Differential Metabolism of Glycerol Based on Oral versus Intravenous Administration in Humans
Abstract
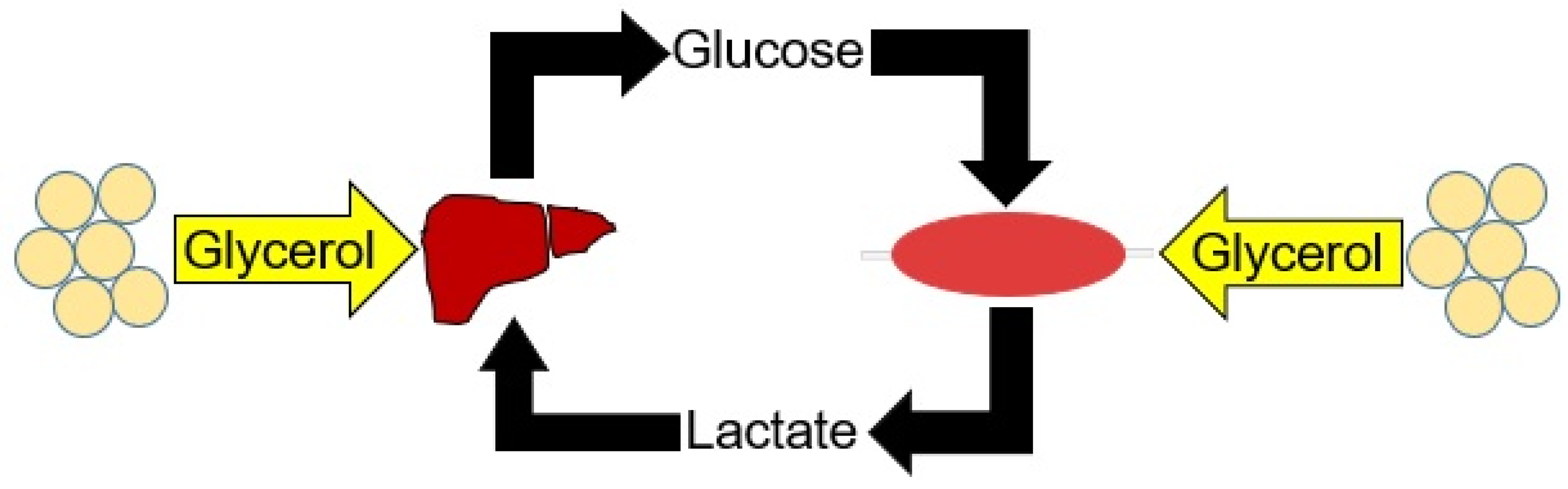
1. Introduction
2. Materials and Methods
2.1. Human Participants
2.2. Experimental Protocol
2.3. Glycerol and Glucose Derivatization
2.4. LC-MS Analysis
2.5. Calculations
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
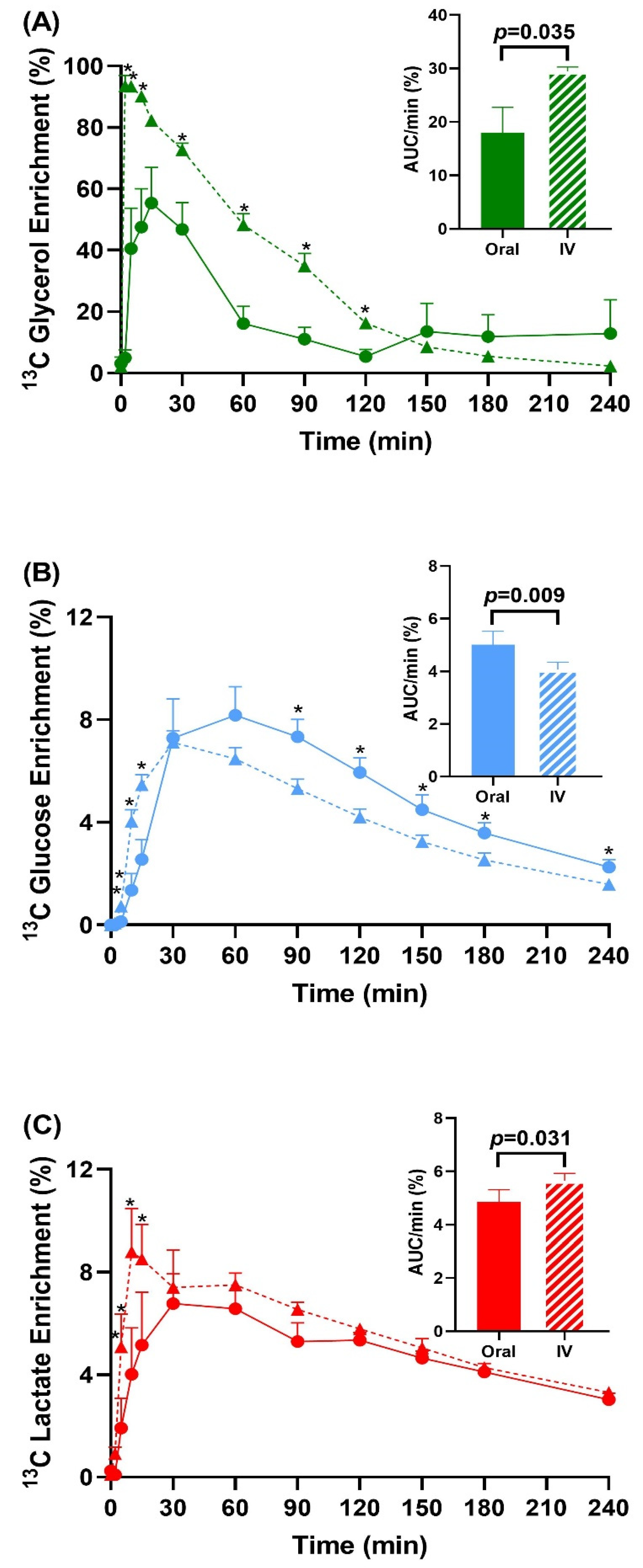
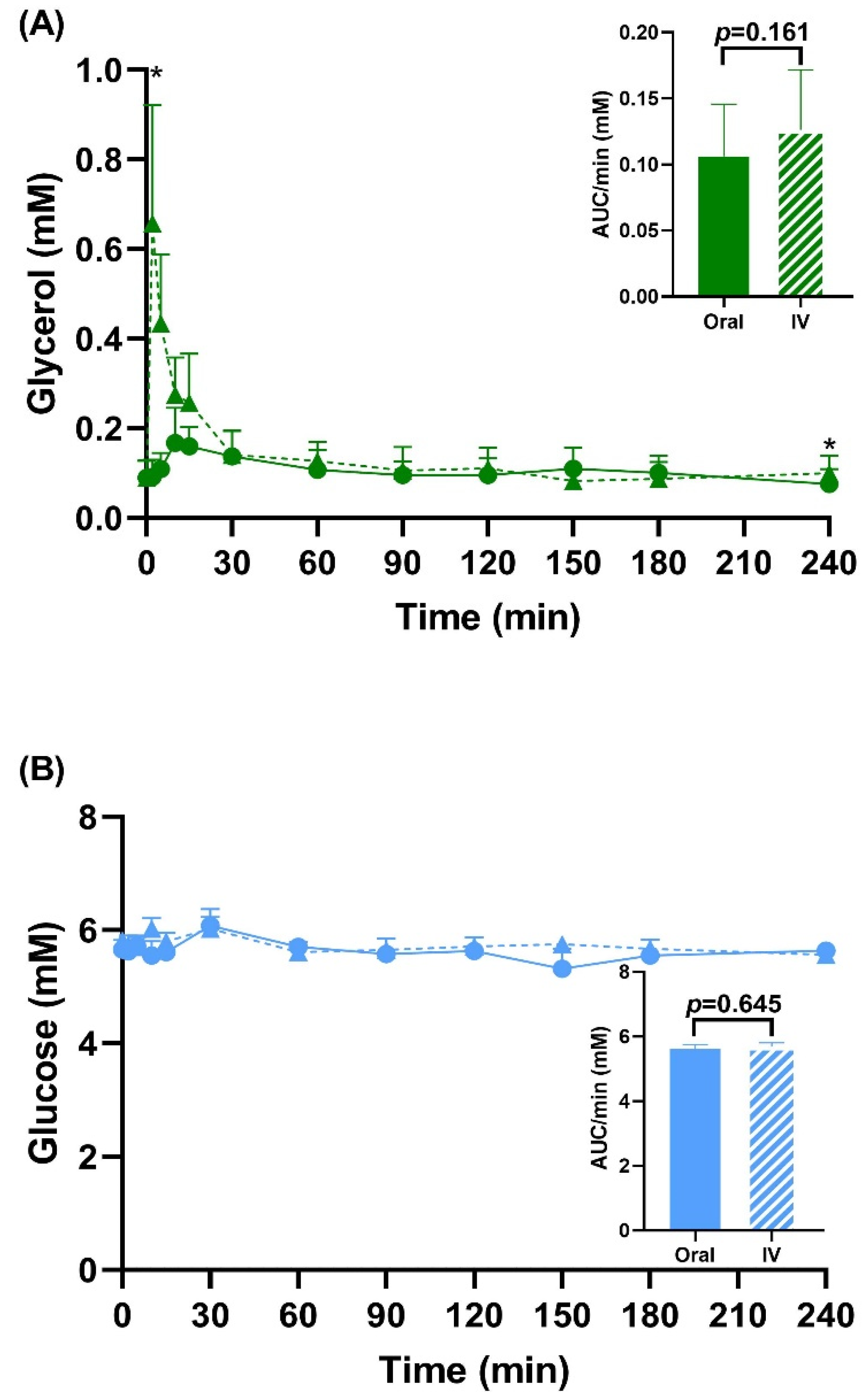
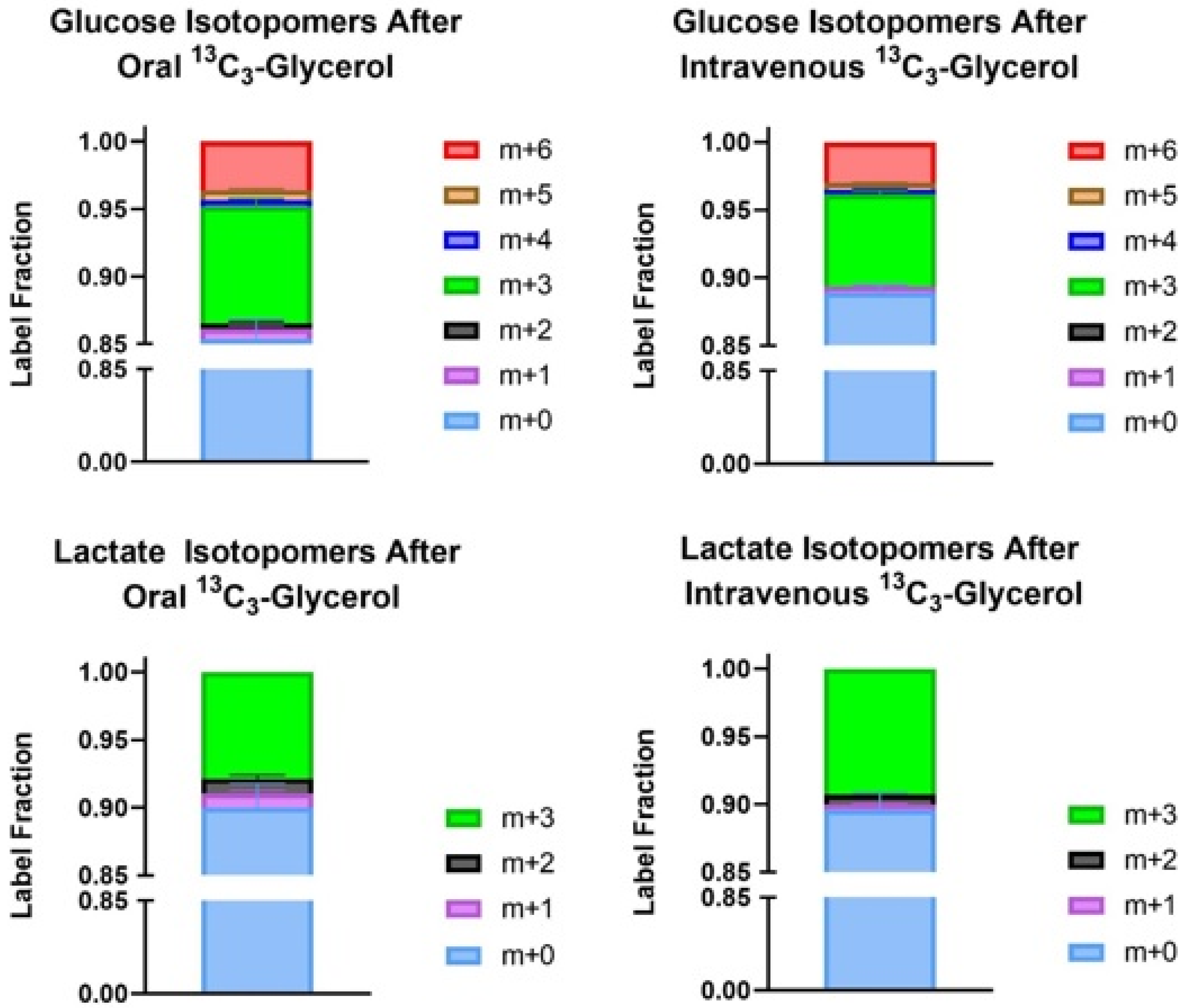
3.2. Enrichment Data and Total Pool Size
3.3. Peak Glucose and Lactate Enrichment Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Kwon, H.; Su, X.; Wondisford, F.E. Glycerol not lactate is the major net carbon source for gluconeogenesis in mice during both short and prolonged fasting. Mol. Metab. 2020, 31, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Wang, Y.; Su, X.; Wondisford, F.E. Glycerol’s contribution to lactate production outside of a glucose intermediate in fasting humans. Metabolism 2022, 132, 155214. [Google Scholar] [CrossRef] [PubMed]
- Dipple, K.M.; Zhang, Y.H.; Huang, B.L.; McCabe, L.L.; Dallongeville, J.; Inokuchi, T.; Kimura, M.; Marx, H.J.; Roederer, G.O.; Shih, V.; et al. Glycerol kinase deficiency: Evidence for complexity in a single gene disorder. Hum. Genet. 2001, 109, 55–62. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar]
- Hibuse, T.; Maeda, N.; Nagasawa, A.; Funahashi, T. Aquaporins and glycerol metabolism. Biochim. Biophys. Acta 2006, 1758, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Neeland, I.J.; Hughes, C.; Ayers, C.R.; Malloy, C.R.; Jin, E.S. Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism 2017, 67, 80–89. [Google Scholar] [CrossRef]
- Diniz Behn, C.; Jin, E.S.; Bubar, K.; Malloy, C.; Parks, E.J.; Cree-Green, M. Advances in stable isotope tracer methodology part 1: Hepatic metabolism via isotopomer analysis and postprandial lipolysis modeling. J. Investig. Med. 2020, 68, 3–10. [Google Scholar] [CrossRef]
- Carreau, A.M.; Jin, E.S.; Garcia-Reyes, Y.; Rahat, H.; Nadeau, K.J.; Malloy, C.R.; Cree-Green, M. A simple method to monitor hepatic gluconeogenesis and triglyceride synthesis following oral sugar tolerance test in obese adolescents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R134–R142. [Google Scholar] [CrossRef]
- Jin, E.S.; Sherry, A.D.; Malloy, C.R. An Oral Load of [13C3] Glycerol and Blood NMR Analysis Detect Fatty Acid Esterification, Pentose Phosphate Pathway, and Glycerol Metabolism through the Tricarboxylic Acid Cycle in Human Liver. J. Biol. Chem. 2016, 291, 19031–19041. [Google Scholar] [CrossRef]
- Chiles, E.; Wang, Y.; Kalemba, K.M.; Kwon, H.; Wondisford, F.E.; Su, X. Fast LC-MS quantitation of glucose and glycerol via enzymatic derivatization. Anal. Biochem. 2019, 575, 40–43. [Google Scholar] [CrossRef]
- Su, X.; Chiles, E.; Maimouni, S.; Wondisford, F.E.; Zong, W.X.; Song, C. In-Source CID Ramping and Covariant Ion Analysis of Hydrophilic Interaction Chromatography Metabolomics. Anal. Chem. 2020, 92, 4829–4837. [Google Scholar] [CrossRef]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinf. 2012, 37, 14.11.1–14.11.23. [Google Scholar] [CrossRef]
- Su, X.; Lu, W.; Rabinowitz, J.D. Metabolite Spectral Accuracy on Orbitraps. Anal. Chem. 2017, 89, 5940–5948. [Google Scholar] [CrossRef]
- Allison, D.B.; Paultre, F.; Maggio, C.; Mezzitis, N.; Pi-Sunyer, F.X. The use of areas under curves in diabetes research. Diabetes Care 1995, 18, 245–250. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Baldi, S.; Pettiti, M.; Toschi, E.; Camastra, S.; Natali, A.; Landau, B.R.; Ferrannini, E. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: A quantitative study. Diabetes 2000, 49, 1367–1373. [Google Scholar] [CrossRef]
- Puhakainen, I.; Koivisto, V.A.; Yki-Jarvinen, H. Lipolysis and gluconeogenesis from glycerol are increased in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1992, 75, 789–794. [Google Scholar] [CrossRef]
- Nurjhan, N.; Consoli, A.; Gerich, J. Increased lipolysis and its consequences on gluconeogenesis in non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1992, 89, 169–175. [Google Scholar] [CrossRef]
- Mahendran, Y.; Cederberg, H.; Vangipurapu, J.; Kangas, A.J.; Soininen, P.; Kuusisto, J.; Uusitupa, M.; Ala-Korpela, M.; Laakso, M. Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care 2013, 36, 3732–3738. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Miyazaki, Y.; Pettiti, M.; Matsuda, M.; Mahankali, S.; Santini, E.; DeFronzo, R.A.; Ferrannini, E. Metabolic effects of visceral fat accumulation in type 2 diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 5098–5103. [Google Scholar] [CrossRef]
- Foster, M.T.; Pagliassotti, M.J. Metabolic alterations following visceral fat removal and expansion: Beyond anatomic location. Adipocyte 2012, 1, 192–199. [Google Scholar] [CrossRef]
- DeGuzman, A.; Lorenson, M.Y.; Walker, A.M. Bittersweet: Relevant amounts of the common sweet food additive, glycerol, accelerate the growth of PC3 human prostate cancer xenografts. BMC Res. Notes 2022, 15, 101. [Google Scholar] [CrossRef]
- Previs, S.F.; Martin, S.K.; Hazey, J.W.; Soloviev, M.V.; Keating, A.P.; Lucas, D.; David, F.; Koshy, J.; Kirschenbaum, D.W.; Tserng, K.Y.; et al. Contributions of liver and kidneys to glycerol production and utilization in the dog. Am. J. Physiol. 1996, 271, E1118–E1124. [Google Scholar] [CrossRef]
- Moller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Katayama, S.; Tonai, K.; Goto, Y.; Koyama, K.; Koinuma, T.; Shima, J.; Wada, M.; Nunomiya, S. Transient hyperlactatemia during intravenous administration of glycerol: A prospective observational study. J. Intensive Care 2018, 6, 55. [Google Scholar] [CrossRef]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 4th ed.; W.H. Freeman: New York, NY, USA, 2005. [Google Scholar]

| Value | Reference | |
|---|---|---|
| Gender (M/F) | 4/4 | |
| Age (years) | 29.1 ± 7.5 | |
| Weight (kg) | 69.9 ± 8.51 | |
| BMI (kg/m2) | 22.8 ± 2.78 | 18.5–24.9 |
| Fasting glucose (mmol/L) | 4.72 ± 0.32 | 3.6–5.6 |
| Creatinine (mg/dL) | 0.86 ± 0.19 | 0.65–1.35 |
| Total cholesterol (mg/dL) | 195.3 ± 34.7 | <200 |
| Triglycerides (mg/dL) | 82.4 ± 22.6 | <150 |
| High-density lipoprotein cholesterol (mg/dL) | 56.0 ± 16.8 | >40 |
| Low-density lipoprotein cholesterol (mg/dL) | 122.9 ± 35.0 | <160 |
| Aspartate aminotransferase level (IU/L) | 21.5 ± 8.00 | 10–40 |
| Alanine aminotransferase level (IU/L) | 16.9 ± 5.94 | 9–46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.; Wang, Y.; Wondisford, F.E. Differential Metabolism of Glycerol Based on Oral versus Intravenous Administration in Humans. Metabolites 2022, 12, 890. https://doi.org/10.3390/metabo12100890
Shah A, Wang Y, Wondisford FE. Differential Metabolism of Glycerol Based on Oral versus Intravenous Administration in Humans. Metabolites. 2022; 12(10):890. https://doi.org/10.3390/metabo12100890
Chicago/Turabian StyleShah, Ankit, Yujue Wang, and Fredric E. Wondisford. 2022. "Differential Metabolism of Glycerol Based on Oral versus Intravenous Administration in Humans" Metabolites 12, no. 10: 890. https://doi.org/10.3390/metabo12100890
APA StyleShah, A., Wang, Y., & Wondisford, F. E. (2022). Differential Metabolism of Glycerol Based on Oral versus Intravenous Administration in Humans. Metabolites, 12(10), 890. https://doi.org/10.3390/metabo12100890






