Untargeted Metabolomics Combined with Solid Phase Fractionation for Systematic Characterization of Bioactive Compounds in Hemp with Methane Mitigation Potential
Abstract
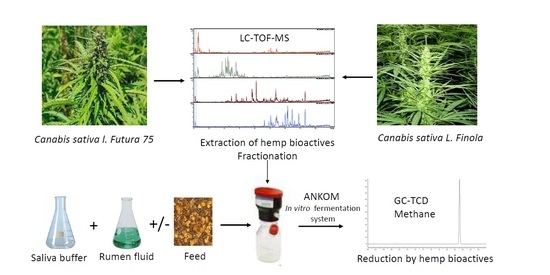
:1. Introduction
2. Results and Discussion
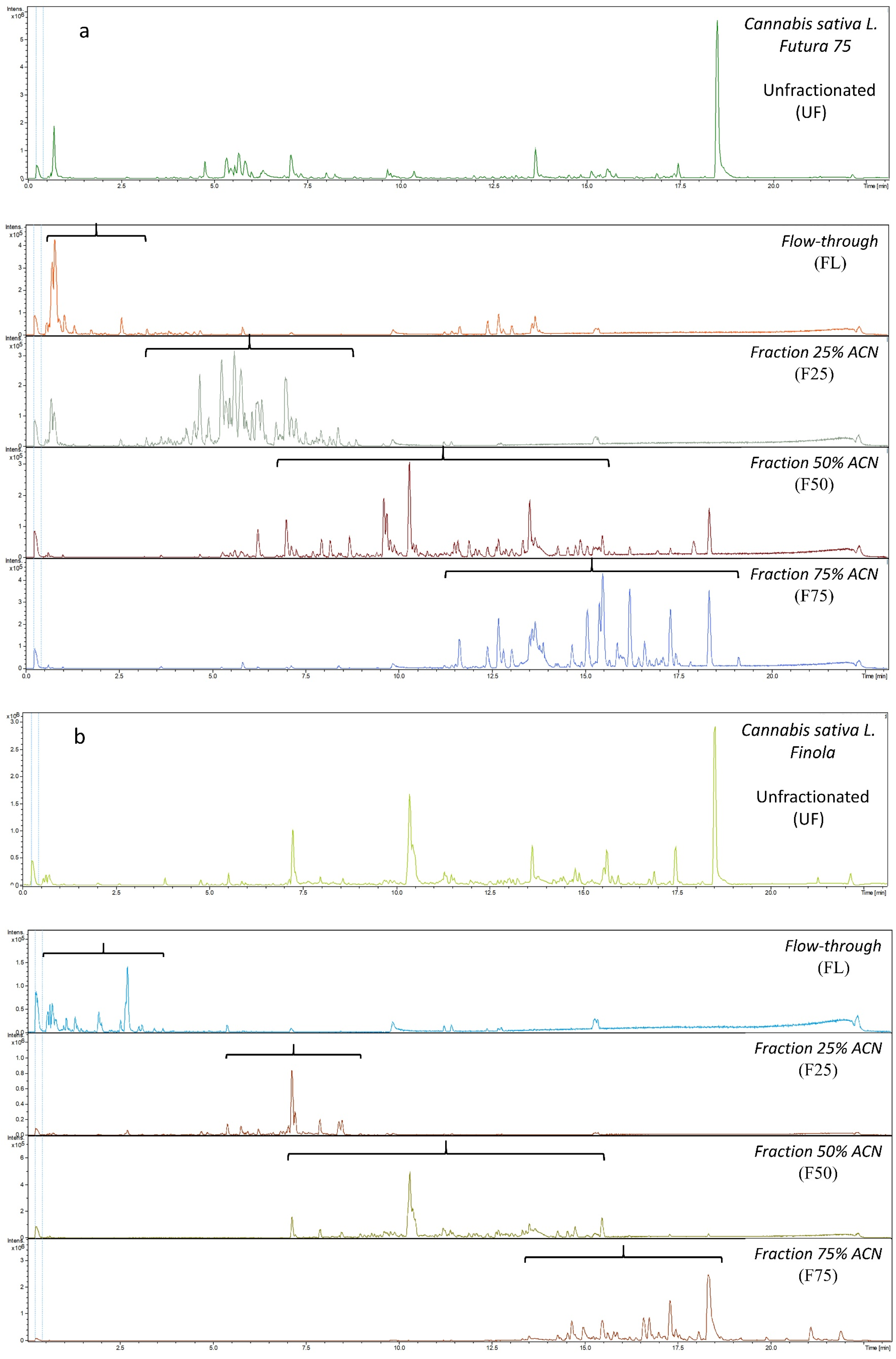
2.1. Metabolic Profiling of Extracts and Fractions of the Hemp Varieties Futura 75 and Finola
2.2. Screening for Methane Mitigation Potential and Identification of the Bioactive Compounds in Hemp Varieties Futura 75 and Finola
3. Materials and Methods
3.1. Chemicals
3.2. Sample Information
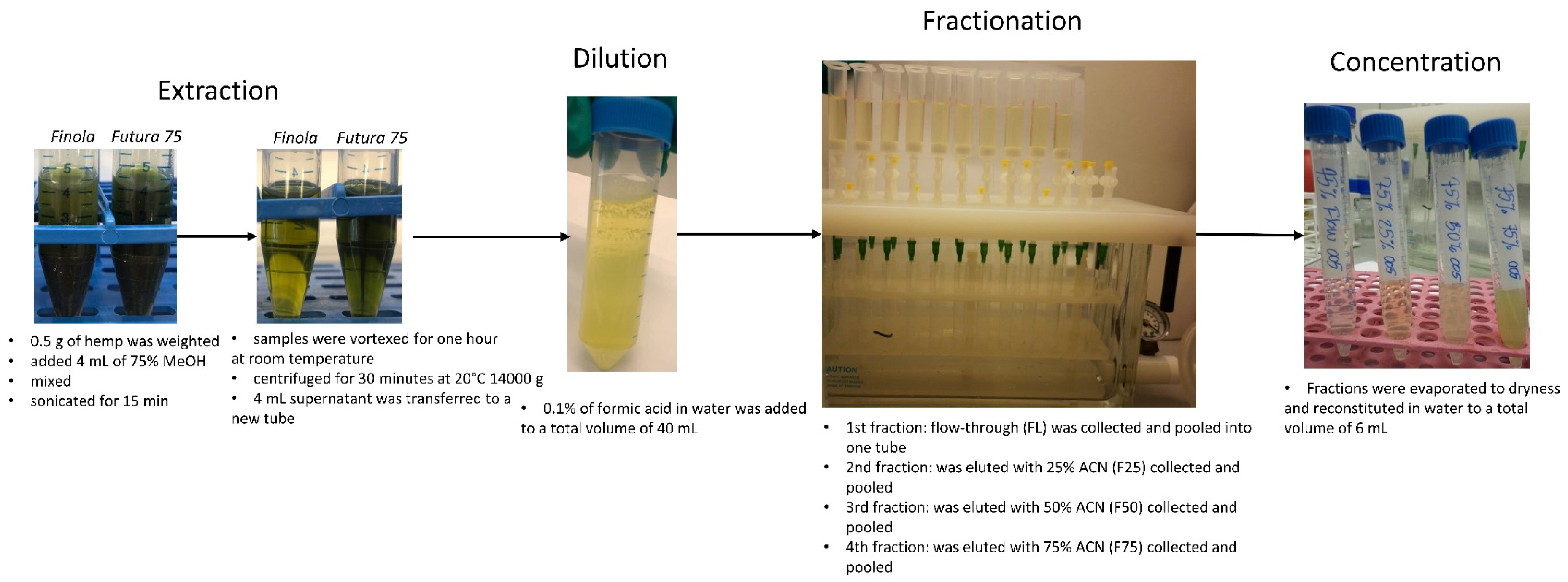
3.3. Extraction and Fractionation Protocol
3.4. Metabolic Profiling Using LC-TOF-MS
3.5. Working Solutions for In Vitro Fermentation System
3.6. Rumen Fluid
3.7. In Vitro Fermentation System
3.8. CH4, CO2 and VFA Analyses Using GC-TCD
3.9. Chemical Analyses
3.10. Calculations and Statistical Analyses
3.11. Preprocessing of Metabolomics Data and Multivariate Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Liu, S.; Ge, L.; Gao, S.; Zhuang, L.; Zhu, Z.; Wang, H. Activated carbon derived from bio-waste hemp hurd and retted hemp hurd for CO2 adsorption. Compos. Commun. 2017, 5, 27–30. [Google Scholar] [CrossRef]
- Jørgensen, J.R.; Boelt, B.; Kristensen, E.F. Produktion af Biopolymerer-Danske afgrøder der kan være relevante. 2020. Available online: https://dcapub.au.dk/djfpublikation/djfpdf/DCArapport168.pdf (accessed on 10 October 2021).
- Mastali, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Carbon dioxide sequestration of fly ash alkaline-based mortars containing recycled aggregates and reinforced by hemp fibres. Constr. Build. Mater. 2018, 160, 48–56. [Google Scholar] [CrossRef]
- EU. Rådets Forordning (EF) Nr. 1420/98. 1998. Available online: https://eur-lex.europa.eu/legal-content/DA/TXT/?uri=CELEX%3A31998R1420 (accessed on 20 October 2021).
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, G.-K.D., Plattner, M., Tignor, S.K., Allen, J., Boschung, A., Nauels, Y., Xia, V., Bex Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Oh, D.-G.; Singh, D.; Lee, H.J.; Kim, G.R.; Lee, S.; Lee, J.S.; Lee, C.H. Untargeted Metabolomics Toward Systematic Characterization of Antioxidant Compounds in Betulaceae Family Plant Extracts. Metabolites 2019, 9, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povedano, M.D.M.D.; Callado, C.S.-C.; Priego-Capote, F.; Ferreiro-Vera, C. Untargeted characterization of extracts from Cannabis sativa L. cultivars by gas and liquid chromatography coupled to mass spectrometry in high resolution mode. Talanta 2019, 208, 120384. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309–3314. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Abdah, S.N.M.; Sarmidi, M.R.; Yaakob, H.; Ware, I. Fractionation of Labisia Pumila using Solid-Phase Extraction for Extraction of Gallic Acid. J. Teknol. 2014, 69, 65–68. [Google Scholar]
- Naldi, M.; Brusotti, G.; Massolini, G.; Andrisano, V.; Temporini, C.; Bartolini, M. Bio-Guided Fractionation of Stem Bark Extracts from Phyllanthus muellarianus: Identification of Phytocomponents with Anti-Cholinesterase Activity. Molecules 2021, 26, 4376. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Ramírez, J.A.M.; Monroy, Z.J.R.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Nørskov, N.; Bruhn, A.; Cole, A.; Nielsen, M. Targeted and Untargeted Metabolic Profiling to Discover Bioactive Compounds in Seaweeds and Hemp Using Gas and Liquid Chromatography-Mass Spectrometry. Metabolites 2021, 11, 259. [Google Scholar] [CrossRef]
- Li, H.; Qin, Q.; Shi, X.; He, J.; Xu, G. Modified metabolites mapping by liquid chromatography-high resolution mass spectrometry using full scan/all ion Cheek for fragmentation/neutral loss acquisition. J. Chromatogr. A 2019, 1583, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, J.G.; Dill, K.A. The molecular mechanism of retention in reversed-phase liquid chromatography. Chem. Rev. 1989, 89, 331–346. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [Green Version]
- Kazuno, S.; Yanagida, M.; Shindo, N.; Murayama, K. Mass spectrometric identification and quantification of glycosyl flavonoids, including dihydrochalcones with neutral loss scan mode. Anal. Biochem. 2005, 347, 182–192. [Google Scholar] [CrossRef]
- Pikulski, M.; Brodbelt, J.S. Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1437–1453. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Kind, T.; Yang, D.; Leon, C.; Fiehn, O. MS2Analyzer: A Software for Small Molecule Substructure Annotations from Accurate Tandem Mass Spectra. Anal. Chem. 2014, 86, 10724–10731. [Google Scholar] [CrossRef] [Green Version]
- Bodas, R.; Lopez, S.; Fernández, M.; García-González, R.; Rodríguez, A.; Wallace, R.; González, J. In vitro screening of the potential of numerous plant species as antimethanogenic feed additives for ruminants. Anim. Feed. Sci. Technol. 2008, 145, 245–258. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar] [CrossRef]
- Kamra, D.N.; Patra, A.K.; Chatterjee, P.N.; Kumar, R.; Agarwal, N.; Chaudhary, L.C. Effect of plant extracts on methanogenesis and microbial profile of the rumen of buffalo: A brief overview. Aust. J. Exp. Agric. 2008, 48, 175–178. [Google Scholar] [CrossRef]
- Patra, A.; Kamra, D.; Agarwal, N. Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim. Feed Sci. Technol. 2006, 128, 276–291. [Google Scholar] [CrossRef]
- Patra, A.K.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broudiscou, L.-P.; Papon, Y.; Broudiscou, A.F. Effects of dry plant extracts on fermentation and methanogenesis in continuous culture of rumen microbes. Anim. Feed. Sci. Technol. 2000, 87, 263–277. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of Flavonoids on Rumen Fermentation Activity, Methane Production, and Microbial Population. BioMed Res. Int. 2013, 2013, 349129. [Google Scholar] [CrossRef] [Green Version]
- Becker, P.M.; Van Wikselaar, P.G.; Franssen, M.C.R.; De Vos, R.C.H.; Hall, R.; Beekwilder, J. Evidence for a hydrogen-sink mechanism of (+)catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics 2013, 10, 179–189. [Google Scholar] [CrossRef]
- Seradj, A.R.; Gimeno, A.; Fondevila, M.; Crespo, J.; Armengol, R.; Balcells, J. Effects of the citrus flavonoid extract Bioflavex or its pure components on rumen fermentation of intensively reared beef steers. Anim. Prod. Sci. 2018, 58, 553. [Google Scholar] [CrossRef]
- Nørskov, N.P.; Knudsen, K.E.B. Validated LC-MS/MS Method for the Quantification of Free and Bound Lignans in Cereal-Based Diets and Feces. J. Agric. Food Chem. 2016, 64, 8343–8351. [Google Scholar] [CrossRef]
- Vangsøe, C.T.; Nørskov, N.P.; Devaux, M.-F.; Bonnin, E.; Knudsen, K.E.B. Carbohydrase Complexes Rich in Xylanases and Arabinofuranosidases Affect the Autofluorescence Signal and Liberate Phenolic Acids from the Cell Wall Matrix in Wheat, Maize, and Rice Bran: An In Vitro Digestion Study. J. Agric. Food Chem. 2020, 68, 9878–9887. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, N.; Côrtes, C.; da Silva, D.; Kazama, R.; Benchaar, C.; dos Santos, G.; Zeoula, L.; Petit, H.V. Ruminal metabolism of flaxseed (Linum usitatissimum) lignans to the mammalian lignan enterolactone and its concentration in ruminal fluid, plasma, urine and milk of dairy cows. Br. J. Nutr. 2009, 102, 1015–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peekhaus, N.; Conway, T. What’s for Dinner?: Entner-Doudoroff Metabolism in Escherichia coli. J. Bacteriol. 1998, 180, 3495–3502. [Google Scholar] [CrossRef] [Green Version]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Andre, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.; Stengass, H.; Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Brask, M.; Lund, P.; Hellwing, A.L.F.; Poulsen, M.; Weisbjerg, M.R. Enteric methane production, digestibility and rumen fermentation in dairy cows fed different forages with and without rapeseed fat supplementation. Anim. Feed. Sci. Technol. 2013, 184, 67–79. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Danfær, A.; Tetens, V.; Agergaard, N. Portal Recovery of Intraruminally Infused Short-chain Fatty Acids in Sheep. Acta Agric. Scand. Sect. A—Anim. Sci. 1996, 46, 26–38. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A.; IUPNC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| CH4/Sample OM (mL/g) | TGP (mL) | Degradable DM (%) | Total VFA (mmol/L) | Acetic Acid (% of Total VFA) | Propionic Acid (% of Total VFA) | Butyric Acid (% of Total VFA) | Total Other 1 VFAs (mmol/L) | Other VFAs (% of Total VFA) | |
|---|---|---|---|---|---|---|---|---|---|
| Maize silage | 61.6 ab | 79.7 b | 60.5 ab | 127 | 40.2 | 30.2 | 2.28 b | 3.19 | 2.52 |
| Futura 75 UF | 41.2 c | 60.6 c | 58.6 ab | 113 | 46.3 | 26.0 | 3.19 ab | 3.67 | 3.31 |
| Futura 75 FL | 58.9 abc | 81.5 abc | 64.3 ab | 110 | 47.0 | 25.1 | 3.49 a | 3.86 | 3.56 |
| Futura 75 F25 | 59.0 abc | 77.4 ab | 62.1 ab | 104 | 50.0 | 23.7 | 3.79 a | 3.98 | 3.82 |
| Futura 75 F50 | 58.3 abc | 80.4 ab | 66.4 a | 113 | 46.3 | 25.6 | 3.46 a | 4.03 | 3.64 |
| Futura 75 F75 | 57.9 abc | 76.3 ab | 65.3 ab | 109 | 49.3 | 24.6 | 3.66 a | 4.04 | 3.81 |
| Futura 75 Pulp | 62.1 abc | 86.6 ab | 51.2 b | 114 | 47.6 | 25.4 | 3.68 a | 4.07 | 3.63 |
| Finola UF | 46.3 bc | 63.1 ab | 68.9 ab | 112 | 47.7 | 25.6 | 3.29 ab | 3.67 | 3.43 |
| Finola FL | 62.5 abc | 83.7 abc | 63.1 ab | 111 | 46.9 | 25.2 | 3.50 ab | 3.92 | 3.56 |
| Finola F25 | 59.5 abc | 77.0 ab | 69.2 a | 120 | 44.7 | 27.1 | 3.40 ab | 4.03 | 3.38 |
| Finola F50 | 59.7 abc | 79.8 ab | 63.2 ab | 107 | 50.3 | 24.1 | 3.81 a | 4.01 | 3.80 |
| Finola F75 | 62.9 ab | 81.3 ab | 61.7 ab | 117 | 45.0 | 26.3 | 3.35 ab | 3.90 | 3.37 |
| Finola Pulp | 73.7 a | 97.2 a | 57.4 ab | 121 | 47.1 | 27.4 | 3.61 a | 4.30 | 3.62 |
| SEM | 4.38 | 4.74 | 6.11 | 11.3 | 5.37 | 2.45 | 0.463 | 0.347 | 0.579 |
| p value | 0.04 | <0.001 | 0.03 | 0.50 | 0.58 | 0.17 | 0.03 | 0.12 | 0.18 |
| RT 1 (min) | M-H− (m/z) (Δppm) | Fragments (m/z) | Neutral Loss (Da) | AutoMS/MS (eV) 2 | Tentatively Identified |
|---|---|---|---|---|---|
| 5.35 | 609.1458 (0) | 489.1042, 429.0823, 357.0611, 327.0510, 309.0409 | 120, 60 | 22–28 | Kaempferol 7-sophoroside (HMDB) |
| 5.46 | 593.1506 (1) | 473.1092, 429.0813, 357.0619, 327.0509, 309.0399 | 120, 44, 72 | 22–27 | Kaempferol-3-O-neohesperidoside [19] |
| 5.55 | 447.0930 (1) | 357.0618, 327.0511, 297.0405 | 90, 120 | 19–24 | Orientin [20,21] |
| 5.55 | 895.1917 | 447.0929, 357.0610, 327.0505 | 448, 90, 120 | 28–35 | Orientin + sugar moiety |
| 5.66 | 593.1506 (1) | 473.1068, 413.0869, 293.0449 | 120, 60, 120 | 22–27 | Kaempferol diglycoside (HMDB) |
| 5.66 | 875.2248 | 593.1506, 473.1076, 413.0881, 293.0452 | 282, 120, 60, 120 | 28–34 | Kaempferol diglycoside + sugar moiety |
| 5.77 | 577.1559 (1) | 457.1139, 413.0880, 311.0563, 293.0453 | 120, 44, 120 | 22–27 | Daidzein diglycoside (HMDB) |
| 6.01 | 431.0989 (1) | 341.0672, 311.0561, 283.0611 | 90, 120 | 19–23 | Vitexin [21] |
| 6.01 | 863.2014 | 431.0989, 341.0654, 311.0557 | 432, 90, 120 | 27–34 | Vitiexin + sugar moiety |
| 6.30 | 461.0719 (2) | 285.0400 | 176 | 19–24 | Kaempferol glucuronide (HMDB) |
| 6.30 | 923.1496 | 461.0726, 285.0403 | 462, 176 | 29–36 | Kaempferol glucuronide + sugar moiety |
| 7.05 | 445.0774 (1) | 269.0451 | 176 | 19–24 | Genistein glucuronide (HMDB) |
| 7.05 | 668.1190 | 445.0768, 269.0453 | 223, 176 | 23–30 | Genistein glucuronide + sugar moiety |
| 7.05 | 891.1607 | 445.0769, 269.0450 | 446, 176 | 28–35 | Genistein glucuronide + sugar moiety |
| 7.32 | 475.0876 (2) | 299.0556 | 176 | 19–24 | Diosmetin glucuronide (HMDB) |
| 7.32 | 951.1803 | 475.0876, 299.0553 | 476, 176 | 29–36 | Diosmetin glucuronide + sugar moiety |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, R.H.; Rønn, M.; Thorsteinsson, M.; Olijhoek, D.W.; Nielsen, M.O.; Nørskov, N.P. Untargeted Metabolomics Combined with Solid Phase Fractionation for Systematic Characterization of Bioactive Compounds in Hemp with Methane Mitigation Potential. Metabolites 2022, 12, 77. https://doi.org/10.3390/metabo12010077
Jensen RH, Rønn M, Thorsteinsson M, Olijhoek DW, Nielsen MO, Nørskov NP. Untargeted Metabolomics Combined with Solid Phase Fractionation for Systematic Characterization of Bioactive Compounds in Hemp with Methane Mitigation Potential. Metabolites. 2022; 12(1):77. https://doi.org/10.3390/metabo12010077
Chicago/Turabian StyleJensen, Rikke Hald, Marie Rønn, Mirka Thorsteinsson, Dana W. Olijhoek, Mette Olaf Nielsen, and Natalja P. Nørskov. 2022. "Untargeted Metabolomics Combined with Solid Phase Fractionation for Systematic Characterization of Bioactive Compounds in Hemp with Methane Mitigation Potential" Metabolites 12, no. 1: 77. https://doi.org/10.3390/metabo12010077
APA StyleJensen, R. H., Rønn, M., Thorsteinsson, M., Olijhoek, D. W., Nielsen, M. O., & Nørskov, N. P. (2022). Untargeted Metabolomics Combined with Solid Phase Fractionation for Systematic Characterization of Bioactive Compounds in Hemp with Methane Mitigation Potential. Metabolites, 12(1), 77. https://doi.org/10.3390/metabo12010077