Analytical Validation of an Assay for Concurrent Measurement of Amino Acids in Dog Serum and Comparison of Amino Acid Concentrations between Whole Blood, Plasma, and Serum from Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Amino acid Analysis
2.3. Validation
2.4. Statistical Analysis
3. Results
3.1. Analytical Validation for Dog Serum
3.2. Intra-Assay Variability (Precision)
3.3. Inter-Assay Variability (Reproducibility)
3.4. Spiking Recovery (Accuracy/Matrix Effect)
3.5. Dilutional Parallelism (Linearity)
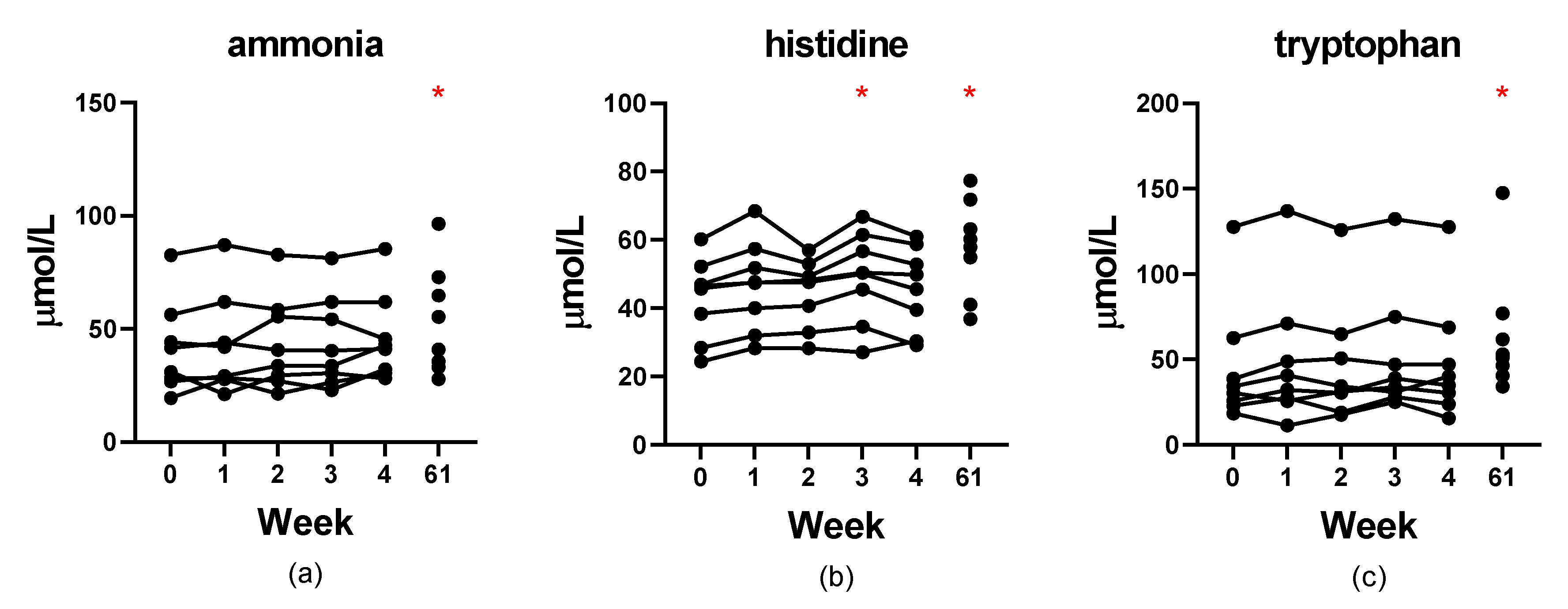
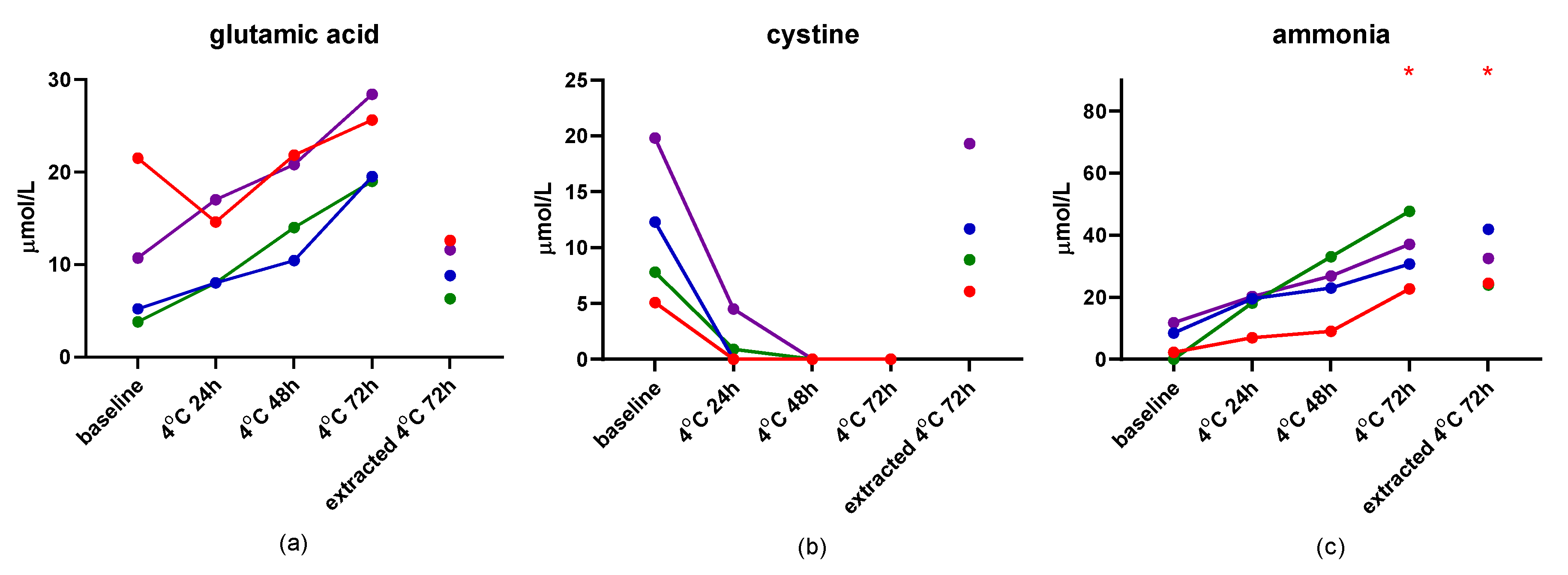
3.6. Stability
3.7. Effect of Hemolysis
3.8. Other Correlations
3.9. Comparison of Results in Whole Blood, Plasma, and Serum
4. Discussion
4.1. Analytical Validation of Dog Serum
4.2. Whole Blood, Plasma, Serum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, X.; Hu, C.-A.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A Gut Bacterial Pathway Metabolizes Aromatic Amino Acids into Nine Circulating Metabolites. Nature 2017, 551, 648. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Williams, B.B.; Battaglioli, E.J.; Whitaker, W.R.; Till, L.; Grover, M.; Linden, D.R.; Akiba, Y.; Kandimalla, K.K.; Zachos, N.C.; et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018, 23, 775–785.e775. [Google Scholar] [CrossRef] [PubMed]
- Dewolfe, M.S.; Baskurt, S.; Cochrane, W.A. Automatic Amino Acid Analysis of Blood Serum and Plasma. Clin. Biochem. 1967, 1, 75–81. [Google Scholar] [CrossRef]
- Dossin, O. Laboratory Tests for Diagnosis of Gastrointestinal and Pancreatic Diseases. Top. Companion Anim. Med. 2011, 26, 86–97. [Google Scholar] [CrossRef]
- Allenspach, K. Diagnosis of Small Intestinal Disorders in Dogs and Cats. Clin. Lab. Med. 2015, 35, 521–534. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A Dysbiosis Index to Assess Microbial Changes in Fecal Samples of Dogs with Chronic Inflammatory Enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Appierto, V.; Callari, M.; Cavadini, E.; Morelli, D.; Daidone, M.G.; Tiberio, P. A Lipemia-Independent Nanodrop((R))-Based Score to Identify Hemolysis in Plasma and Serum Samples. Bioanalysis 2014, 6, 1215–1226. [Google Scholar] [CrossRef]
- Delaney, S.J.; Kass, P.H.; Rogers, Q.R.; Fascetti, A.J. Plasma and Whole Blood Taurine in Normal Dogs of Varying Size Fed Commercially Prepared Food. J. Anim. Physiol. Anim. Nutr. 2003, 87, 236–244. [Google Scholar] [CrossRef]
- Sakamoto, T.; Qiu, Z.; Inagaki, M.; Fujimoto, K. Simultaneous Amino Acid Analysis Based on (19)F Nmr Using a Modified Opa-Derivatization Method. Anal. Chem. 2020, 92, 1669–1673. [Google Scholar] [CrossRef]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated Gc–Ms Analysis of Free Amino Acids in Biological Fluids. J. Chromatogr. B 2008, 870, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Ayon, N.J.; Sharma, A.D.; Gutheil, W.G. Lc-Ms/Ms-Based Separation and Quantification of Marfey’s Reagent Derivatized Proteinogenic Amino Acid Dl-Stereoisomers. J. Am. Soc. Mass Spectrom. 2019, 30, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Ng, A.; Kwan, T.; Cusmano-Ozog, K.; Cowan, T.M. A Rapid, Sensitive Method for Quantitative Analysis of Underivatized Amino Acids by Liquid Chromatography-Tandem Mass Spectrometry (Lc-Ms/Ms). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 944, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, H.M.; Dejong, C.H.; Deutz, N.E.; Soeters, P.B. Influence of Storage Conditions on Normal Plasma Amino-Acid Concentrations. Clin. Nutr. 1994, 13, 374–380. [Google Scholar] [CrossRef]
- Takehana, S.; Yoshida, H.; Ozawa, S.; Yamazaki, J.; Shimbo, K.; Nakayama, A.; Mizukoshi, T.; Miyano, H. The Effects of Pre-Analysis Sample Handling on Human Plasma Amino Acid Concentrations. Clin. Chim. Acta 2016, 455, 68–74. [Google Scholar] [CrossRef]
- Hansen, B.; DiBartola, S.P.; Chew, D.J.; Brownie, C.; Berrie, H.K. Amino Acid Profiles in Dogs with Chronic Renal Failure Fed Two Diets. Am. J. Vet. Res. 1992, 53, 335–341. [Google Scholar]
- Downes, A.M. The Fate of Intravenous Doses of Free and Plasma Protein-Bound [35s]Cystine in the Sheep. Aust. J. Biol. Sci. 1961, 14, 427–439. [Google Scholar] [CrossRef]
- An, Z.; Shi, C.; Li, P.; Liu, L. Stability of Amino Acids and Related Amines in Human Serum under Different Preprocessing and Pre-Storage Conditions Based on Itraq®-Lc-Ms/Ms. Biol. Open 2021, 10, bio055020. [Google Scholar] [CrossRef]
- Yu, B.L.; Han, J.; Hammond, M.; Wang, X.; Zhang, Q.; Clausen, A.; Forster, R.; Eu, M. Kinetic Modeling of the Release of Ethylene Oxide from Sterilized Plastic Containers and Its Interaction with Monoclonal Antibodies. PDA J. Pharm. Sci. Technol. 2017, 71, 11–19. [Google Scholar] [CrossRef]
- Holtrop, S.; Abeling, N.G.G.; van Gennip, A.H. Recommendations to Improve the Quality of Diagnostic Quantitative Analysis of Amino Acids in Plasma and Urine Using Cation-Exchange Liquid Chromatography with Post Column Ninhydrin Reaction and Detection. ERNDIM 2002. Available online: https://www.yumpu.com/en/document/read/19477578/amino-acid-analysis-recommendations-erndim (accessed on 8 September 2022).
- Schaefer, A.; Piquard, F.; Haberey, P. Plasma Amino-Acids Analysis: Effects of Delayed Samples Preparation and of Storage. Clin. Chim. Acta 1987, 164, 163–169. [Google Scholar] [CrossRef]
- Li, J.; Piao, C.; Jin, H.; Wongpanit, K.; Manabe, N. Delayed Deproteinization Causes Methodological Errors in Amino Acid Levels in Plasma Stored at Room Temperature or −20 °C. Asian-Aust. J. Anim. Sci. 2009, 22, 1703–1708. [Google Scholar] [CrossRef]
- Hester, J.R.; Korzun, W.J.; Mabry, L.U. Blood Ammonia Stability Revisited. Clin. Lab. Sci. 2015, 28, 173. [Google Scholar] [CrossRef]
- Olek, K.; Uhlhaas, S.; Wardenbach, P.; Yamaguchi, M. Influence of Storing Conditions on the Amino Acid Concentration in Human Serum (Author’s Transl). J. Clin. Chem. Clin. Biochem. 1979, 17, 599–604. [Google Scholar] [PubMed]
- Kochlik, B.; Gerbracht, C.; Grune, T.; Weber, D. The Influence of Dietary Habits and Meat Consumption on Plasma 3-Methylhistidine—A Potential Marker for Muscle Protein Turnover. Mol. Nutr. Food Res. 2018, 62, 1701062. [Google Scholar] [CrossRef]
- Young, V.R.; Munro, H.N. Ntau-Methylhistidine (3-Methylhistidine) and Muscle Protein Turnover: An Overview. Fed. Proc. 1978, 37, 2291–2300. [Google Scholar] [PubMed]
- Jepson, R.E.; Syme, H.M.; Vallance, C.; Elliott, J. Plasma Asymmetric Dimethylarginine, Symmetric Dimethylarginine, L-Arginine, and Nitrite/Nitrate Concentrations in Cats with Chronic Kidney Disease and Hypertension. J. Vet. Intern. Med. 2008, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Klessens, C.Q.F.; Baelde, H.J.; Singler, B.; Veraar, K.A.M.; Zutinic, A.; Drozak, J.; Zschocke, J.; Schmitt, C.P.; de Heer, E. Intrinsic Carnosine Metabolism in the Human Kidney. Amino Acids 2015, 47, 2541–2550. [Google Scholar] [CrossRef]
- Mitry, P.; Wawro, N.; Rohrmann, S.; Giesbertz, P.; Daniel, H.; Linseisen, J. Plasma Concentrations of Anserine, Carnosine and Pi-Methylhistidine as Biomarkers of Habitual Meat Consumption. Eur. J. Clin. Nutr. 2019, 73, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Seip, M.; Lindemann, R.; Gjesdahl, P.; Gjessing, L.R. Amino Acid Concentrations in Plasma and Erythrocytes in Aregeneratory and Haemolytic Anaemias. Scand. J. Haematol. 1975, 15, 178–186. [Google Scholar] [CrossRef]
- Fukuda, K.; Nishi, Y.; Usui, T. Free Amino Acid Concentrations in Plasma, Erythrocytes, Granulocytes, and Lymphocytes in Umbilical Cord Blood, Children, and Adults. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 432–439. [Google Scholar] [CrossRef]
- Perry, T.L.; Hansen, S. Technical Pitfalls Leading to Errors in the Quantitation of Plasma Amino Acids. Clin. Chim. Acta 1969, 25, 53–58. [Google Scholar] [CrossRef]
- Davids, M.; Peters, J.H.; de Jong, S.; Teerlink, T. Measurement of Nitric Oxide-Related Amino Acids in Serum and Plasma: Effects of Blood Clotting and Type of Anticoagulant. Clin. Chim. Acta 2013, 421, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Sotelo-Orozco, J.; Chen, S.-Y.; Hertz-Picciotto, I.; Slupsky, C.M. A Comparison of Serum and Plasma Blood Collection Tubes for the Integration of Epidemiological and Metabolomics Data. Front. Mol. Biosci. 2021, 8, 650. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, E.S.; Whelchel, B.D.; Yu, J.; Kaplan, J.L.; Sharpe, A.N.; Fousse, S.L.; Crofton, A.E.; Fascetti, A.J.; Stern, J.A. Development of Plasma and Whole Blood Taurine Reference Ranges and Identification of Dietary Features Associated with Taurine Deficiency and Dilated Cardiomyopathy in Golden Retrievers: A Prospective, Observational Study. PLoS ONE 2020, 15, e0233206. [Google Scholar] [CrossRef]
- Williams, A.P. General Problems Associated with the Analysis of Amino Acids by Automated Ion-Exchange Chromatography. J. Chromatogr. A 1986, 373, 175–190. [Google Scholar] [CrossRef]
- Holecek, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Donadelli, R.A.; Pezzali, J.G.; Oba, P.M.; Swanson, K.S.; Coon, C.; Varney, J.; Pendlebury, C.; Shoveller, A.K. A Commercial Grain-Free Diet Does Not Decrease Plasma Amino Acids and Taurine Status but Increases Bile Acid Excretion When Fed to Labrador Retrievers. Transl. Anim. Sci. 2020, 4, txaa141. [Google Scholar] [CrossRef]
- Parvy, P.; Bardet, J.; Gasquet, M.; Rabier, D.; Kamoun, P. Stability of Free Amino Acids in Sulfosalicylic Filtrates. Clin. Chem. 1995, 41, 465–466. [Google Scholar] [CrossRef]
- Kornhuber, M.E.; Balabanova, S.; Heiligensetzer, G.V.; Kornhuber, C.; Zettlmeissl, H.; Kornhuber, A.W. Stability of Human Blood Serum Aminoacids after Storage at Different Ph and Temperature Conditions. Clin. Chim. Acta 1991, 197, 189–200. [Google Scholar] [CrossRef]



| Matrix Spike Level | |||
|---|---|---|---|
| Low | Medium | High | |
| components | 220 µL serum | 190 µL serum | 160 µL serum |
| 10 µL acidics/neutrals | 20 µL acidics/neutrals | 30 µL acidics/neutrals | |
| 10 µL basics | 20 µL basics | 30 µL basics | |
| 10 µL glutamine | 20 µL glutamine | 30 µL glutamine | |
| Dilution Factor | |||
| 0.88 | 0.76 | 0.64 | |
| components | 220 µL serum | 190 µL serum | 160 µL serum |
| 30 µL lithium loading buffer | 60 µL lithium loading buffer | 90 µL lithium loading buffer | |
| Serum Volume (µL) | ID | Day 0—Collect | Day 1 | Day 2 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|---|---|---|
| 600 | A | deprot, run Ae | |||||||
| B | store Ae 4 °C 72 h | run Ae | |||||||
| C | store Ae −20 °C 1 w | run Ae | |||||||
| 200 | D | Aliquot, store 4 °C 24 h | deprot, run | ||||||
| 200 | E | Aliquot, store 4 °C 48 h | deprot, run | ||||||
| 200 | F | Aliquot, store 4 °C 72 h | deprot, run | ||||||
| 200 | G | Aliquot, store −20 °C 24 h | deprot, run | ||||||
| 200 | H | Aliquot, store −20 °C 48 h | deprot, run | ||||||
| 200 | I | Aliquot, store −20 °C 1 w | deprot, run | ||||||
| 200 | J | Aliquot, store −20 °C 2 w | deprot, run | ||||||
| 200 | K | Aliquot, store −20 °C 3 w | deprot, run | ||||||
| 200 | L | Aliquot, store −20 °C 4 w | deprot, run |
| Whole Blood | Plasma | Serum | |||||
|---|---|---|---|---|---|---|---|
| Interval | Median | Interval | Median | Interval | Median | p-Value | |
| phospho-serine | 7–13 | 9 a | 3–10 | 3 b | 4–14 | 9 a | <0.001 |
| taurine | 135–359 | 208 a | 50–145 | 85 b | 89–326 | 175 c | <0.001 |
| phosphoethanolamine | 5–14 | 10 a | 0–4 | 2 b | 0–10 | 4 c | <0.001 |
| urea | 2656–7943 | 4867 a | 2961–8632 | 5109 b | 2937–8585 | 5149 b | <0.001 |
| aspartic acid | 143–458 | 295 a | 4–10 | 6 b | 5–12 | 9 c | <0.001 |
| hydroxyproline | 2–106 | 11 | 2–99 | 10 | 0–106 | 12 | 0.590 |
| threonine | 115–340 | 178 a | 94–346 | 173 b | 101–354 | 182 a | <0.001 |
| serine | 84–176 | 120 a | 66–149 | 105 b | 69–170 | 112 c | <0.001 |
| asparagine | 16–55 | 35 a | 31–88 | 61 b | 32–93 | 64 c | <0.001 |
| glutamic acid | 40–80 | 58 a | 16–42 | 25 b | 24–45 | 34 c | <0.001 |
| glutamine | 479–996 | 658 a | 490–1055 | 704 a | 494–1079 | 704 b | <0.001 |
| α-aminoadipic acid | 465–1070 | 685 a | 1–11 | 4 b | 1–12 | 5 b | <0.001 |
| proline | 81–256 | 134 a | 74–294 | 129 a | 80–312 | 135 b | <0.001 |
| glycine | 139–362 | 204 a | 118–371 | 216 a | 128–390 | 222 b | <0.001 |
| alanine | 241–570 | 364 a | 192–544 | 371 b | 207–553 | 367 a | <0.001 |
| citrulline | 27–102 | 55 a | 20–86 | 48 b | 20–88 | 50 b | <0.001 |
| α-aminobutyric acid | 9–48 | 23 a | 10–53 | 26 b | 11–53 | 27 b | <0.001 |
| valine | 114–220 | 159 a | 101–215 | 157 a | 106–223 | 164 b | <0.001 |
| cystine | 1–10 | 5 a | 2–17 | 7 b | 3–14 | 7 b | <0.001 |
| methionine | 31–68 | 46 a | 34–73 | 46 a | 37–76 | 49 b | <0.001 |
| cystathionine | 4–24 | 9 a | 2–14 | 6 b | 2–14 | 6 b | <0.001 |
| isoleucine | 39–88 | 55 a | 35–88 | 54 b | 38–90 | 58 a | <0.001 |
| leucine | 69–181 | 113 a | 68–189 | 117 a | 75–196 | 123 b | <0.001 |
| tyrosine | 51–90 | 63 a | 24–73 | 43 b | 26–76 | 44 c | <0.001 |
| β-alanine | 0–5 | 0 | 0–5 | 0 | 0–5 | 0 | N/A |
| phenylalanine | 39–76 | 52 a | 38–81 | 54 a | 40–86 | 56 b | <0.001 |
| homocystine | 0–2 | 0 | 0–4 | 0 | 0–4 | 0 | <0.001 |
| ethanolamine | 0–23 | 4 | 0–49 | 0 | 0–34 | 3 | 0.089 |
| ammonia | 62–117 | 83 a | 37–81 | 51 b | 55–104 | 67 c | <0.001 |
| hydroxylysine | 10–17 | 13 | 9–17 | 14 | 10–17 | 14 | 0.423 |
| ornithine | 13–35 | 21 a | 6–23 | 12 b | 7–24 | 12 b | <0.001 |
| lysine | 157–541 | 272 a | 57–288 | 147 b | 64–305 | 154 c | <0.001 |
| 1-methylhistidine | 4–38 | 7 a | 4–31 | 8 b | 5–33 | 8 b | <0.001 |
| histidine | 59–96 | 79 a | 50–84 | 70 b | 52–88 | 74 c | <0.001 |
| tryptophan | 18–52 | 29 a | 31–110 | 55 b | 30–114 | 58 c | <0.001 |
| 3-methylhistidine | 4–19 | 8 a | 3–22 | 9 b | 4–22 | 10 b | <0.001 |
| anserine | 0–10 | 3 | 2–19 | 3 | 0–16 | 3 | 0.144 |
| carnosine | 11–25 | 16 a | 11–45 | 25 b | 11–42 | 26 b | <0.001 |
| arginine | 121–242 | 182 a | 62–157 | 109 b | 96–197 | 147 c | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, A.B.; Ishii, P.E.; Phillips, R.K.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Analytical Validation of an Assay for Concurrent Measurement of Amino Acids in Dog Serum and Comparison of Amino Acid Concentrations between Whole Blood, Plasma, and Serum from Dogs. Metabolites 2022, 12, 891. https://doi.org/10.3390/metabo12100891
Blake AB, Ishii PE, Phillips RK, Lidbury JA, Steiner JM, Suchodolski JS. Analytical Validation of an Assay for Concurrent Measurement of Amino Acids in Dog Serum and Comparison of Amino Acid Concentrations between Whole Blood, Plasma, and Serum from Dogs. Metabolites. 2022; 12(10):891. https://doi.org/10.3390/metabo12100891
Chicago/Turabian StyleBlake, Amanda B., Patricia E. Ishii, Robert K. Phillips, Jonathan A. Lidbury, Joerg M. Steiner, and Jan S. Suchodolski. 2022. "Analytical Validation of an Assay for Concurrent Measurement of Amino Acids in Dog Serum and Comparison of Amino Acid Concentrations between Whole Blood, Plasma, and Serum from Dogs" Metabolites 12, no. 10: 891. https://doi.org/10.3390/metabo12100891
APA StyleBlake, A. B., Ishii, P. E., Phillips, R. K., Lidbury, J. A., Steiner, J. M., & Suchodolski, J. S. (2022). Analytical Validation of an Assay for Concurrent Measurement of Amino Acids in Dog Serum and Comparison of Amino Acid Concentrations between Whole Blood, Plasma, and Serum from Dogs. Metabolites, 12(10), 891. https://doi.org/10.3390/metabo12100891







