Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review
Abstract
1. Introduction
2. Search Method and Systematic Literature Review
3. Results
4. Pathogenesis and Transmission
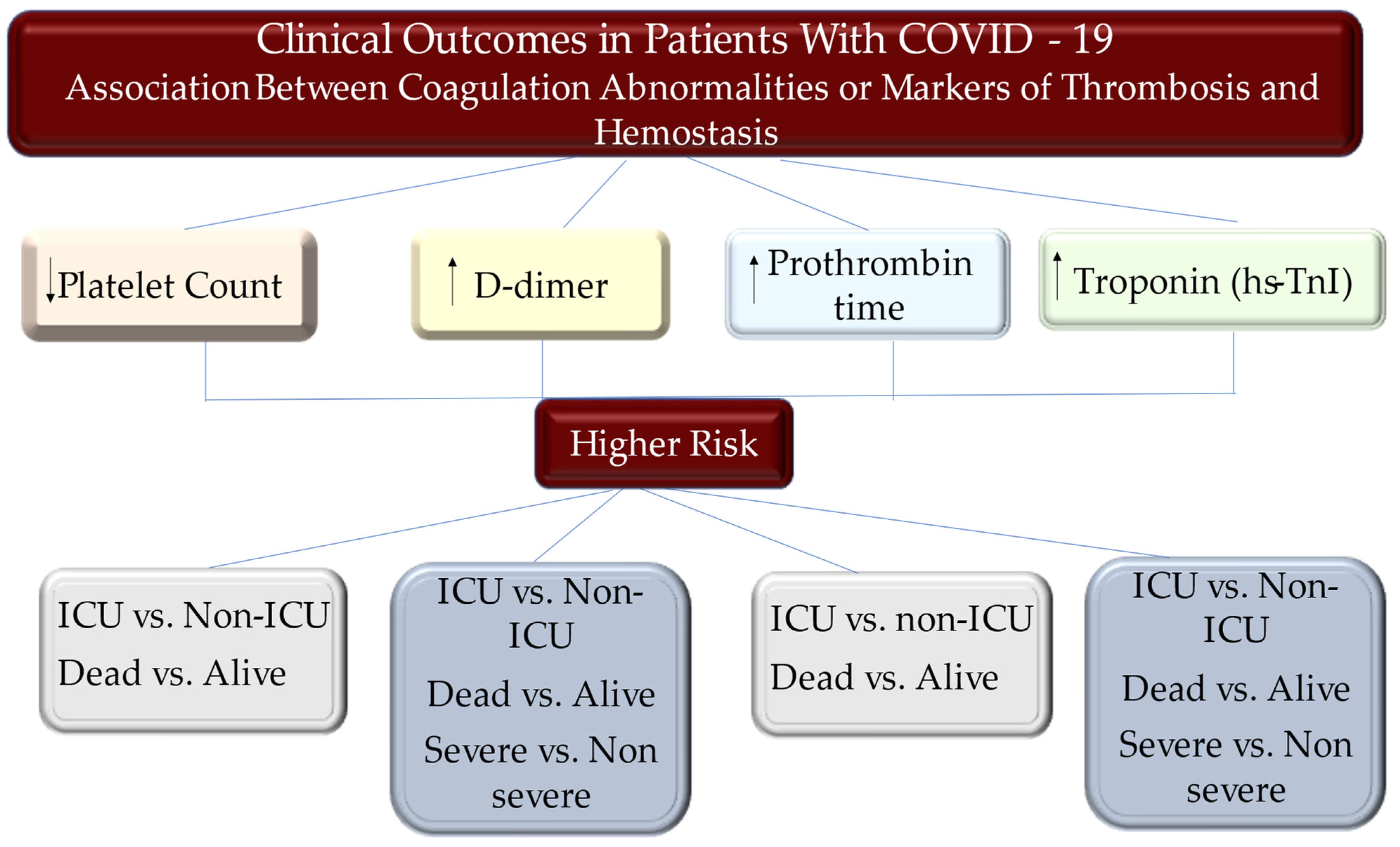
5. Hemostasis Parameters during COVID-19
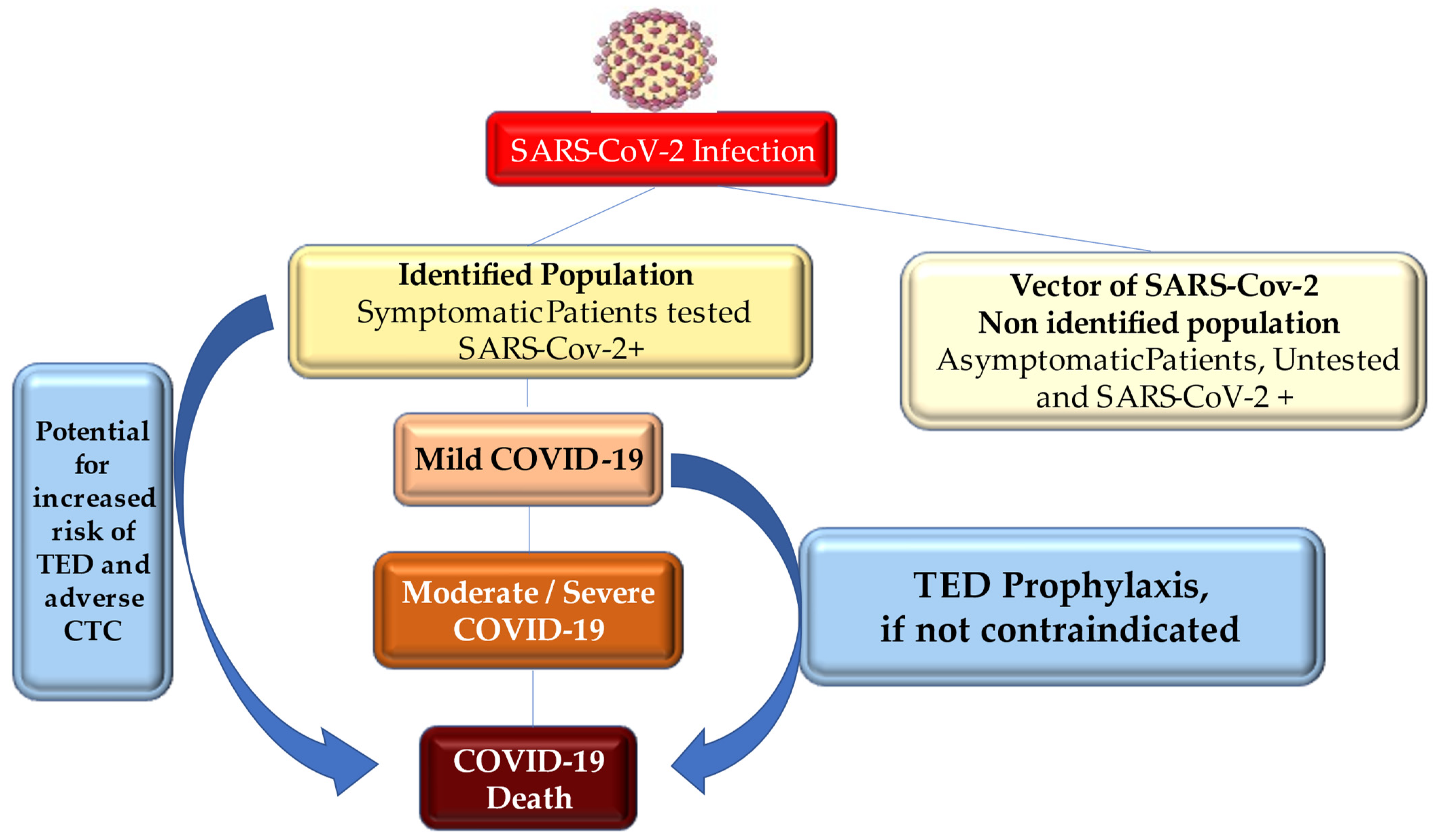
Thromboembolic Disease Diagnosed in COVID-19 Patients
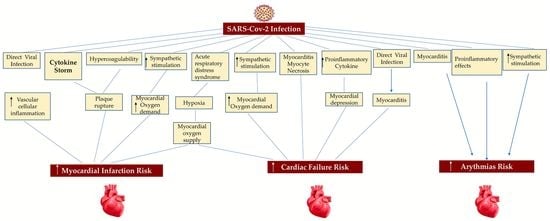
6. Cardiac Thrombotic Complication
6.1. Myocardial Injury
6.2. Acute Coronary Syndrome
7. Principles of Therapy of COVID-19 Heart Disease
7.1. COVID-19 and Antithrombotic Therapy in Occurring Acute Coronary Syndrome
7.2. Interaction between COVID-19 Investigational Therapies and Antiplatelet Medicaments
7.3. New Strategies for Patients with STEMI and Indications for the Percutaneous Coronary Intervention during COVID-19 Pandemic
8. NETs and Autoantibodies May Drive COVID-19 Blood Clots: A New Investigation and Therapy Plan
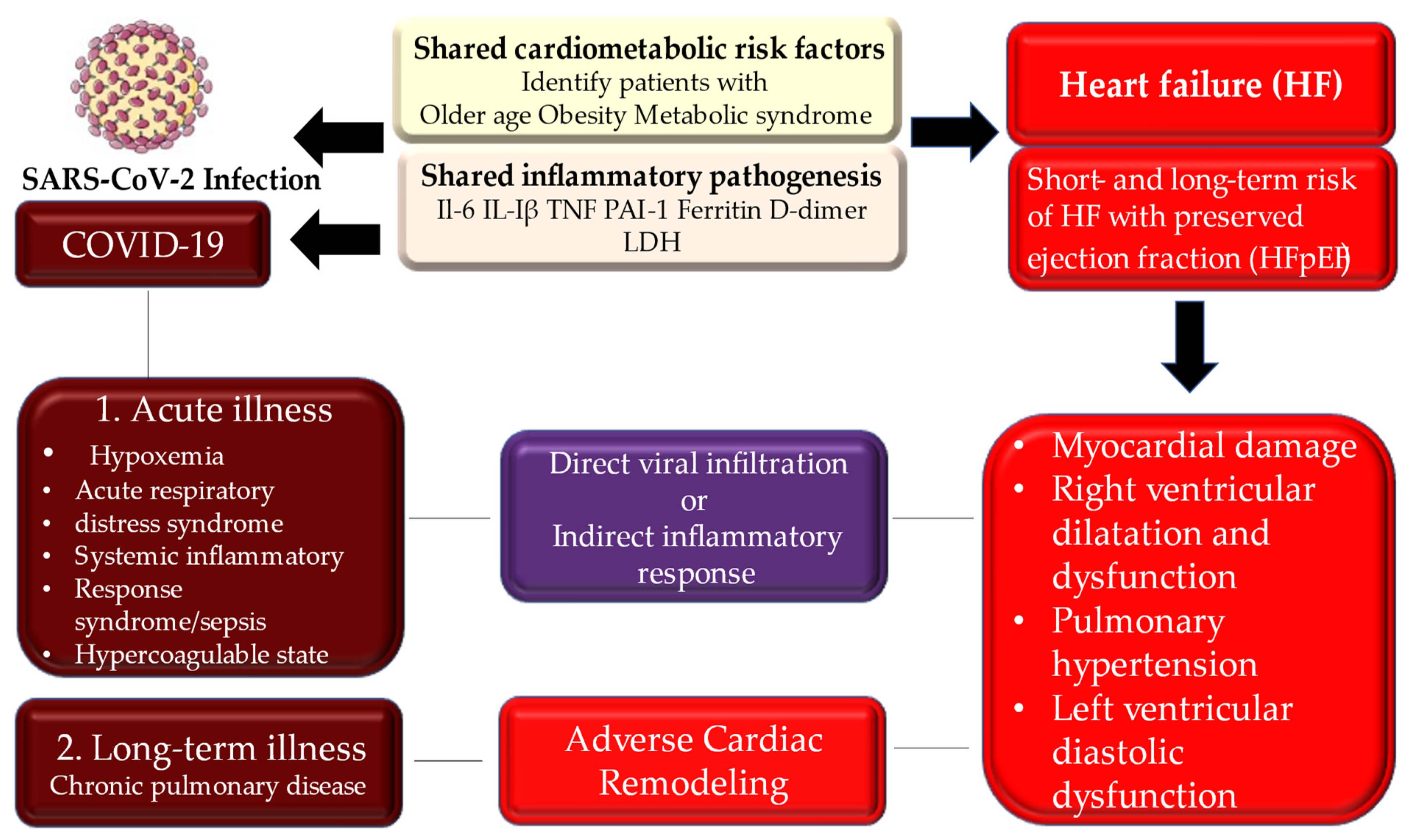
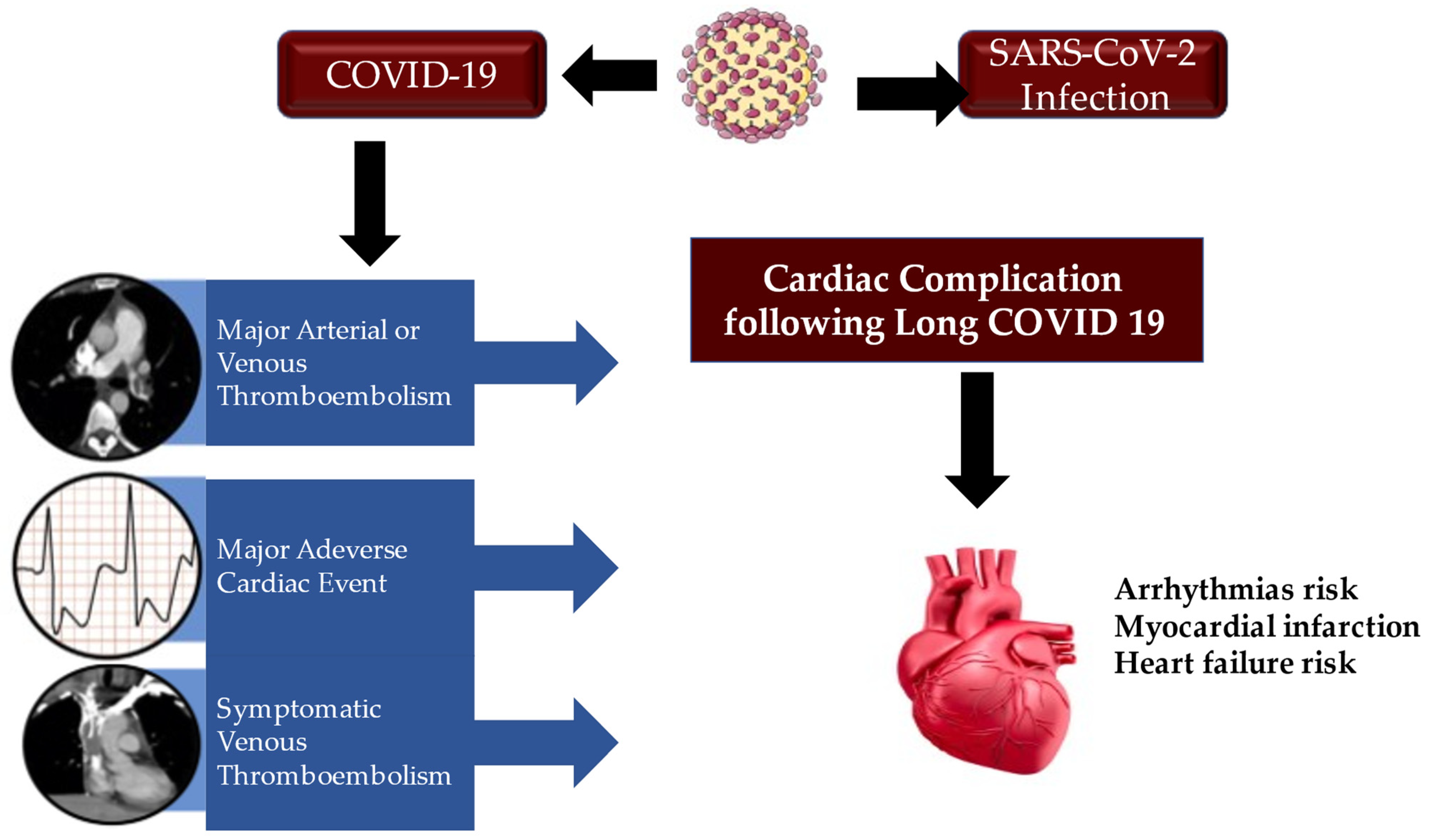
9. Long Term Consequences of COVID-19 Heart Disease
10. Discussion
11. Limitations
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations/Acronyms
| ACC | American College of Cardiology |
| ACE | angiotensin I-converting enzyme |
| ACS | acute coronary syndrome |
| ADP | P2Y12 receptor: adenosine 5′diphosphate P2Y12 receptor |
| AHA | American Heart Association |
| APTT | activated partial thromboplastin time |
| aPL | antiphospholipid |
| APS | antiphospholipid syndrome |
| ARDS | acute respiratory distress syndrome |
| CAD | coronary artery disease |
| Cit-H3 | citrullinated histone H3 |
| CTC | cardiac thrombotic complication |
| CVD | cardiovascular disease |
| COVID-19 | coronavirus disease 2019 |
| CYP3A4 | cytochrome P450 3A4 |
| CYP2C19 | Cytochrome P450 2C19 |
| CRP | C-reactive protein |
| DIC | disseminated intravascular coagulation |
| DILI | drug-induced liver injury |
| dsDNA | double-stranded DNA |
| ESC | European Society of Cardiology |
| ICU | intensive care unit |
| IL | interleukine |
| INR | international normalized ratio |
| MI | myocardial infarction |
| MPO-DNA | myeloperoxidase-DNA |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NETs | neutrophil extracellular traps. |
| PAI-1 | plasminogen activator inhibitor 1 |
| PCI | percutaneous coronary intervention |
| PE | pulmonary embolism |
| PT | prothrombin time |
| RAAS | renin-angiotensin-aldosterone system |
| RUCAM | Roussel Uclaf Causality Assessment Method |
| SARS-CoV-2 | severe acute respiratory syndrome-coronavirus-2 |
| SIRS | systemic inflammatory response syndrome |
| STEMI | ST elevation myocardial infarction |
| TED | thromboembolic disease |
| TNF | tumor necrosis factor |
| TT | thrombin time |
References
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; Fonarow, G.C.; O’Gara, P.T.; Yancy, C.W. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020, 5, 751–753. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Cleland, J.G.F.; Katus, H.A. Myocardial injury in severe COVID-19 infection. Eur. Heart J. 2020, 41, 2080–2082. [Google Scholar] [CrossRef]
- Dhakal, B.P.; Sweitzer, N.K.; Indik, J.H.; Acharya, D.; William, P. SARS-CoV-2 Infection and Cardiovascular Disease: COVID-19 Heart. Heart Lung Circ. 2020, 29, 973–987. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular considerations for patients, healthcare workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Zonzin, P. Age-adjusted D-dimer cut-off levels to rule out venous thromboembolism in COVID-19 patients. Thromb. Res. 2020, 190, 102. [Google Scholar] [CrossRef]
- Ciavarella, A.; Peyvandi, F.; Martinelli, I. Where do we stand with antithrombotic prophylaxis in patients with COVID-19? Thromb. Res. 2020, 191, 29. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; Van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020, 18, 786–787. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Amgalan, A.; Othman, M. Exploring possible mechanisms for COVID-19 induced thrombocytopenia: Unanswered questions. J. Thromb. Haemost. 2020, 18, 1514–1516. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Levi, M.; Clark, C.; Iba, T. Laboratory haemostasis monitoring in COVID-19. J. Thromb. Haemost. 2020, 18, 2058–2060. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Llitjos, J.-F.; Leclerc, M.; Chochois, C.; Monsallier, J.-M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Marietta, M.; Ageno, W.; Artoni, A.; De Candia, E.; Gresele, P.; Marchetti, M.; Marcucci, R.; Tripodi, A. COVID-19 and haemostasis: A position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020, 18, 167–169. [Google Scholar]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Müller, M.C.; Bouman, C.C.; Beenen, L.F.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Moll, M.; Zon, R.L.; Sylvester, K.W.; Chen, E.C.; Cheng, V.; Connell, N.T.; Fredenburgh, L.E.; Baron, R.M.; Cho, M.H.; Woolley, A.E.; et al. VTE in ICU Patients with COVID-19. Chest 2020, 158, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar] [CrossRef]
- Sofia, R.; Carbone, M.; Landoni, G.; Zangrillo, A.; Dagna, L. Anticoagulation as secondary prevention of massive lung thromboses in hospitalized patients with COVID-19. Eur. J. Intern. Med. 2022, 100, 21–24. [Google Scholar] [CrossRef]
- Poletto, F.; Spiezia, L.; Simion, C.; Campello, E.; Valle, F.D.; Tormene, D.; Camporese, G.; Simioni, P. Risk Factors of Venous Thromboembolism in Noncritically Ill Patients Hospitalized for Acute COVID-19 Pneumonia Receiving Prophylactic-Dose Anticoagulation. Viruses 2022, 14, 737. [Google Scholar] [CrossRef]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Jimenez, D.; Le Gal, G.; Rali, P.; et al. Prevention, diagnosis and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest 2020, 158, 1143–1163. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Thierry, A.R.; Roch, B. Neutrophil Extracellular Traps and By-Products Play a Key Role in COVID-19: Pathogenesis, Risk Factors, and Therapy. J. Clin. Med. 2020, 9, 2942. [Google Scholar] [CrossRef]
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with COVID-19—A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480. [Google Scholar] [CrossRef] [PubMed]
- Khazaal, S.; Harb, J.; Rima, M.; Annweiler, C.; Wu, Y.; Cao, Z.; Khattar, Z.A.; Legros, C.; Kovacic, H.; Fajloun, Z.; et al. The Pathophysiology of Long COVID throughout the Renin-Angiotensin System. Molecules 2022, 27, 2903. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943, Erratum in JAMA Intern. Med. 2020, 180, 1031. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Lymperaki, E.; Kazeli, K.; Variti, G.; Gerothanasi, M.; Gkinoudis, A.; Tsamesidis, I.; Vagdatli, E. Potential Role of Certain Biomarkers Such as Vitamin B12, ROS, Albumin, as Early Predictors for Prognosis of COVID-19 Outcomes. Medicines 2022, 9, 36. [Google Scholar] [CrossRef]
- Garma, L.D.; Deng, H.; Goldschmidt, E. Integrated analysis of transcriptomic data reveals the platelet response in COVID-19 disease. Sci. Rep. 2022, 12, 6851. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Varshney, S.; Dutt, N.; Munikumar, M.; Asfahan, S.; Kulkarni, P.P.; Gupta, P. D-dimer, disease severity, and deaths (3D-study) in patients with COVID-19: A systematic review and meta-analysis of 100 studies. Sci. Rep. 2021, 11, 21888. [Google Scholar] [CrossRef]
- Du, W.N.; Zhang, Y.; Yu, Y.; Zhang, R.M. D-dimer levels is associated with severe COVID-19 infections: A meta-analysis. Int. J. Clin. Pract. 2021, 75, e14031. [Google Scholar] [CrossRef]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.L.; Li, J.; Liu, X.H.; Zhu, C.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Nappi, F.; Bellomo, F.; Avtaar Singh, S.S. Insights into the Role of Neutrophils and Neutrophil Extracellular Traps in Causing Cardiovascular Complications in Patients with COVID-19: A Systematic Review. J. Clin. Med. 2022, 11, 2460. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, M.; Lin, W.; Cai, Q.; Zhang, L.; Chen, D.; Liu, F.; Xiong, X.; Chu, J.; Peng, J.; et al. Cardiac biomarkers, cardiac injury, and comorbidities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Immun. Inflamm. Dis. 2021, 9, 1071–1100. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed]
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beasley, M.B.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; AlRasheed, M.R.; et al. Pathophysiology of SARS-CoV-2: The Mount Sinai COVID-19 autopsy experience. Mod. Pathol. 2021, 34, 1–12. [Google Scholar] [CrossRef]
- Schaefer, I.M.; Padera, R.F.; Solomon, I.H.; Kanjilal, S.; Hammer, M.M.; Hornick, J.L.; Sholl, L.M. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod. Pathol. 2020, 33, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Delorey, T.M.; Ziegler, C.G.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstolpe, Å.; Abbondanza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.T.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.; Coronado, M.-J.; Hernández-Terciado, F.; Martín, P.; Royuela, A.; Ramil, E.; García, D.; Goicolea, J.; Del Trigo, M.; Ortega, J.; et al. Assessment of Neutrophil Extracellular Traps in Coronary Thrombus of a Case Series of Patients With COVID-19 and Myocardial Infarction. JAMA Cardiol. 2021, 6, 469. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef]
- Szekely, Y.; Lichter, Y.; Taieb, P. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19), A systematic echocardiographic study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative; GlobalSurg Collaborative. SARS-CoV-2 infection and venous thromboembolism after surgery: An international prospective cohort study. Anaesthesia 2022, 77, 28–39. [Google Scholar]
- COVIDSurg Collaborative; GlobalSurg Collaborative. Effects of pre-operative isolation on postoperative pulmonary complications after elective surgery: An international prospective cohort study. Anesthesia 2021, 76, 1454–1464. [Google Scholar]
- COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 vaccination modelling for safe surgery to save lives: Data from an international prospective cohort study. Br. J. Surg. 2021, 108, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative; GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anaesthesia 2021, 76, 748–758. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 18, 281–292. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2. (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Blasco, A.; Bellas, C.; Goicolea, L.; Muñiz, A.; Abraira, V.; Royuela, A.; Mingo, S.; Oteo, J.F.; García-Touchard, A.; Goicolea, F.J. Immunohistological Analysis of Intracoronary Thrombus Aspirate in STEMI Patients: Clinical Implications of Pathological Findings. Rev. Española De Cardiol. (Engl. Ed.) 2017, 70, 170–177. [Google Scholar] [CrossRef]
- Langseth, M.S.; Helseth, R.; Ritschel, V.; Hansen, C.H.; Andersen, G.; Eritsland, J.; Halvorsen, S.; Fagerland, M.W.; Solheim, S.; Arnesen, H.; et al. Double-Stranded DNA and NETs Components in Relation to Clinical Outcome After ST-Elevation Myocardial Infarction. Sci. Rep. 2020, 10, 5007. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes Jr, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Youssry, I.; Elaziz, D.A.; Ayad, N.; Eyada, I. The Cause–Effect Dilemma of Hematologic Changes in COVID-19: One Year after the Start of the Pandemic. Hematol. Rep. 2022, 14, 95–102. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M.; Favaloro, E.J. Mean Platelet Volume Predicts Severe COVID-19 Illness. Semin. Thromb. Hemost. 2021, 47, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Favaloro, E.J. D-dimer is associated with severity of coronavirus disease 2019 (COVID-19): A Pooled analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar]
- Thachil, J.; Longstaff, C.; Favaloro, E.J.; Lippi, G.; Urano, T.; Kim, P.Y. the SSC Subcommittee on Fibrinolysis of the International Society on Thrombosis and Haemostasis The need for accurate D-dimer reporting in COVID-19: Communication from the ISTH SSC on fibrinolysis. J. Thromb. Haemost. 2020, 18, 2408–2411. [Google Scholar] [CrossRef]
- Perini, P.; Nabulsi, B.; Massoni, C.B.; Azzarone, M.; Freyrie, A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet 2020, 395, 1546. [Google Scholar] [CrossRef]
- Lippi, G.; Salvagno, G.L.; Ippolito, L.; Franchini, M.; Favaloro, E.J. Shortened activated partial thromboplastin time: Causes and management. Blood Coagul. Fibrinolysis 2010, 21, 459–463. [Google Scholar] [CrossRef]
- Levi, M.M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S. Thromboembolic Complications of SARS-CoV-2 and Metabolic Derangements: Suggestions from Clinical Practice Evidence to Causative Agents. Metabolites 2021, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Stoneham, S.M.; Milne, K.M.; Nuttall, E.; Frew, G.H.; Sturrock, B.R.; Sivaloganathan, H.; Ladikou, E.E.; Drage, S.; Phillips, B.; Chevassut, T.J.; et al. Thrombotic risk in COVID-19: A case series and case-control study. Clin. Med. 2020, 20, e76–e81. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2021, 51, 446–453. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Planquette, B.; Le Berre, A.; Khider, L.; Yannoutsos, A.; Gendron, N.; de Torcy, M.; Mohamedi, N.; Jouveshomme, S.; Smadja, D.M.; Lazareth, I.; et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID-19 patients with respiratory symptoms: A nested case-control study. Thromb. Res. 2021, 197, 94–99. [Google Scholar] [CrossRef]
- Trimaille, A.; Curtiaud, A.; Marchandot, B.; Matsushita, K.; Sato, C.; Leonard-Lorant, I.; Sattler, L.; Grunebaum, L.; Ohana, M.; Von Hunolstein, J.J.; et al. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb. Res. 2020, 193, 166–169. [Google Scholar] [CrossRef]
- Shah, A.; Donovan, K.; McHugh, A.; Pandey, M.; Aaron, L.; Bradbury, C.A.; Stanworth, S.J.; Alikhan, R.; Von Kier, S.; Maher, K.; et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: A multicentre observational study. Crit. Care 2020, 24, 561. [Google Scholar] [CrossRef]
- Koleilat, I.; Galen, B.; Choinski, K.; Hatch, A.N.; Jones, D.B.; Billett, H.; Indes, J.; Lipsitz, E. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 36–46. [Google Scholar] [CrossRef]
- Kampouri, E.; Filippidis, P.; Viala, B.; Méan, M.; Pantet, O.; Desgranges, F.; Tschopp, J.; Regina, J.; Karachalias, E.; Bianchi, C.; et al. Predicting Venous Thromboembolic Events in Patients with Coronavirus Disease 2019 Requiring Hospitalization: An Observational Retrospective Study by the COVIDIC Initiative in a Swiss University Hospital. Biomed. Res. Int. 2020, 2020, 9126148. [Google Scholar] [CrossRef]
- Koupenova, M. Potential role of platelets in COVID-19: Implications for thrombosis. Res. Pract. Thromb. Haemost. 2020, 4, 737–740. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on Behalf of theHLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Espinosa, G.; Serrano, A.; Cervera, R. Antigens and Antibodies of the Antiphospholipid Syndrome as New Allies in the Pathogenesis of COVID-19 Coagulopathy. Int. J. Mol. Sci. 2022, 23, 4946. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 2022, 44, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Teschke, R.; Méndez-Sánchez, N.; Eickhoff, A. Liver Injury in COVID-19 Patients with Drugs as Causatives: A Systematic Review of 996 DILI Cases Published 2020/2021 Based on RUCAM as Causality Assessment Method. Int. J. Mol. Sci. 2022, 23, 4828. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, F.M.; De Bruyne, B.; Pijls, N.H.; Desai, M.; Oldroyd, K.G.; Park, S.-J.; Reardon, M.J.; Wendler, O.; Woo, J.; Yeung, A.C.; et al. Rationale and design of the Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) 3 Trial: A comparison of fractional flow reserve–guided percutaneous coronary intervention and coronary artery bypass graft surgery in patients with multivessel coronary artery disease. Am. Heart J. 2015, 170, 619–626.e2. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Rivara, M.B.; Bajwa, E.K.; Januzzi, J.L.; Gong, M.N.; Thompson, B.T.; Christiani, D.C. Prognostic Significance of Elevated Cardiac Troponin-T Levels in Acute Respiratory Distress Syndrome Patients. PLoS ONE 2012, 7, e40515. [Google Scholar] [CrossRef]
- Poe, S.; Vandivier-Pletsch, R.H.; Clay, M.; Wong, H.R.; Haynes, E.; Rothenberg, F.G. Cardiac Troponin Measurement in the Critically Ill: Potential for Guiding Clinical Management. J. Investig. Med. 2015, 63, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, E.K.; Januzzi, J.L.; Gong, M.N.; Thompson, B.T.; Christiani, D.C. Prognostic value of plasma N-terminal probrain natriuretic peptide levels in the acute respiratory distress syndrome. Crit. Care Med. 2008, 36, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Gentile, F.; Aimo, A.; Januzzi JLJr Richards, A.M.; Lam, C.S.P.; de Boer, R.A.; Meems, L.M.G.; Latini, R.; Staszewsky, L.; Anand, I.S.; et al. Circulating levels and prognostic cut-offs of sST2, hs-cTnT, and NT-proBNP in women vs. men with chronic heart failure. ESC Heart Fail. 2022, 9, 2084–2095. [Google Scholar] [CrossRef]
- Raynor, A.; Vallée, C.; Belkarfa, A.-L.; Lunte, K.; Laney, M.; Belhadjer, Z.; Vicca, S.; Boutten, A.; Bonnet, D.; Nivet-Antoine, V. Multisystem inflammatory syndrome in children: Inputs of BNP, NT-proBNP and Galectin-3. Clin. Chim. Acta 2022, 529, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Hampton, T. Autoantibodies May Drive COVID-19 Blood Clots. JAMA 2021, 325, 425. [Google Scholar] [CrossRef]
- Nappi, F. Incertitude Pathophysiology and Management During the First Phase of the COVID-19 Pandemic. Ann. Thorac. Surg. 2022, 113, 693. [Google Scholar] [CrossRef]
- Nappi, F.; Giacinto, O.; Ellouze, O.; Nenna, A.; Singh, S.S.A.; Chello, M.; Bouzguenda, A.; Copie, X. Association between COVID-19 Diagnosis and Coronary Artery Thrombosis: A Narrative Review. Biomedicines 2022, 10, 702. [Google Scholar] [CrossRef]
- Nappi, F.; Avtaar Singh, S.S. Endothelial Dysfunction in SARS-CoV-2 Infection. Biomedicines 2022, 10, 654. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S. Molecular Insights of SARS-CoV-2 Antivirals Administration: A Balance between Safety Profiles and Impact on Cardiovascular Phenotypes. Biomedicines 2022, 10, 437. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Hussaniy, H.A.; Al-Harcan, N.A.H.; Alexiou, A.; Batiha, G.E. Neutrophil Extracellular Traps (NETs) and COVID-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022, 104, 108516. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Welt, F.G.; Shah, P.B.; Aronow, H.D.; Bortnick, A.E.; Henry, T.D.; Sherwood, M.W.; Young, M.N.; Davidson, L.J.; Kadavath, S.; Mahmud, E.; et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: From ACC’s Interventional Council and SCAI. J. Am. Coll Cardiol. 2020, 75, 2372–2375. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, J.; Pan, L. How to balance acute myocardial infarction and COVID-19: The protocols from Sichuan Provincial People’s Hospital. Intensiv. Care Med. 2020, 46, 1111–1113. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients with Cancer Treated with Bevacizumab: A Meta-Analysis of More Than 20000 Patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kentepozidis, N.; Kotsakis, A.; Kapiris, I. Cancer therapy and cardiovascular risk: Focus on bevacizumab. Cancer Manag. Res. 2015, 7, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fu, Y.; Tian, D.; Sun, N.; Han, W.; Chang, G.; Dong, Y.; Xu, X.; Liu, Q.; Huang, D.; et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: A pilot trial. Circulation 2015, 132, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Olsen, N.J.; Schleich, M.A.; Karp, D.R. Multifaceted effects of hydroxychloroquine in human disease. Semin. Arthritis Rheum. 2013, 43, 264–272. [Google Scholar] [CrossRef]
- Prescribing Information. Brilinta (Ticagrelor); AstraZeneca LP: Wilmington, DE, USA, 2011.
- Product Monograph. Brilinta (Ticagrelor); AstraZeneca Canada: Mississauga, ON, Canada, 2011.
- Itkonen, M.; Tornio, A.; Lapatto-Reiniluoto, O.; Neuvonen, M.; Neuvonen, P.; Niemi, M.; Backman, J.T. Clopidogrel Increases Dasabuvir Exposure With or Without Ritonavir, and Ritonavir Inhibits the Bioactivation of Clopidogrel. Clin. Pharmacol. Ther. 2018, 105, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Marsousi, N.; Daali, Y.; Fontana, P.; Reny, J.-L.; Ancrenaz-Sirot, V.; Calmy, A.; Rudaz, S.; Desmeules, J.A.; Samer, C.F. Impact of Boosted Antiretroviral Therapy on the Pharmacokinetics and Efficacy of Clopidogrel and Prasugrel Active Metabolites. Clin. Pharmacokinet. 2018, 57, 1347–1354. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Ranard, L.S.; Berg, D.D. Cardiopulmonary Resuscitation During the COVID-19 Pandemic: A View From Trainees on the Front Line. Circulation 2020, 141, 1833–1835. [Google Scholar] [CrossRef]
- Valente, S.; Anselmi, F.; Cameli, M. Acute coronary syndromes during COVID-19. Eur. Heart J. 2020, 41, 2047–2049. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Mozzini, C.; Garbin, U.; Pasini, A.M.F.; Cominacini, L. An exploratory look at NETosis in atherosclerosis. Intern. Emerg. Med. 2016, 12, 13–22. [Google Scholar] [CrossRef]
- Almyroudis, N.G.; Grimm, M.J.; Davidson, B.A.; Rohm, M.; Urban, C.F.; Segal, B.H. NETosis and NADPH oxidase: At the intersection of host defence, inflammation, and injury. Front. Immunol. 2013, 4, 45. [Google Scholar] [CrossRef]
- Ali, R.A.; Gandhi, A.A.; Meng, H.; Yalavarthi, S.; Vreede, A.P.; Estes, S.K.; Palmer, O.R.; Bockenstedt, P.L.; Pinsky, D.J.; Greve, J.M.; et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019, 10, 1916. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Liu, S.; Sun, J.; Chen, Z.; Jiang, M.; Zhang, Q.; Wei, Y.; Wang, X.; Huang, Y.-Y.; et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B 2020, 10, 1205–1215. [Google Scholar] [CrossRef]
- Knight, J.S. Dipyridamole to Prevent Coronavirus Exacerbation of Respiratory Status (DICER) in COVID-19 (DICER). ClinicalTrials.Gov Identifier. Available online: https://clinicaltrials.gov/ct2/show/NCT04391179 (accessed on 25 August 2022).
- Harvala, H.; Nguyen, D.; Simmonds, P.; Lamikanra, A.A.; Tsang, H.P.; Otter, A.; Maes, P.; Webster, M.; Clarkson, A.; Kaloyirou, F.; et al. Convalescent plasma donors show enhanced cross-reactive neutralising antibody response to antigenic variants of SARS-CoV-2 following immunisation. Transfusion 2022, 62, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Simonovich, V.A.; Pratx, L.D.B.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Lavie, C.J.; Sanchis-Gomar, F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jin, Z. An Acute Respiratory Infection Runs Into the Most Common Noncommunicable Epidemic—COVID-19 and Cardiovascular Diseases. JAMA Cardiol. 2020, 5, 743. [Google Scholar] [CrossRef] [PubMed]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a large series of COVID-19 cases from Northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Singh, S.A. COVID-19 Pathogenesis: From Molecular Pathway to Vaccine Administration. Biomedicines 2021, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 2016, 1, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of Myocardial Infarction and Stroke after Acute Infection or Vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.; Penninger, J.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| First Author/Year Ref | Type of Study | Number of Patients | Mean Age, years | Finding |
|---|---|---|---|---|
| Klok (2020) Thromb. Res. [9] | Retrospective Single Center (Netherlands) | 184 | 64 (12) | Higher incidence (31%) of TED in ICU patients. VTE in 27% (95%CI 17–37%) and arterial thrombotic events in 3.7% (95%CI 0–8.2%). |
| Tang (2020) J. Thromb. Haemost [11] | Prospective Single Center Wuan (China) | 183 | 54.1 ± 16.2 | Elevated D-dimer and FDP are common in deaths with NCP fibrin degradation product (FDP) novel coronavirus pneumonia (NCP). |
| Cui (2020) J. Thromb Haemost [14] | Prospective Single Center Wuan (China) | 81 | 59.9 (14.1) | Higher incidence of VTE (25%) in severe NCP, with poor prognosis. High-risk groups of VTE identified for increased D-dimer. |
| Klok (2020) Thromb Res. [17] | Retrospective Single Center (Netherlands) | 184 | 64 (12) | Higher cumulative incidence of thrombotic complications in critically ill patients with NCP. Total 95% confidence interval [CI] 41–57%. Pulmonary embolism (PE) (65/75; 87%). |
| Lodigiani (2020) Thromb Res. [15] | Prospective Single Center Milan (Italy) | 388 | 61 (55–69) | High rate of TED within 24 h of admission. High rate of positive VTE imaging suggested to improve specific thromboprophylaxis. |
| Middeldorp (2020) J Thromb Haemost. [20] | Prospective Single Center Amsterdam (Netherlands) | 75 | 62 (10) | Higher risk of VTE in ICU patients 42% (95% CI 30–54) at 21 days. |
| Tang (2020) J. Thromb Haemost [29] | Prospective Single Center Wuan (China) | 449 | 65.1 ± 12.0 | Anticoagulant therapy, mainly using low molecular weight heparin, is associated with better prognosis. SIC criteria were relevant or D-dimer were markedly elevated. |
| Huang (2022) Lancet Respir Med. [33] | Retrospective Single Center Wuan (China) | 2469 | 57.0 (48.0–65.0) | Within 2 years, COVID-19 survivors had longitudinal improvements in physical and mental health; however, this population had a remarkably lower health status. |
| Wu (2020) JAMA [34] | Retrospective multicenter (China) | 72,314 | 30 to 79 years of age (87%) | Draconian measures may be considered to limit the spread of infection. |
| Wu (2020) JAMA Intern. Med. [35] | Retrospective Multicenter (China) | 201 | 51 (43–60) | Older had greater risk of progression toward ARDS and death HR, 6.17; 95% CI, 3.26–11.67. Higher D-dimer HR, 1.02; 95% CI, 1.01–1.04. |
| Zhou (2020) Lancet [36] | Retrospective Multicenter (China) | 191 | 56 (46–67) Non-survivor 69 (63–76) Survivor 52 (45–58) | Older age is an increased risk factors (p < 0.0001), as well high SOFA score and d-dimer greater than 1 μg/mL These factors can identify poor prognosis at an early stage. |
| Lymperaki (2022) Medicines [37] | Prospective Single center | 199 Non COVID (60) COVID (139) | Non COVID 9–89 COVID 28–91 | Biomarkers, such as vitamin B12 (p = 0.0029), ROS (p < 0.0001), and albumin (p = 0.046), are useful as possible prognosis tools for an early diagnosis. |
| Garma (2022) Sci. Rep. [38] | Prospective Single center | 22 | ACE-2 was not expressed by infected or control platelets. | |
| Lippi (2020) Clin. Chim. Acta. [39] | Retrospective Multicenter (Study level meta-analysis) | 1779 | 38–67 | Low platelet count is associated with increased risk of severe disease and mortality in patients with COVID-19. |
| Varikasuvu (2021) Sci. Rep. [40] | Retrospective Multicenter (Study level meta-analysis) | Unadjusted 26,960 Adjusted 15,653 | 41–73 | Higher D-dimer levels provide early assess COVID-19 patients at risk for disease progression and mortality outcomes. |
| Du (2021) Int. J. Clin. Pract. [41] | Retrospective Multicenter (Study level meta-analysis) | 1430 Non severe COVID (1025) Severe COVID (378) | Non severe COVID 29–74 Severe COVID 41–83 | Severe COVID-19 patients reveal a higher concentration of D-dimer, when compared with non-severe patients. |
| Han (2020) Clin Chem Lab Med [42] | Prospective Single Center Wuan (China) | 94 | Patients with SARS-CoV-2 reveal significant changes in coagulation function, as compared with healthy people. Monitoring D-dimer and FDP values may be helpful to identify severe cases. | |
| Yang (2020) Lancet Respir Med. [43] | Retrospective Single Center Wuan (China) | 710 52 critically | 59·7 (13·3) | Older patients (>65 years) with comorbidities and ARDS are at increased risk of death. |
| Gao (2020) J. Med. Virol. [44] | Retrospective Single Center Wuan (China) | 43 | Severe COVID 45.20 ± 7.68 Mild COVID 42.96 ± 14.00 | IL-6 and d-D tandem testing predict severity of COVID (sensitivity 93.3%, for IL-6 and 96.4%.d-D). |
| Wang (2020) JAMA [45] | Retrospective Single Center Wuan (China) | 138 | 56 (42–68) | A total of 41% of patients with COVID-19 have presumed hospital-related transmission. A total of 26% of patients received ICU care, and mortality was 4.3%. |
| Yang (2020) J. Thromb Haemost [46] | Retrospective Single Center Wuan (China) | 1476 | Survivors 56 (46–65) Non survivors 67 (59–75) | Thrombocytopenia is marked in patients with COVID-19, and it is associated with increased risk of in-hospital mortality. |
| Nappi (2022) J. Clin. Med. [47] | Retrospective Multicenter (Systematic review) | 38,485 | (29–86) | NETs are implicated in the pathogenesis of the inflammatory response during COVID-19, and long-term effects requires ongoing monitoring and research. |
| Guo (2020) JAMA cardiology [48] | Retrospective Single Center Wuan (China) | 187 | 58.50 (14.66) | Myocardial injury is significantly associated with fatal outcome of COVID-19 with increased cardiac dysfunction and arrhythmias. |
| Zhu (2021) Immun. Inflamm. Dis. [49] | Retrospective Multicenter (Study level meta-analysis) | 15,354 | 40 (1–96) | Hypertension, cardiovascular disease, acute cardiac injury, and related laboratory indicators are associated with the severity of COVID-19. |
| Lala (2020) JACC [50] | Prospective single center | 985 | 66.4 (18–100) | Myocardial injury is prevalent among patients hospitalized with COVID-19. Low levels of troponin are revealed. |
| Zuo (2020) Sci. Transl. Med. [51] | Prospective single center | 172 | 61 ± 17 (25–95) | Patients hospitalized with COVID-19 reveal transient positivity for APL antibodies. APL autoantibodies are potentially pathogenic. |
| Zuo (2020) JCI Insight [52] | Prospective single center | 80 | 61 ± 15 (29–91) | Sera from patients with COVID-19 disclose NET release. |
| Bryce et al. (2021) Mod. Pathol [53] | Retrospective Single center | 100 | 68 (29 to 94) | A total of 82 cases were DAD. Hemphagocytosis, higher cytokines IL-6, IL-8, and TNFα. |
| Schaefer et al. (2020) Mod. Pathol. [54] | Retrospective Single center | 7 | 66 (50 to 77) | A total of 5 cases diffused DAD. Two cases alveolar injuries. SARS-CoV-2 infection involving epithelial lung cell in acute phase. No endothelial cell infection. |
| Delorey et al. (2021) Nature [55] | Retrospective Single center | 32 | 30 to 89 | Higher viral RNAs in phagocytic mononuclear and endothelial lung cells. Transcriptional alterations in multiple cell types in the heart tissue. |
| Lindner et al. (2020) JAMA Cardiol. [56] | Prospective Single center | 39 | 68 (78–89) | SARS-CoV-2 directly infects the myocardium. Absence of inflammatory cell infiltrates in patient with SARS-CoV-2 infection. Higher cytokine response. |
| Varga et al. (2020) Lancet [22] | Retrospective Single center | 3 | 63 (58–61) | Lymphocytic endotheliitis in lung, heart, kidney, and liver. Apoptotic bodies in the heart; mononuclear cells in lung. |
| Ackerman et al. (2020) N. Engl. J. Med. [24] | Retrospective Single center | 14 SARS-CoV-2 7 H1N1 7 | 68 ± 9.2 years (female) 80 ± 11.5 years (male) | Alveolar capillary microthrombi 9 times more in SARS-CoV-2. Higher CD3, CD4, and CD-8 positive T cells in SARS-CoV-2. Lower neutrophils (CD15). |
| Blasco (2020) JAMA Cardiology [57] | Prospective Single center | 55 | COVID 62 (14) Non COVID 58 (12) | In patients with COVID-19 and myocardial infarction, NETs seem to play a major role in the pathogenesis of STEMI. |
| Chen (2020) Lancet [58] | Retrospective Multicenter center Wuan (China) | 99 | 55.5 (13.1) | The COVID-19 infection is more likely to affect older males with comorbidities, resulting in severe and even fatal acute respiratory distress syndrome. |
| Shi (2020) JAMA Cardiol. [59] | Retrospective Multicenter center Wuan (China) | 416 | 64 (21–95) | Cardiac injury is a common evidence among hospitalized patients with COVID-19, and it is associated with higher risk of in-hospital mortality. |
| Szekely (2020) Circulation [60] | Prospective Single center | 100 | 66.1 ± 17.2 | Preservation of LV systolic function is in the majority of COVID-19 patients. Impairement of LV diastolic and RV functions. Elevated troponin and poorer clinical grade are associated with worse RV function. |
| Xie (2022) Nat. Med. [61] | Retrospective Multicenter center | 153,760 | 61.42 (15.64) | Risk and 1-year burden of cardiovascular disease in survivors of acute COVID-19 are substantial. |
| Guan (2020) NEJM [61] | Retrospective Multicenter center Wuan (China) | 1099 | 47 (35.0–58.0) | COVID-19 spread rapidly throughout China and caused varying degrees of illness. Many patients without fever did not have abnormal radiologic findings. |
| COVIDSurg Collaborative (2022) Anaesthesia [62] | Prospective Multicenter | 128,013 | 55.6 (18–49) | High risk of thromboembolic complication in COVID-19 patients. |
| COVIDSurg Collaborative (2021) Anaesthesia [63] | Prospective Multicenter | 96,454 | Isolation before elective surgery might be associated with a small, but clinically important, increased risk of postoperative pulmonary complications. | |
| COVIDSurg Collaborative (2021) Br. J. Surg. [64] | Prospective Multicenter | 56,589 | (18–69) | As global roll out of SARS-CoV-2 vaccination proceeds, patients needing elective surgery should be prioritized ahead of the general population. |
| COVIDSurg Collaborative (2021) Anaesthesia [65] | Prospective Multicenter | 140,231 | (31.4–87.4) | After a ≥7 week delay in undertaking surgery, following SARS-CoV-2 infection, patients with ongoing symptoms had a higher mortality than patients whose symptoms had resolved or who had been asymptomatic 6.0% (95% CI 3.2–8.7) vs. 2.4% (95% CI 1.4–3.4) vs. 1.3% (95% CI 0.6–2.0). |
| Authors | Total SARS-CoV-2 + Hospitalized Patients | VTE, ATE Cases | Risk Factors More Present in Cases (p < 0.05) | Risk Factors Similar in Cases and Controls (p > 0.05) | Conclusions |
|---|---|---|---|---|---|
| Stoneham et al., 2020 [82] | 208 | 21 | High WBCs, high D-dimer, high INR. | APTT ratio, fibrinogen. | Comorbidities were not associated with a higher risk of thrombosis. Monitoring of D-dimer and anti-factor Xa levels may be relevant for management. |
| Zuo et al., 2020 [83] | 44 | 11 | High calprotectin, markers of NETs (myeloperoxidase-DNA complexes) high D-dimer, high platelets. | Troponins, WBCs. | There was a significant difference between peak D-dimer, calprotectin and cell free DNA levels between the populations. |
| Zhang et al., 2020 [84] | 143 | 66 | High WBCs, older age, low oxygenation index, high rate of cardiac injury, CURB-65 score 3 to 5, Padua score ≥ 4, high D-dimer. | Platelets count. | COVID-19 is suspected to cause an additional risk factor for DVT in hospitalized patients. |
| Planquette et al., 2020 [85] | 1042 | 59 | High CRP, fibrinogen, d-dimer. IMV. | Comorbidities: BMI, previous VTE, ATE, cancer, hypertension, cardiovascular diseases. | No higher prevalence for VTE risk factors in cases group compared to both cases and control was found. Altered coagulation parameters were found. |
| Trimaille et al., 2020 [86] | 289 | 49 | High Improve score, high WBCs, d-dimer, low haemoglobin at discharge. | Padua score of 4 or more, CRP- | Lack of thromboprophylaxis is a major determinant of VTE in non-ICU COVID-19 patients. Comorbidities were not found to affect the event occurrence. |
| Shah et al., 2020 [87] | 187 | 81 | High troponins, ferritin, d-dimer. | Platelets count, WBCs, thromboelastography parameters. | Elevated D-dimer, ferritin, troponin and white cell count at ICU admission may reflect undiagnosed altered coagulation and be used to identify patients for CTPA. |
| Kolielat et al., 2020 [88] | 117 | 18 | High d-dimer, fibrinogen, ferritin. | WBCs, platelets, troponins, Il-6. | Elevated d-dimer and a less elevated fibrinogen are associated with DVT despite conventional thromboprophylactic treatment. |
| Kampuori et al., 2020 [89] | 443 | 41 | High d-dimer, positive Wells criteria, bilateral infiltrates on X-rays or CT scan, mechanical ventilation. | Wbcs, platelets, CRP, Padua score, Geneva score. | The combination of Wells ≥ 2 score and D−dimer ≥ 3000 ng/L is a good predictor of VTE at admission. Hospitalization in the ICU and especially mechanical ventilation were associated with VTE occurrence. The combination of Wells’ score with the D-dimer value at admission can be a useful tool to guide empiric anticoagulation therapy. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Nappi, P.; Gambardella, I.; Avtaar Singh, S.S. Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites 2022, 12, 889. https://doi.org/10.3390/metabo12100889
Nappi F, Nappi P, Gambardella I, Avtaar Singh SS. Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites. 2022; 12(10):889. https://doi.org/10.3390/metabo12100889
Chicago/Turabian StyleNappi, Francesco, Pierluigi Nappi, Ivancarmine Gambardella, and Sanjeet Singh Avtaar Singh. 2022. "Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review" Metabolites 12, no. 10: 889. https://doi.org/10.3390/metabo12100889
APA StyleNappi, F., Nappi, P., Gambardella, I., & Avtaar Singh, S. S. (2022). Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites, 12(10), 889. https://doi.org/10.3390/metabo12100889