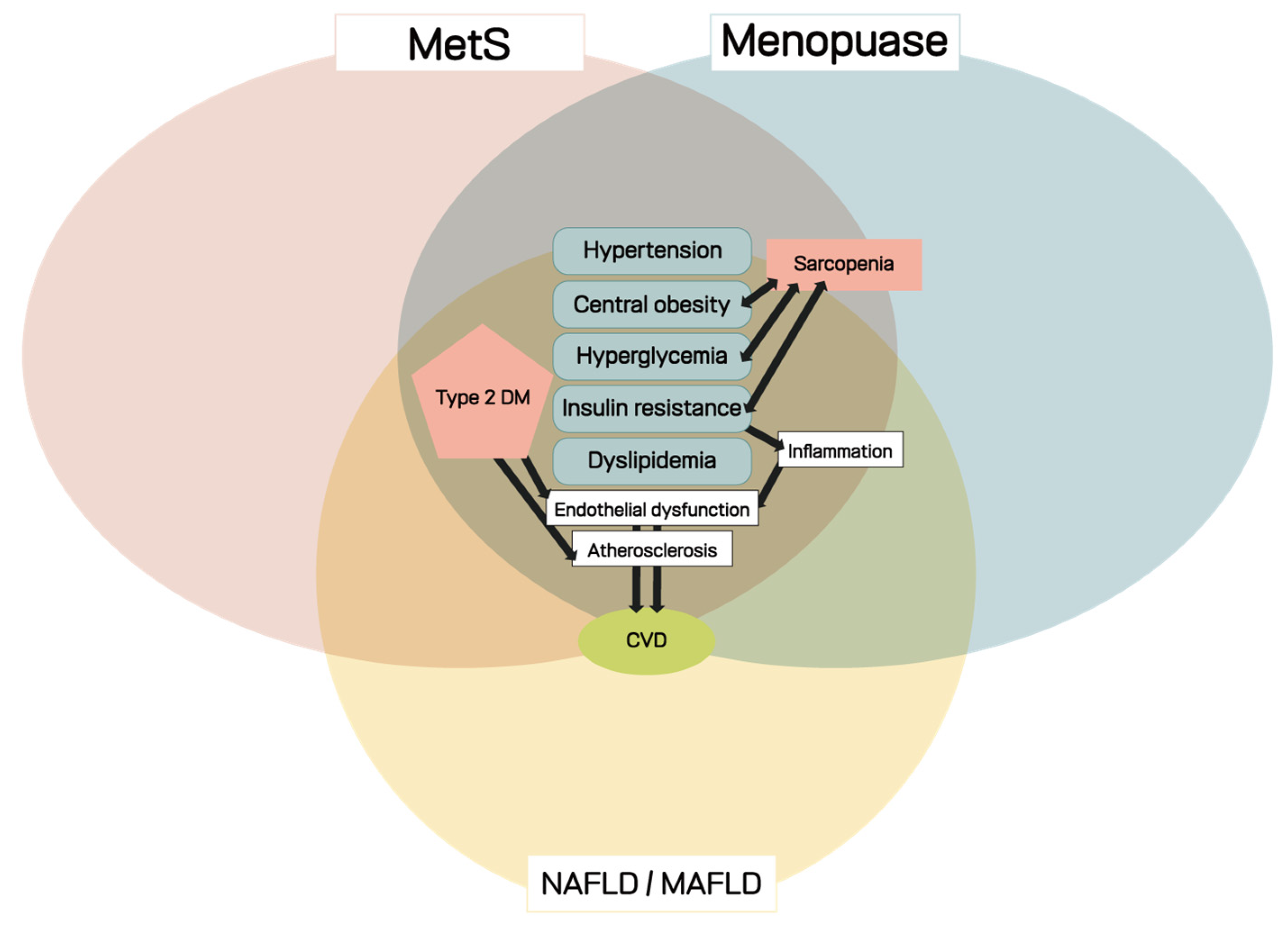
2.3. Metabolic Disorders in Women with Menopause
2.3.1. Definition and Prevalence of MetS
2.3.2. Diagnosis of MetS
2.3.3. Pathophysiology of MetS
2.3.4. NAFLD/MAFLD in Menopausal Women
2.3.5. Other Considerations
3. Diagnosis of Metabolic Disorders in Menopausal Women
3.1. History Taking and Physical Examination
3.2. Laboratory Examination
3.3. Imaging Test
3.4. Other Biomarkers Associated with MetS
4. Management of Metabolic Disorders in Menopausal Women
5. Prevention of Metabolic Disorders in Menopausal Women
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019. Available online: https://population.un.org/wpp/Download/Standard/Population (accessed on 8 April 2020).
- Hetzel, L.; Smith, A. The 65 Years and over Population: 2000; Census 2000 Brief, C2KBR/01-10; U.S. Census Bureau: Washington, DC, USA, 2001.
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 136–147. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Mumusoglu, S.; Yildiz, B.O. Metabolic Syndrome During Menopause. Curr. Vasc. Pharmacol. 2019, 17, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.A.; Bachmann, G.A. The genitourinary syndrome of menopause. Menopause 2021, 28, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Pan, X.; Zhou, J.; Wang, J.; Qi, Q.; Wang, L. Update on hormone therapy for the management of postmenopausal women. Biosci. Trends 2022, 16, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Hyvarinen, M.; Juppi, H.K.; Taskinen, S.; Karppinen, J.E.; Karvinen, S.; Tammelin, T.H.; Kovanen, V.; Aukee, P.; Kujala, U.M.; Rantalainen, T.; et al. Metabolic health, menopause, and physical activity-a 4-year follow-up study. Int. J. Obes. 2022, 46, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Khalfa, A.; Tiali, A.; Zemour, L.; Fatah, A.; Mekki, K. Prevalence of metabolic syndrome and its association with lifestyle and cardiovascular biomarkers among postmenopausal women in western Algeria. Int. J. Gynaecol. Obstet. 2017, 138, 201–206. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance. Adv. Clin. Chem. 2015, 72, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Avis, N.E.; McKinlay, S.M. A longitudinal analysis of women’s attitudes toward the menopause: Results from the Massachusetts Women’s Health Study. Maturitas 1991, 13, 65–79. [Google Scholar] [CrossRef]
- Treloar, A.E. Menarche, menopause, and intervening fecundability. Hum. Biol. 1974, 46, 89–107. [Google Scholar]
- Meschia, M.; Pansini, F.; Modena, A.B.; de Aloysio, D.; Gambacciani, M.; Parazzini, F.; Campagnoli, C.; Maiocchi, G.; Peruzzi, E. Determinants of age at menopause in Italy: Results from a large cross-sectional study. ICARUS Study Group. Italian Climacteric Research Group Study. Maturitas 2000, 34, 119–125. [Google Scholar] [CrossRef]
- Van Noord, P.A.; Dubas, J.S.; Dorland, M.; Boersma, H.; te Velde, E. Age at natural menopause in a population-based screening cohort: The role of menarche, fecundity, and lifestyle factors. Fertil. Steril. 1997, 68, 95–102. [Google Scholar] [CrossRef]
- Gosden, R.G. Follicular status at the menopause. Hum. Reprod. 1987, 2, 617–621. [Google Scholar] [CrossRef]
- Sherwin, B.B. Estrogen and cognitive functioning in women. Endocr. Rev. 2003, 24, 133–151. [Google Scholar] [CrossRef]
- Patel, S. Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2017, 168, 19–25. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.B.; Hong, Y.J.; Kim, Y.; Han, K.; Park, J.W. The influence of endogenous and exogenous hormonal factors on migraine in spontaneous postmenopausal women: A nationwide population-based study in South Korea. Cephalalgia 2022, 42, 376–384. [Google Scholar] [CrossRef]
- Christakis, M.K.; Hasan, H.; De Souza, L.R.; Shirreff, L. The effect of menopause on metabolic syndrome: Cross-sectional results from the Canadian Longitudinal Study on Aging. Menopause 2020, 27, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Okeke, T.; Anyaehie, U.; Ezenyeaku, C. Premature menopause. Ann. Med. Health Sci. Res. 2013, 3, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, L.; Yao, N. Management of cardiovascular disease in women with premature ovarian insufficiency: Critical quality appraisal of clinical guidelines and algorithm development. Menopause 2022, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Canning, K.L.; Brown, R.E.; Jamnik, V.K.; Kuk, J.L. Relationship between obesity and obesity-related morbidities weakens with aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 87–92. [Google Scholar] [CrossRef]
- Choi, D.; Choi, S.; Son, J.S.; Oh, S.W.; Park, S.M. Impact of Discrepancies in General and Abdominal Obesity on Major Adverse Cardiac Events. J. Am. Heart Assoc. 2019, 8, e013471. [Google Scholar] [CrossRef]
- Farahmand, M.; Bahri Khomamid, M.; Rahmati, M.; Azizi, F.; Ramezani Tehrani, F. Aging and changes in adiposity indices: The impact of menopause. J. Endocrinol. Investig. 2022, 45, 69–77. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Jasielec, M.S.; Kazlauskaite, R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity 2015, 23, 488–494. [Google Scholar] [CrossRef]
- Thurston, R.C.; Sowers, M.R.; Chang, Y.; Sternfeld, B.; Gold, E.B.; Johnston, J.M.; Matthews, K.A. Adiposity and reporting of vasomotor symptoms among midlife women: The study of women’s health across the nation. Am. J. Epidemiol. 2008, 167, 78–85. [Google Scholar] [CrossRef]
- Guthrie, J.R.; Dennerstein, L.; Taffe, J.R.; Ebeling, P.R.; Randolph, J.F.; Burger, H.G.; Wark, J.D. Central abdominal fat and endogenous hormones during the menopausal transition. Fertil. Steril. 2003, 79, 1335–1340. [Google Scholar] [CrossRef]
- Liu, X.M.; Chan, H.C.; Ding, G.L.; Cai, J.; Song, Y.; Wang, T.T.; Zhang, D.; Chen, H.; Yu, M.K.; Wu, Y.T.; et al. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca(2+)/CREB pathway. Aging Cell 2015, 14, 409–420. [Google Scholar] [CrossRef]
- Lobo, R.A. Surgical menopause and cardiovascular risks. Menopause 2007, 14, 562–566. [Google Scholar] [CrossRef]
- Dørum, A.; Tonstad, S.; Liavaag, A.H.; Michelsen, T.M.; Hildrum, B.; Dahl, A.A. Bilateral oophorectomy before 50 years of age is significantly associated with the metabolic syndrome and Framingham risk score: A controlled, population-based study (HUNT-2). Gynecol. Oncol. 2008, 109, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.; Ramezani Tehrani, F.; Bahri Khomami, M.; Noroozzadeh, M.; Azizi, F. Surgical menopause versus natural menopause and cardio-metabolic disturbances: A 12-year population-based cohort study. J. Endocrinol. Investig. 2015, 38, 761–767. [Google Scholar] [CrossRef]
- Farahmand, M.; Ramezani Tehrani, F.; Simbar, M.; Mehrabi, Y.; Khalili, D.; Azizi, F. Does metabolic syndrome or its components differ in naturally and surgically menopausal women? Climacteric 2014, 17, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, G.; Pertynski, T.; Pertynska-Marczewska, M. Metabolic disorders in menopause. Prz. Menopauzalny 2015, 14, 59–64. [Google Scholar] [CrossRef]
- Zuo, H.; Shi, Z.; Hu, X.; Wu, M.; Guo, Z.; Hussain, A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism 2009, 58, 1102–1108. [Google Scholar] [CrossRef]
- Khanam, M.A.; Qiu, C.; Lindeboom, W.; Streatfield, P.K.; Kabir, Z.N.; Wahlin, Å. The metabolic syndrome: Prevalence, associated factors, and impact on survival among older persons in rural Bangladesh. PLoS ONE 2011, 6, e20259. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Powell, L.H.; Crawford, S.; Lasley, B.; Sutton-Tyrrell, K. Menopause and the metabolic syndrome: The Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008, 168, 1568–1575. [Google Scholar] [CrossRef]
- Jouyandeh, Z.; Nayebzadeh, F.; Qorbani, M.; Asadi, M. Metabolic syndrome and menopause. J. Diabetes Metab. Disord. 2013, 12, 1. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, U. Hyperinsulinaemia, a key factor of the metabolic syndrome in postmenopausal women. Maturitas 2009, 62, 362–365. [Google Scholar] [CrossRef] [PubMed]
- McGown, C.; Birerdinc, A.; Younossi, Z.M. Adipose tissue as an endocrine organ. Clin. Liver Dis. 2014, 18, 41–58. [Google Scholar] [CrossRef]
- Esteghamati, A.; Morteza, A.; Khalilzadeh, O.; Noshad, S.; Novin, L.; Nakhjavani, M. Association of serum cortisol levels with parameters of metabolic syndrome in men and women. Clin. Investig. Med. 2011, 34, E131–E137. [Google Scholar] [CrossRef]
- Cho, G.J.; Lee, J.H.; Park, H.T.; Shin, J.H.; Hong, S.C.; Kim, T.; Hur, J.Y.; Lee, K.W.; Park, Y.K.; Kim, S.H. Postmenopausal status according to years since menopause as an independent risk factor for the metabolic syndrome. Menopause 2008, 15, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heshka, S.; Carnethon, M.R.; Heymsfield, S.B. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Wamala, S.P.; Lynch, J.; Horsten, M.; Mittleman, M.A.; Schenck-Gustafsson, K.; Orth-Gomér, K. Education and the metabolic syndrome in women. Diabetes Care 1999, 22, 1999–2003. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Mantovani, A.; Dalbeni, A. NAFLD, MAFLD and DAFLD. Dig. Liver Dis. 2020, 52, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.; Waked, I.; Bollipo, S.; Gomaa, A.; Ajlouni, Y.; Attia, D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020, 40, 1254–1261. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- Molina-Molina, E.; Furtado, G.E.; Jones, J.G.; Portincasa, P.; Vieira-Pedrosa, A.; Teixeira, A.M.; Barros, M.P.; Bachi, A.L.L.; Sardao, V.A. The advantages of physical exercise as a preventive strategy against NAFLD in postmenopausal women. Eur. J. Clin. Investig. 2022, 52, e13731. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Heber, D.; Ingles, S.; Ashley, J.M.; Maxwell, M.H.; Lyons, R.F.; Elashoff, R.M. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am. J. Clin. Nutr. 1996, 64, 472S–477S. [Google Scholar] [CrossRef] [PubMed]
- Stenholm, S.; Harris, T.B.; Rantanen, T.; Visser, M.; Kritchevsky, S.B.; Ferrucci, L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 693–700. [Google Scholar] [CrossRef]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef]
- Sternfeld, B.; Wang, H.; Quesenberry, C.P., Jr.; Abrams, B.; Everson-Rose, S.A.; Greendale, G.A.; Matthews, K.A.; Torrens, J.I.; Sowers, M. Physical activity and changes in weight and waist circumference in midlife women: Findings from the Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2004, 160, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Karvonen-Gutierrez, C.; Kim, C. Association of Mid-Life Changes in Body Size, Body Composition and Obesity Status with the Menopausal Transition. Healthcare 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Fuh, J.L.; Wang, S.J.; Lee, S.J.; Lu, S.R.; Juang, K.D. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas 2006, 53, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, H.; Paillard-Borg, S.; Zhu, H.; Qi, X.; Rizzuto, D. Prevalence of Overweight and Obesity among Chinese Adults: Role of Adiposity Indicators and Age. Obes. Facts 2016, 9, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Albala, C.; Lera, L.; García, C.; Arroyo, P.; Pérez-Bravo, F.; Angel, B.; Peláez, M. Anthropometric measurements in the elderly population of Santiago, Chile. Nutrition 2004, 20, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Corbatón Anchuelo, A.; Martínez-Larrad, M.T.; Serrano-García, I.; Fernández Pérez, C.; Serrano-Ríos, M. Body fat anthropometric indexes: Which of those identify better high cardiovascular risk subjects? A comparative study in Spanish population. PLoS ONE 2019, 14, e0216877. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Hwang, S.Y.; Kim, J.A.; Lee, Y.B.; Roh, E.; Kim, N.H.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; et al. Comparison of anthropometric indices for the screening of nonalcoholic fatty liver disease in pre- and postmenopausal women. Menopause 2020, 27, 88–94. [Google Scholar] [CrossRef]
- Ramezani Tehrani, F.; Minooee, S.; Azizi, F. Comparison of various adiposity indexes in women with polycystic ovary syndrome and normo-ovulatory non-hirsute women: A population-based study. Eur. J. Endocrinol. 2014, 171, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Maessen, M.F.; Eijsvogels, T.M.; Verheggen, R.J.; Hopman, M.T.; Verbeek, A.L.; de Vegt, F. Entering a new era of body indices: The feasibility of a body shape index and body roundness index to identify cardiovascular health status. PLoS ONE 2014, 9, e107212. [Google Scholar] [CrossRef]
- Kahn, H.S. The lipid accumulation product is better than BMI for identifying diabetes: A population-based comparison. Diabetes Care 2006, 29, 151–153. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef]
- Thomson, C.A.; Garcia, D.O.; Wertheim, B.C.; Hingle, M.D.; Bea, J.W.; Zaslavsky, O.; Caire-Juvera, G.; Rohan, T.; Vitolins, M.Z.; Thompson, P.A.; et al. Body shape, adiposity index, and mortality in postmenopausal women: Findings from the Women’s Health Initiative. Obesity 2016, 24, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.E.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Yuan, Z.; Liu, X. Effect of hormone replacement therapy on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e62329. [Google Scholar] [CrossRef] [PubMed]
- Sare, G.M.; Gray, L.J.; Bath, P.M. Association between hormone replacement therapy and subsequent arterial and venous vascular events: A meta-analysis. Eur. Heart J. 2008, 29, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Canonico, M.; Plu-Bureau, G.; Lowe, G.D.; Scarabin, P.Y. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: Systematic review and meta-analysis. BMJ 2008, 336, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Rogers, S.L.; Abramson, M.J.; Tonkin, A.M. Hormone therapy and cardiovascular disease: A systematic review and meta-analysis. BJOG 2006, 113, 5–14. [Google Scholar] [CrossRef]
- Kim, J.E.; Chang, J.H.; Jeong, M.J.; Choi, J.; Park, J.; Baek, C.; Shin, A.; Park, S.M.; Kang, D.; Choi, J.Y. A systematic review and meta-analysis of effects of menopausal hormone therapy on cardiovascular diseases. Sci. Rep. 2020, 10, 20631. [Google Scholar] [CrossRef]
- Boardman, H.M.; Hartley, L.; Eisinga, A.; Main, C.; Roqué i Figuls, M.; Bonfill Cosp, X.; Gabriel Sanchez, R.; Knight, B. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst. Rev. 2015, CD002229. [Google Scholar] [CrossRef] [PubMed]
- Nudy, M.; Chinchilli, V.M.; Foy, A.J. A systematic review and meta-regression analysis to examine the ‘timing hypothesis’ of hormone replacement therapy on mortality, coronary heart disease, and stroke. Int. J. Cardiol. Heart Vasc. 2019, 22, 123–131. [Google Scholar] [CrossRef]
- Oliver-Williams, C.; Glisic, M.; Shahzad, S.; Brown, E.; Pellegrino Baena, C.; Chadni, M.; Chowdhury, R.; Franco, O.H.; Muka, T. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: A systematic review. Hum. Reprod. Update 2019, 25, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Millen, B.E.; Pencina, M.J.; Kimokoti, R.W.; Zhu, L.; Meigs, J.B.; Ordovas, J.M.; D’Agostino, R.B. Nutritional risk and the metabolic syndrome in women: Opportunities for preventive intervention from the Framingham Nutrition Study. Am. J. Clin. Nutr. 2006, 84, 434–441. [Google Scholar] [CrossRef]
- Drewnowski, A.; Darmon, N. The economics of obesity: Dietary energy density and energy cost. Am. J. Clin. Nutr. 2005, 82, 265S–273S. [Google Scholar] [CrossRef]
- Cho, Y.A.; Kim, J.; Cho, E.R.; Shin, A. Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 893–900. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Howard, B.; Lu, J.; Tinker, L.F.; Van Horn, L.; Caan, B.; Rohan, T.; Stefanick, M.L.; Thomson, C.A. A low-fat dietary pattern and risk of metabolic syndrome in postmenopausal women: The Women’s Health Initiative. Metabolism 2012, 61, 1572–1581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez-Cano, A.; Mier-Cabrera, J.; Balas-Nakash, M.; Muñoz-Manrique, C.; Legorreta-Legorreta, J.; Perichart-Perera, O. Dietary changes associated with improvement of metabolic syndrome components in postmenopausal women receiving two different nutrition interventions. Menopause 2015, 22, 758–764. [Google Scholar] [CrossRef] [PubMed]
| World Health Organization (1998) [32] | NCEPT: ATPIII (2001) a [33] | AACE (2003) b [34] | AHA/NHLBI (2004) c [35] | IDF (2005) d [36] | Consensus Definition IDF and AHA/NHLBI (2009) [37] | |
|---|---|---|---|---|---|---|
| Required | IR T2DM IFG Fasting glucose ≥ 110 mg/dL 2-h glucose ≥ 140 mg/dL | Impaired glucose tolerance (IGT) or IFG | Elevated WC (depending on population f) in European women ≥ 80 cm g | |||
| Number of abnormalities | And ≥2 of: | ≥3 of: | Any of the following based on clinical judgment: | ≥3 of: | And ≥2 of: | ≥3 of: |
| Glucose | Fasting glucose ≥ 110 mg/dL (includes diabetes) | - IGT or IFG (but not diabetes) - Other features of IR (includeing family history of DM, polycystic ovary syndrome, sedentary lifestyle, advancing age, and ethnic groups susceptible to T2DM.) | Fasting glucose ≥ 100 mg/dL or drug treatment of elevated glucose | Fasting glucose ≥ 100 mg/dL or previously diagnosed T2DM | Fasting glucose ≥ 100 mg/dL or drug treatment of elevated glucose | |
| HDL cholesterol | HDL-C < 50 mg/dL | HDL-C < 50 mg/dL | HDL-C < 50 mg/dL | HDL-C < 50 mg/dL or specific treatment for this lipid abnormality e | HDL-C < 50 mg/dL or specific treatment for this lipid abnormality e | HDL-C < 50 mg/dL or specific treatment for this lipid abnormality e |
| Triglycerides | ≥150 mg/dL | ≥150 mg/dL | ≥150 mg/dL | ≥150 mg/dL or specific treatment for this lipid abnormality | ≥150 mg/dL or specific treatment for this lipid abnormality | ≥150 mg/dL or specific treatment for this lipid abnormality |
| Obesity | Central obesity: WHR > 0.85 or BMI > 30 kg/m2 | WC > 88 cm | BMI ≥ 25 kg/m2 | WC > 88 cm | WC ≥ 80 cm (in European women) (depending on population f) | |
| Hypertension | ≥140/90 mmHg | ≥130/85 mmHg | ≥130/85 mmHg | ≥130/85 mmHg or antihypertensive drug treatment | ≥130/85 mmHg or antihypertensive drug treatment | ≥130/85 mmHg or antihypertensive drug treatment |
| Other | Microalbuminuria: urinary albumin excretion ratio ≥ 20 μg/min or albumin: creatinine ratio ≥ 30 mg/g |
| Waist circumference | ≥102 cm (men), 88 cm (women) |
| Blood pressure | ≥130/85 mmHg or specific drug treatment |
| TG | ≥1.70 mmol/L or specific drug treatment |
| HDL-C | <1.0 mmol/L (male), <1.3 mmol/L (female) |
| Prediabetes | fasting glucose levels: 5.6–6.9 mmol/L 2-h post-load glucose level 7.8–11.0 mmol/L HbA1c 5.7% to 6.4% |
| Homeostasis model assessment-insulin resistance (HOMA-IR) score | ≥2.5 |
| C-reactive protein (CRP) | 2 mg/L |
| EWGSOP | ESPEN SIG | IWGS | Sarcopenia with Limited Mobility | FNIH | AWGS | |
|---|---|---|---|---|---|---|
| Muscle mass (DXA) | SMI M: ≤7.26 kg/m2 F: ≤5.54 kg/m2 | LM total kg | SMI M: ≤7.23 kg/m2 F: ≤5.67 kg/m2 | SMI M: ≤6.81 kg/m2 F: ≤5.18 kg/m2 | LMApp M: <19.75 kg F: <15.02 kg LMApp/BMI M: <0.789 F: <0.512 | ASM DEXA M: <7.0 kg/m2 F: <5.4 kg/m2 |
| Muscular function Handgrip | Kg BMI | - | - | - | M: <26 kg F: <16 kg kg/BMI M: <1 F: <0.56 | M: <28 kg F: <18 kg |
| Waking speed | <0.8 m/s | <0.8 m/s | <1 m/s | <1 m/s 6MWT < 400 m | - | <1 m/s 6MWT Or 5-time chair stand test: ≥12 s or Short physical performance battery: ≤9 |
| Timed up-and-go test | >10 s | - | - | - | - |
| Meta-Analysis (Year) | No. of Trials (No. of Participants) | Conclusions |
|---|---|---|
| Kim, et al. (2020) [92] | RCT (26) Observational (47) | RCTs and observational studies both showed that MHT was associated with an increased risk of VTE and PE, although only the RCTs revealed an increased risk of stroke among those administered MHT. A decrease in the risk of MI due to MHT was identified in the observational studies, but the RCTs did not show this association. The risks and benefits of MHT may vary depending on the characteristics of the women who are treated. MHT is not recommended for prevention of chronic disease. However, it may be beneficial in CVD and for mortality in postmenopausal women with severe menopause symptoms after sufficient consideration of underlying diseases and timing of treatment initiation. It may also suggest use of non-oral MHT in women at high risk of VTE and stroke. |
| Oliver-Williams et al. (2019) [95] | 33 (2,588,327) | Use of low-dose oral and transdermal hormone therapy seems to be safe with respect to CVD risk in women in menopausal transition and within the first years (e.g., 10 years) after menopause onset. In women with increased baseline thromboembolic risk, alternative non-hormonal medications are suggested as first-line treatment, and transdermal estradiol alone or with micronized progesterone only should be considered when the previously mentioned options are not effective. When MHT is initiated >10 years since menopause onset (>60 years old), because of greater absolute risks of coronary heart disease, stroke, and venous thromboembolism, it should be used for the shortest possible time and in the lowest possible dose and should be administered transdermally. However, an individualized treatment approach including baseline CVD risk assessment should be applied when prescribing MHT. |
| Nudy et al. (2019) [94] | 31 (40,521) | When a study with a starting time point of <60 years of age was defined as a younger initiation trial and a study with an average age of >60 was defined as an older initiation trial, younger initiation of MHT may be effective in reducing mortality and cardiac events. However, those in whom younger initiation of HRT was conducted remained at an increased risk of stroke, TIA, and systemic embolism, and this risk increased as average age increased. Younger menopausal women using MHT for treating vasomotor symptoms do not appear to be at an increased risk of mortality or cardiovascular events. |
| Boardman et al. (2015) [93] | 19 (40,410) | There is strong evidence that treatment with hormone therapy in postmenopausal women overall, for either primary or secondary prevention of cardiovascular disease events, has little if any benefit and causes an increase in the risk of stroke and venous thromboembolic events. MHT in both primary and secondary prevention conferred no protective effects for all-cause mortality, cardiovascular death, non-fatal myocardial infarction, angina, or revascularization. |
| Yang et al. (2013) [88] | 10 (38,908) | There is no effect on CVD, such as myocardial infarction, coronary events, and even cardiac and total death, after combination therapy of estrogen combined with medroxyprogesterone acetate therapy. Estrogen monotherapy was related to a 27% increased risk for incident stroke. MHT should not be recommended in women with postmenopause for the purpose of preventing cardiovascular disease. |
| Sare et al. (2008) [89] | 31 (44,113) | MHT is related to an increased risk of CVD (stroke) and VTE. Adding progesterone to estrogen increases the risk of VTE 2-fold. There is no effect of MHT on coronary heart diseases, such as myocardial infarction, unstable angina, and sudden death from cardiac causes. MHT could not be recommended for long-term prophylaxis of vascular events in most women. |
| Canonico et al. (2008) [90] | 9 (38,779) | Oral estrogen increases risk of VTE, especially in obese women and during the first year of treatment. Transdermal estrogen might be safe in VTE. |
| Magliano et al. (2005) [91] | 7 (32,523) | There is no effect of MTH on nonfatal acute myocardial infarction, coronary heart disease mortality, or all-cause mortality. MTH is increasing the risk of stroke in women with menopause. Hormone therapy for reduction or prevention of CVD risk is not supported. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.G.; Park, H. Metabolic Disorders in Menopause. Metabolites 2022, 12, 954. https://doi.org/10.3390/metabo12100954
Jeong HG, Park H. Metabolic Disorders in Menopause. Metabolites. 2022; 12(10):954. https://doi.org/10.3390/metabo12100954
Chicago/Turabian StyleJeong, Hye Gyeong, and Hyuntae Park. 2022. "Metabolic Disorders in Menopause" Metabolites 12, no. 10: 954. https://doi.org/10.3390/metabo12100954
APA StyleJeong, H. G., & Park, H. (2022). Metabolic Disorders in Menopause. Metabolites, 12(10), 954. https://doi.org/10.3390/metabo12100954






