CD74 in Apoptotic Macrophages Is Associated with Inflammation, Plaque Progression and Clinical Manifestations in Human Atherosclerotic Lesions
Abstract
1. Introduction
2. Results
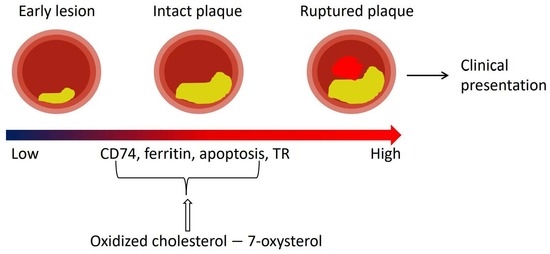
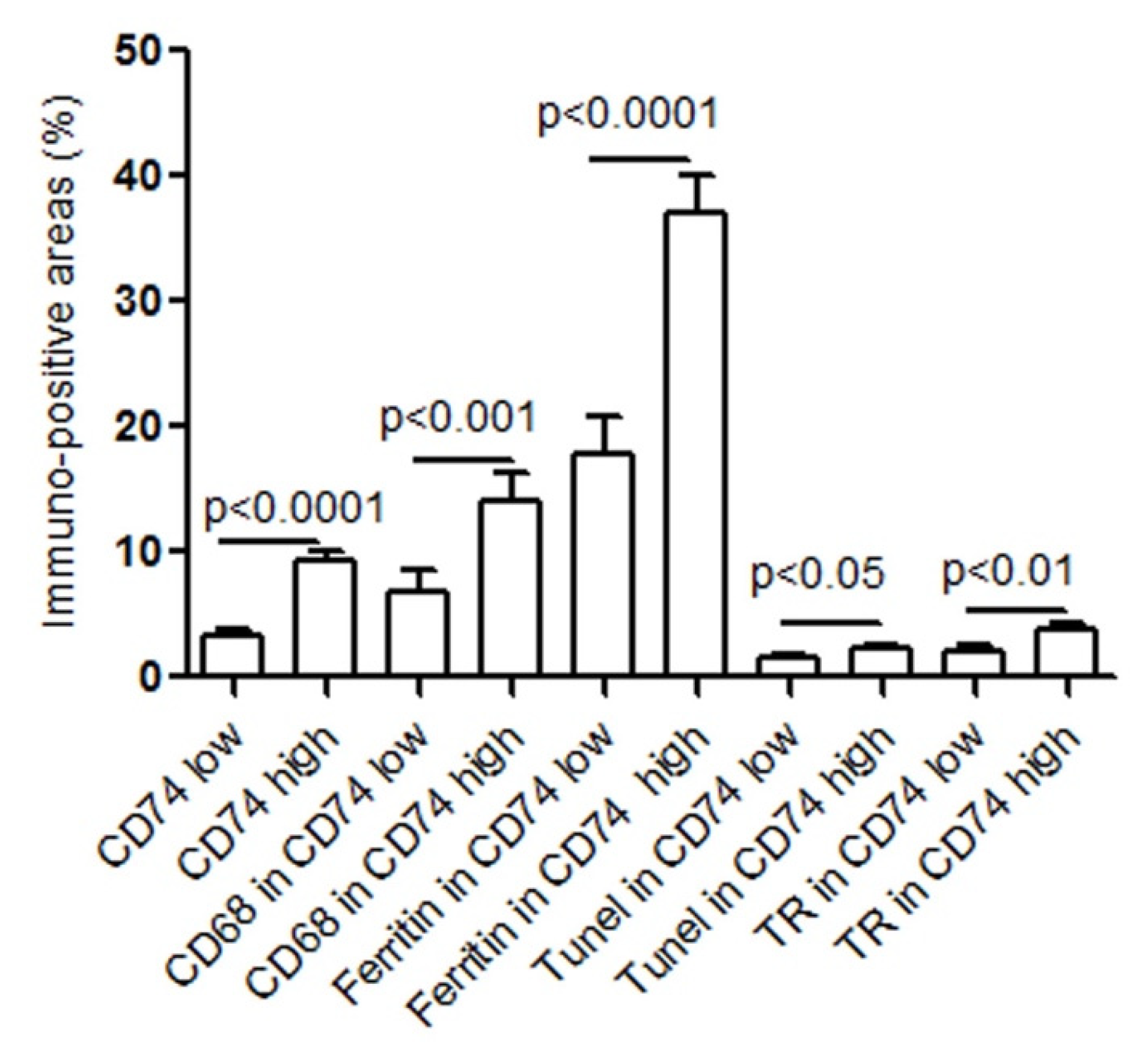
2.1. CD74 Expression Is Significantly Increased with the Progression of Human Atherosclerotic Plaques
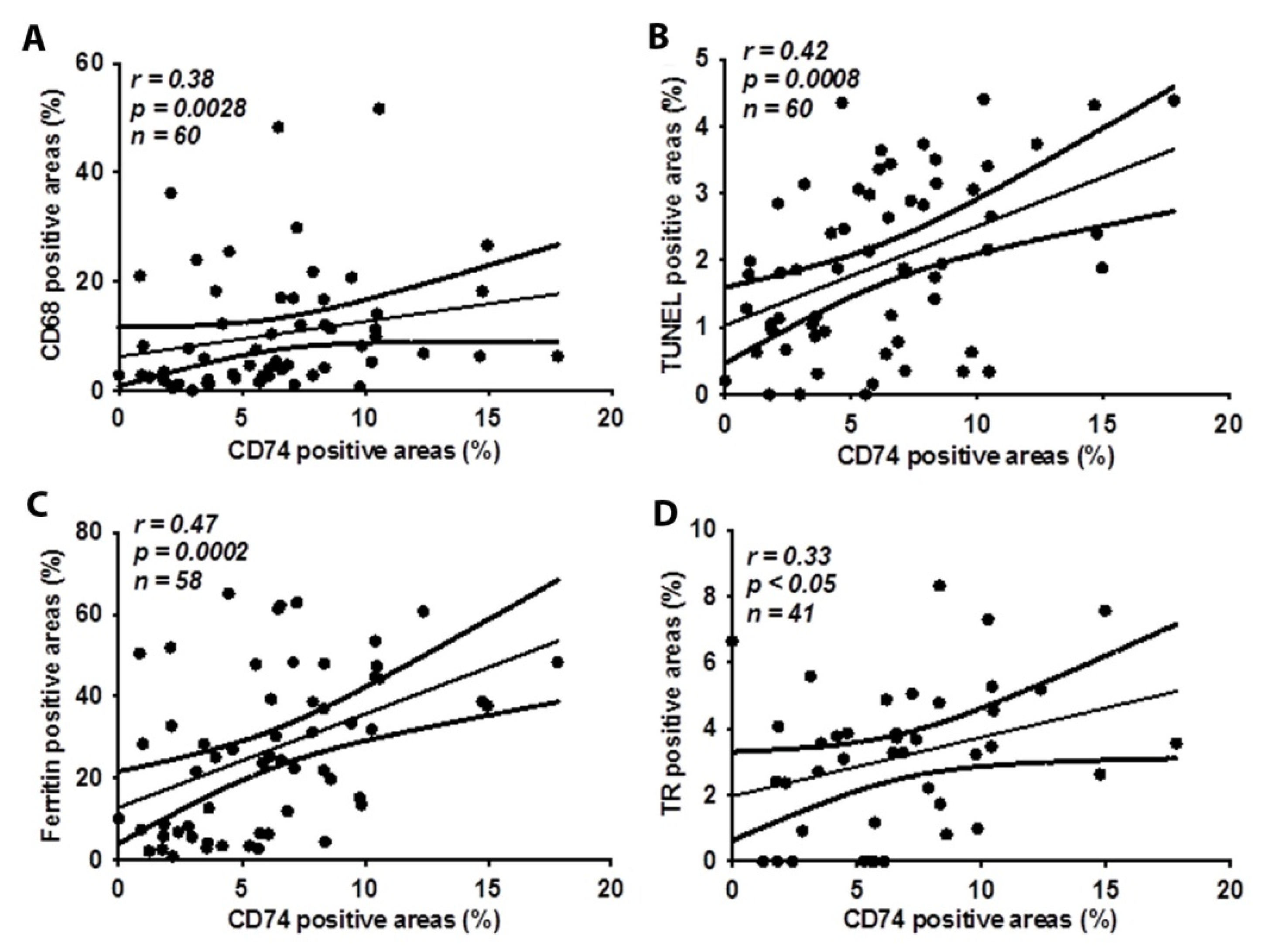
2.2. CD74 Expression in Human Carotid Atheroma Is Significantly Associated with an Accumulation of Macrophages, Apoptosis, Ferritin, and Thrombin Receptor (TR)
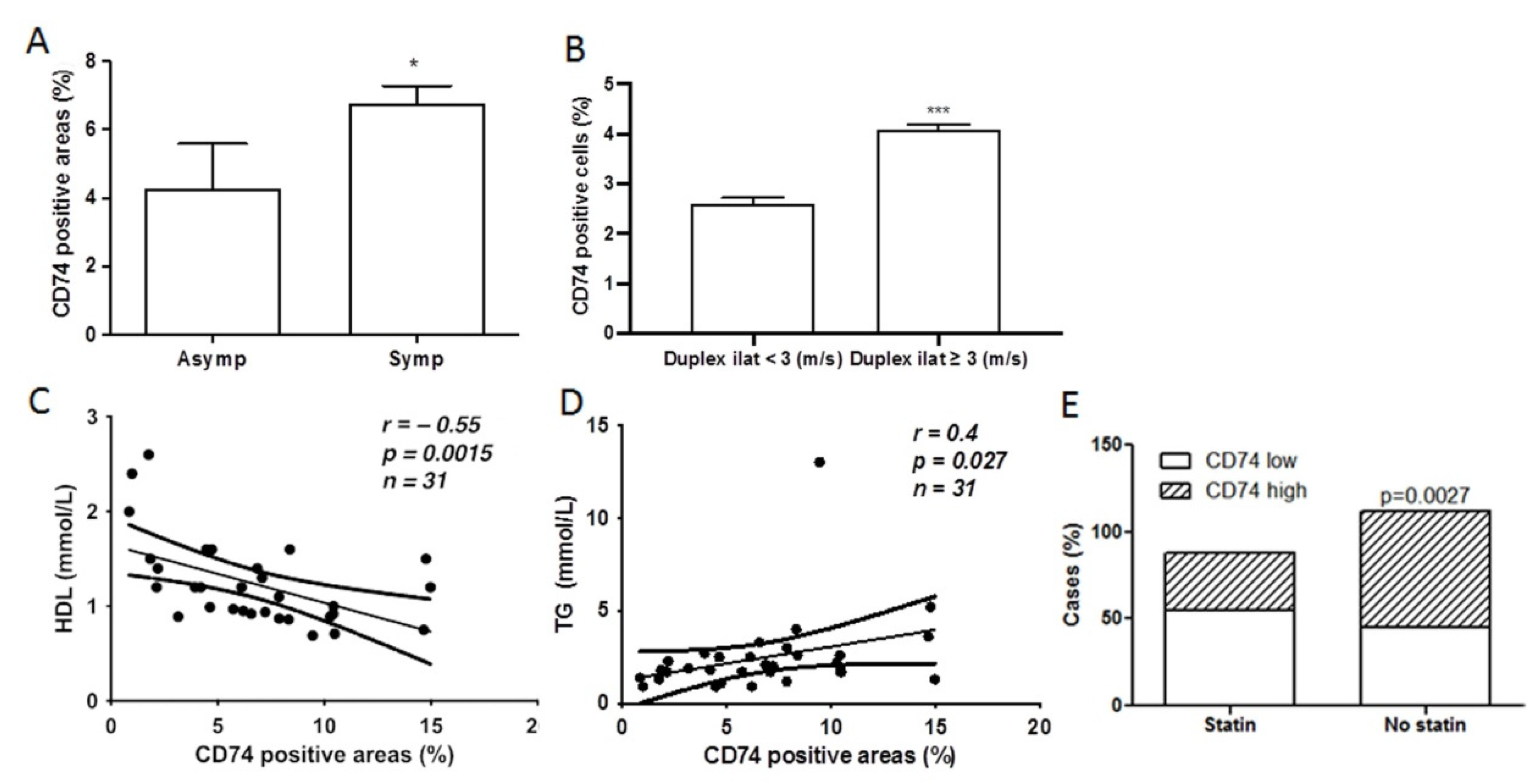
2.3. CD74 Expression Is Significantly Increased in Lesions from Symptomatic Patients and Related to Carotid Stenosis
2.4. CD74 Levels in Atherosclerotic Lesions Is Inversely Related to High Density Lipoprotein (HDL) Levels and Statin Treatment, and Positively Related to Triglyceride (TG) Levels
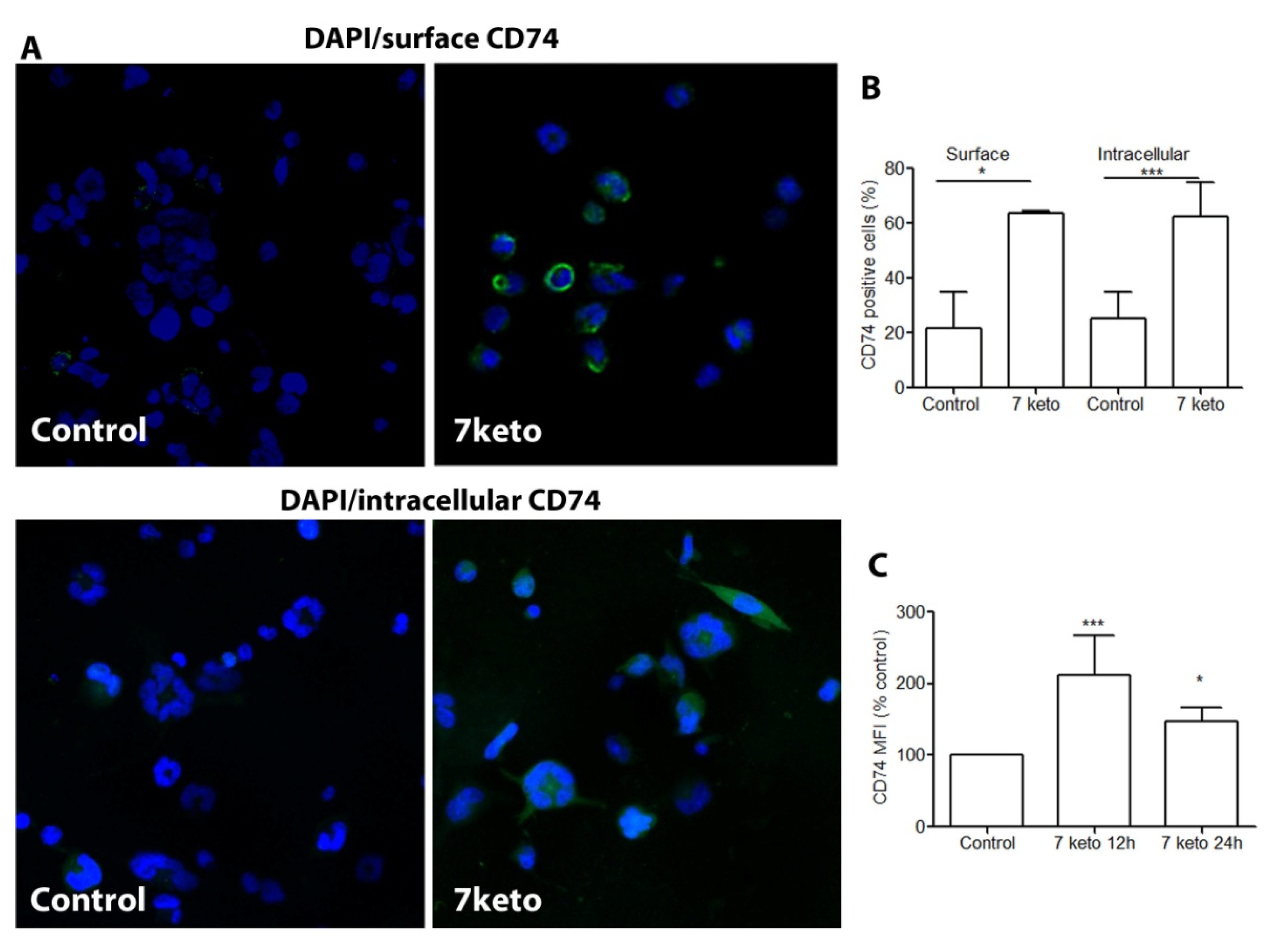
2.5. CD74 Is Significantly Increased in Macrophages Exposed to 7keto
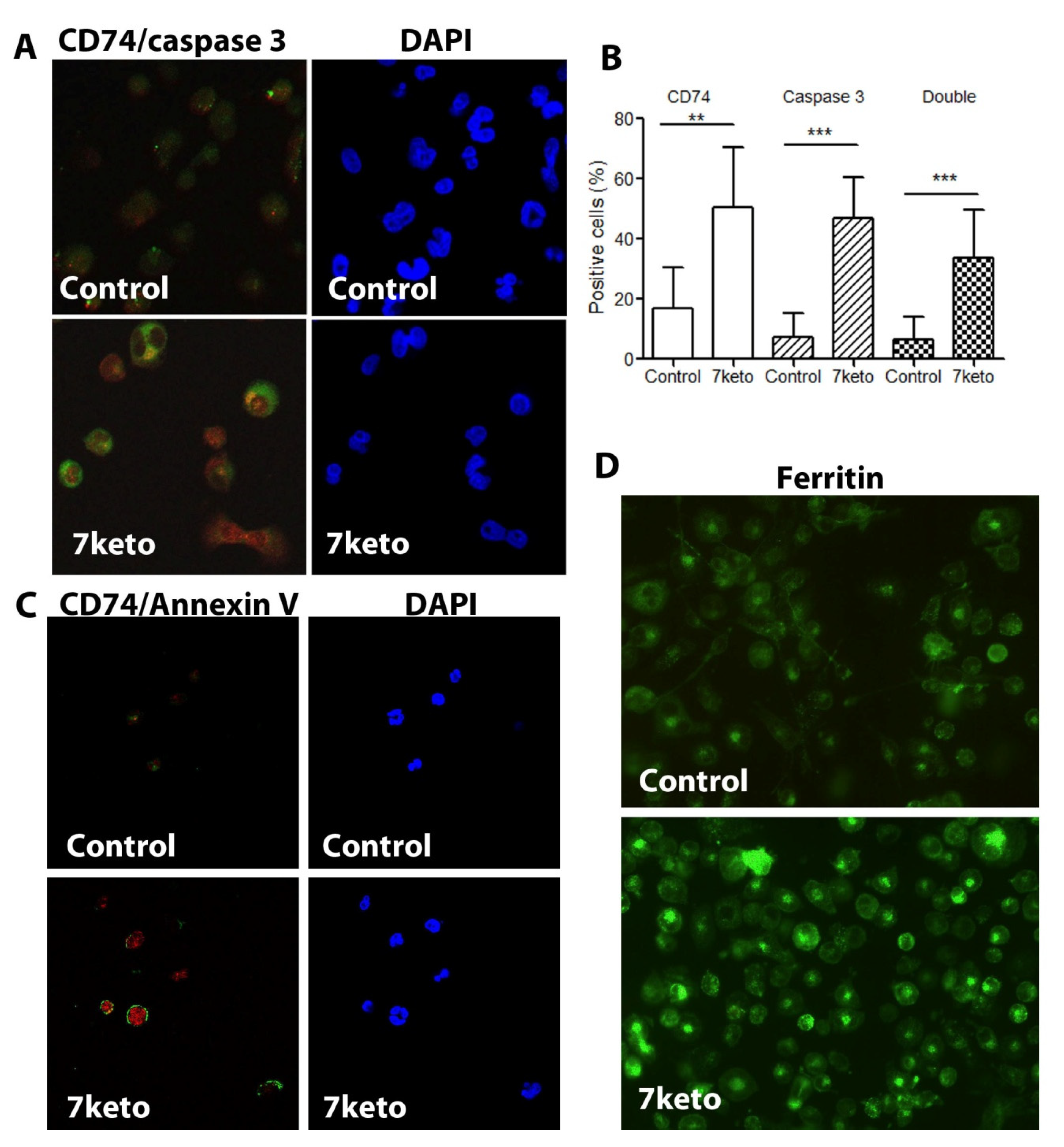
2.6. Increased CD74 Is Significantly Associated with 7keto-Induced Apoptosis in Macrophages
3. Discussion
Limitation
4. Materials and Methods
4.1. Collection of Carotid Artery Samples
4.2. Immunohistochemistry
4.3. Terminal Deoxynucleotidyl-Mediated dUTP Nick End Labeling (TUNEL)
4.4. Classification of the Plaques
4.5. Cell Culture
4.6. Immunocytochemistry
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansson, G.K.; Libby, P.; Schonbeck, U.; Yan, Z.Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Choi, C.; Finlay, D.K. Diverse immunoregulatory roles of oxysterols-the oxidized cholesterol metabolites. Metabolites 2020, 10, 384. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Leonarduzzi, G.; Poli, G. The role of oxysterols in vascular ageing. J. Physiol. 2016, 594, 2095–2113. [Google Scholar] [CrossRef]
- Khatib, S.; Vaya, J. Oxysterols and symptomatic versus asymptomatic human atherosclerotic plaque. Biochem. Biophys. Res. Commun. 2014, 446, 709–713. [Google Scholar] [CrossRef]
- Li, W.; Hellsten, H.; Xu, L.H.; Zhuang, D.M.; Jansson, J.; Brunk, U.T.; Yuan, X.M. Foam cell death induced by 7beta-hydroxycholesterol is mediated by labile iron-driven oxidative injury: Mechanisms underlying induction of ferritin in human atheroma. Free Radic. Biol. Med. 2005, 39, 864–875. [Google Scholar] [CrossRef]
- Depalma, R.G.; Hayes, V.W.; Chow, B.K.; Shamayeva, G.; May, P.E.; Zacharski, L.R. Ferritin levels, inflammatory biomarkers, and mortality in peripheral arterial disease: A substudy of the Iron (Fe) and atherosclerosis study (FeAST) Trial. J. Vasc. Surg. 2010, 51, 1498–1503. [Google Scholar] [CrossRef]
- Saha, S.; Profumo, E.; Togna, R.T.; Riganò, R.; Saso, L.; Buttari, B. Lupeol counteracts the proinflammatory signalling triggered in macrophages by 7keto-cholesterol: New perspectives in the therapy of atherosclerosis. Oxid. Med. Cell Longev. 2020, 1232816. [Google Scholar] [CrossRef]
- Sanchez-Niño, M.D.; Benito-Martin, A.; Ortiz, A. New paradigms in cell death in human diabetic nephropathy. Kidney Int. 2010, 78, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Martín-Ventura, J.L.; Madrigal-Matute, J.; Muñoz-Garcia, B.; Blanco-Colio, L.M.; Oostrom, M.V.; Zalba, G.; Fortuño, A.; Gomez-Guerrero, C.; Ortega, L.; Ortiz, A.; et al. Increased CD74 expression in human atherosclerotic plaques: Contribution to inflammatory responses in vascular cells. Cardiovasc. Res. 2009, 83, 586–594. [Google Scholar] [CrossRef]
- Sun, J.; Hartvigsen, K.; Chou, M.Y.; Zhang, Y.; Sukhova, G.K.; Zhang, J.; Lopez-Ilasaca, M.; Diehl, C.J.; Yakov, N.; Harats, D.; et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation 2010, 122, 808–820. [Google Scholar] [CrossRef]
- Testa, G.; Rossin, D.; Poli, G.; Biasi, F.; Leonarduzzi, G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie 2018, 153, 220–231. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.H.; Forssell, C.; Sullivan, J.L.; Yuan, X.M. Overexpression of transferrin receptor and ferritin related to clinical symptoms and destabilization of human carotid plaques. Exp. Biol. Med. 2008, 233, 818–826. [Google Scholar] [CrossRef]
- Yuan, X.M.; Ward, L.J.; Forssell, C.; Siraj, N.; Li, W. Carotid Atheroma from Men Has Significantly Higher Levels of Inflammation and Iron Metabolism Enabled by Macrophages. Stroke 2018, 49, 419–425. [Google Scholar] [CrossRef]
- Yang, L.; Kong, Y.; Ren, H.; Li, M.; Wei, C.J.; Shi, E.; Jin, W.N.; Hao, J.; Vandenbark, A.A.; Offner, H. Upregulation of CD74 and its potential association with disease severity in subjects with ischemic stroke. Neurochem. Int. 2017, 107, 148–155. [Google Scholar] [CrossRef]
- Welty, F.K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr. Cardiol. Rep. 2013, 15, 400. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.J.; Ljunggren, S.A.; Karlsson, H.; Li, W.; Yuan, X.M. Exposure to atheroma-relevant 7-oxysterols causes proteomic alterations in cell death, cellular longevity, and lipid metabolism in THP-1 macrophages. PLoS ONE 2017, 12, e0174475. [Google Scholar] [CrossRef]
- Beswick, E.J.; Bland, D.A.; Suarez, G.; Barrera, C.A.; Fan, X.; Reyes, V.E. Helicobacter pylori binds to CD74 on gastric epithelial cells and stimulates interleukin-8 production. Infect. Immun. 2005, 73, 2736–2743. [Google Scholar] [CrossRef]
- Wu, G.; Sun, Y.; Wang, K.; Chen, Z.; Wang, X.; Chang, F.; Li, T.; Feng, P.; Xia, Z. Relationship between elevated soluble CD74 and severity of experimental and clinical ali/ards. Sci Rep. 2016, 6, 30067. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Garcia-Cruset, S.; Carpenter, K.L.; Guardiola, F.; Stein, B.K.; Mitchinson, M.J. Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic. Res. 2001, 35, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cruset, S.; Carpenter, K.L.; Guardiola, F.; Mitchinson, M.J. Oxysterols in cap and core of human advanced atherosclerotic lesions. Free Radic. Res. 1999, 30, 341–350. [Google Scholar] [CrossRef]
- Upston, J.M.; Niu, X.; Brown, A.J.; Mashima, R.; Wang, H.; Senthilmohan, R.; Kettle, A.J.; Dean, R.T.; Stocker, R. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Am. J. Pathol. 2002, 160, 701–710. [Google Scholar] [CrossRef]
- Helmschrodt, C.; Becker, S.; Schröter, J.; Hecht, M.; Aust, G.; Thiery, J.; Ceglarek, U. Fast LC-MS/MS analysis of free oxysterols derived from reactive oxygen species in human plasma and carotid plaque. Clin. Chim. Acta 2013, 425, 3–8. [Google Scholar] [CrossRef]
- Albrecht, C.; Soumian, S.; Amey, J.S.; Sardini, A.; Higgins, C.F.; Davies, A.H.; Gibbs, R.G.J. ABCA1 expression in carotid atherosclerotic plaques. Stroke 2004, 35, 2801–2806. [Google Scholar] [CrossRef]
- Liu, H.F.; Cui, K.F.; Wang, J.P.; Zhang, M.; Guo, Y.P.; Li, X.Y.; Jiang, C. Significance of ABCA1 in human carotid atherosclerotic plaques. Exp. Ther. Med. 2012, 4, 297–302. [Google Scholar] [CrossRef][Green Version]
- Weber, S.; Lehmann, L.; Kobilay, M.; Stüber, F.; Hoeft, A. Statins Downregulate the Constitutive Expression of HLA-DR and Reduce Intracellular CD74 in the Monocyte Cell Line Mono Mac 6. Biochem. Pharmacol. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Barter, P.J.; Brandrup-Wognsen, G.; Palmer, M.K.; Nicholls, S.J. Effect of statins on HDL-C: A complex process unrelated to changes in LDL-C: Analysis of the VOYAGER Database. J. Lipid Res. 2010, 51, 1546–1553. [Google Scholar] [CrossRef]
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Li, W.; Xu, L.; Yuan, X.M. Macrophage hemoglobin scavenger receptor and ferritin accumulation in human atherosclerotic lesions. Ann. N. Y. Acad. Sci. 2004, 1030, 196–201. [Google Scholar] [CrossRef]
- Potor, L.; Hendrik, Z.; Patsalos, A.; Katona, É.; Méhes, G.; Póliska, S.; Csősz, É.; Kalló, G.; Komáromi, I.; Combi, Z.; et al. Oxidation of Hemoglobin Drives a Proatherogenic Polarization of Macrophages in Human Atherosclerosis. Antioxid. Redox Signal. 2021, 35, 917–950. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sultana, N.; Yuan, L.; Forssell, C.; Yuan, X.-M. CD74 in Apoptotic Macrophages Is Associated with Inflammation, Plaque Progression and Clinical Manifestations in Human Atherosclerotic Lesions. Metabolites 2022, 12, 54. https://doi.org/10.3390/metabo12010054
Li W, Sultana N, Yuan L, Forssell C, Yuan X-M. CD74 in Apoptotic Macrophages Is Associated with Inflammation, Plaque Progression and Clinical Manifestations in Human Atherosclerotic Lesions. Metabolites. 2022; 12(1):54. https://doi.org/10.3390/metabo12010054
Chicago/Turabian StyleLi, Wei, Nargis Sultana, Linda Yuan, Claes Forssell, and Xi-Ming Yuan. 2022. "CD74 in Apoptotic Macrophages Is Associated with Inflammation, Plaque Progression and Clinical Manifestations in Human Atherosclerotic Lesions" Metabolites 12, no. 1: 54. https://doi.org/10.3390/metabo12010054
APA StyleLi, W., Sultana, N., Yuan, L., Forssell, C., & Yuan, X.-M. (2022). CD74 in Apoptotic Macrophages Is Associated with Inflammation, Plaque Progression and Clinical Manifestations in Human Atherosclerotic Lesions. Metabolites, 12(1), 54. https://doi.org/10.3390/metabo12010054