Lentinan Protects against Nonalcoholic Fatty Liver Disease by Reducing Oxidative Stress and Apoptosis via the PPARα Pathway
Abstract
:1. Introduction
2. Results
2.1. LNT Prevented HFD-Induced Steatosis in Mice
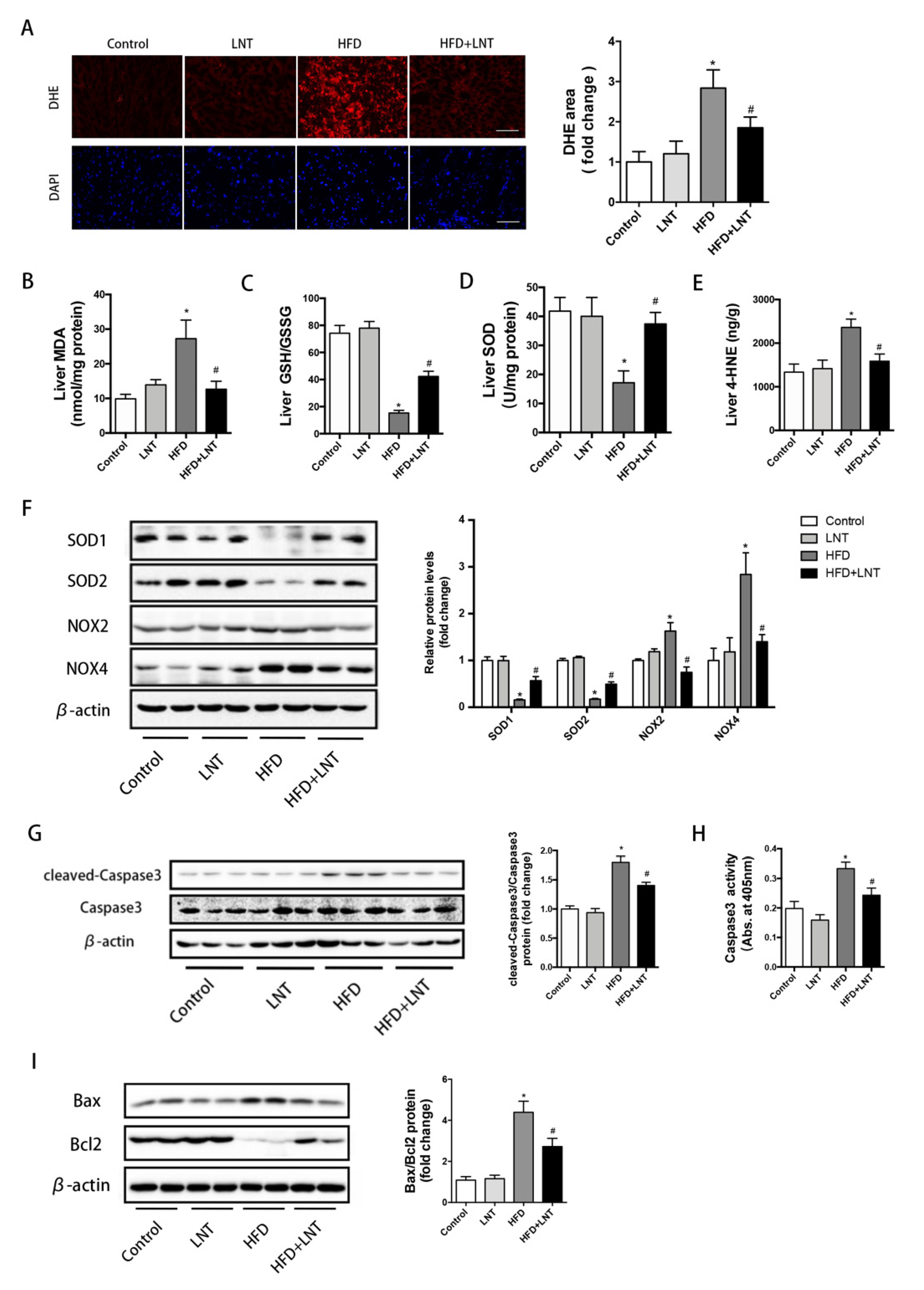
2.2. LNT Protected against HFD-Induced Hepatic Oxidative Stress and Apoptosis in Mice
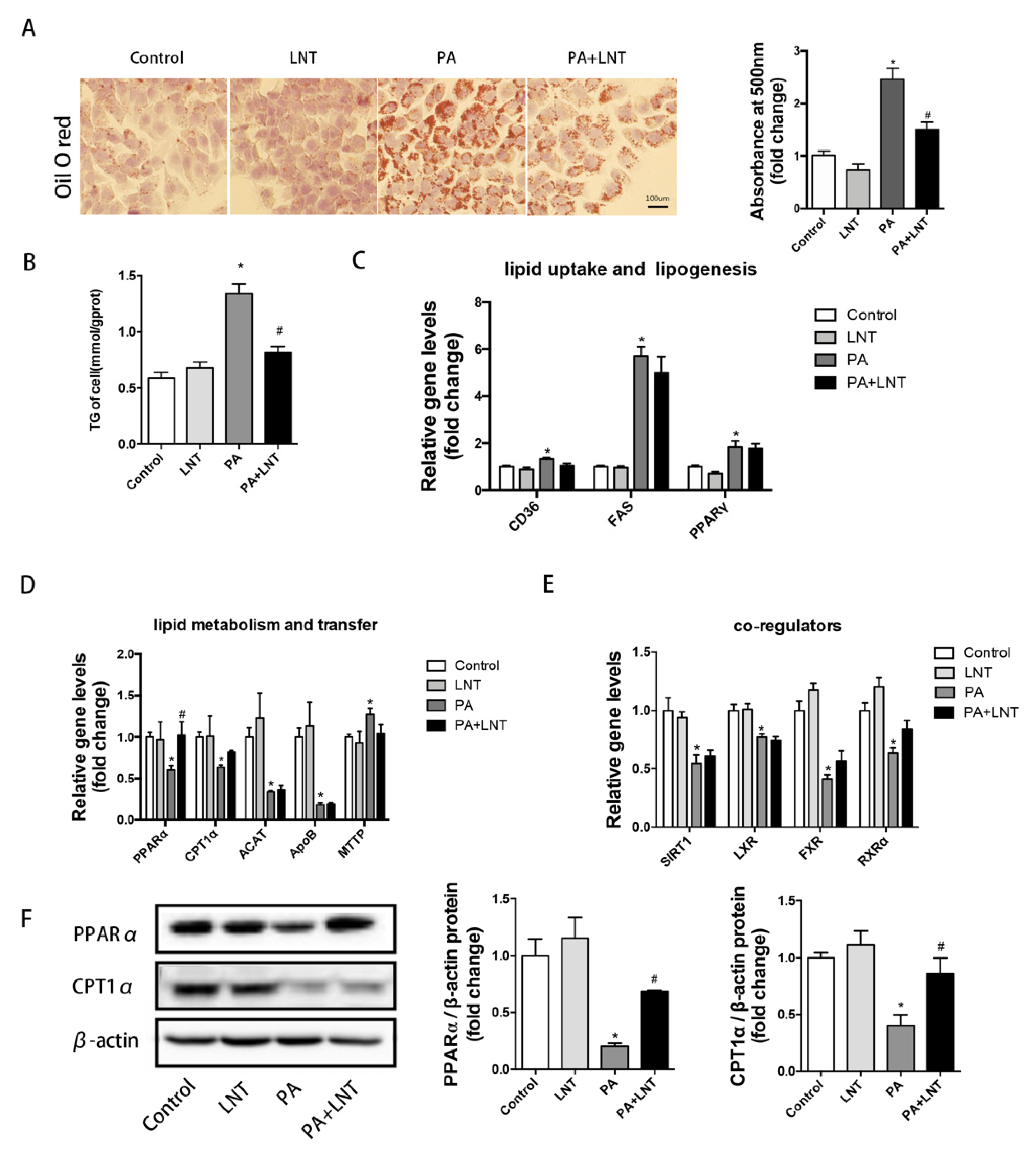
2.3. LNT Prevented Lipid Accumulation in PA-Induced AML12 Cells
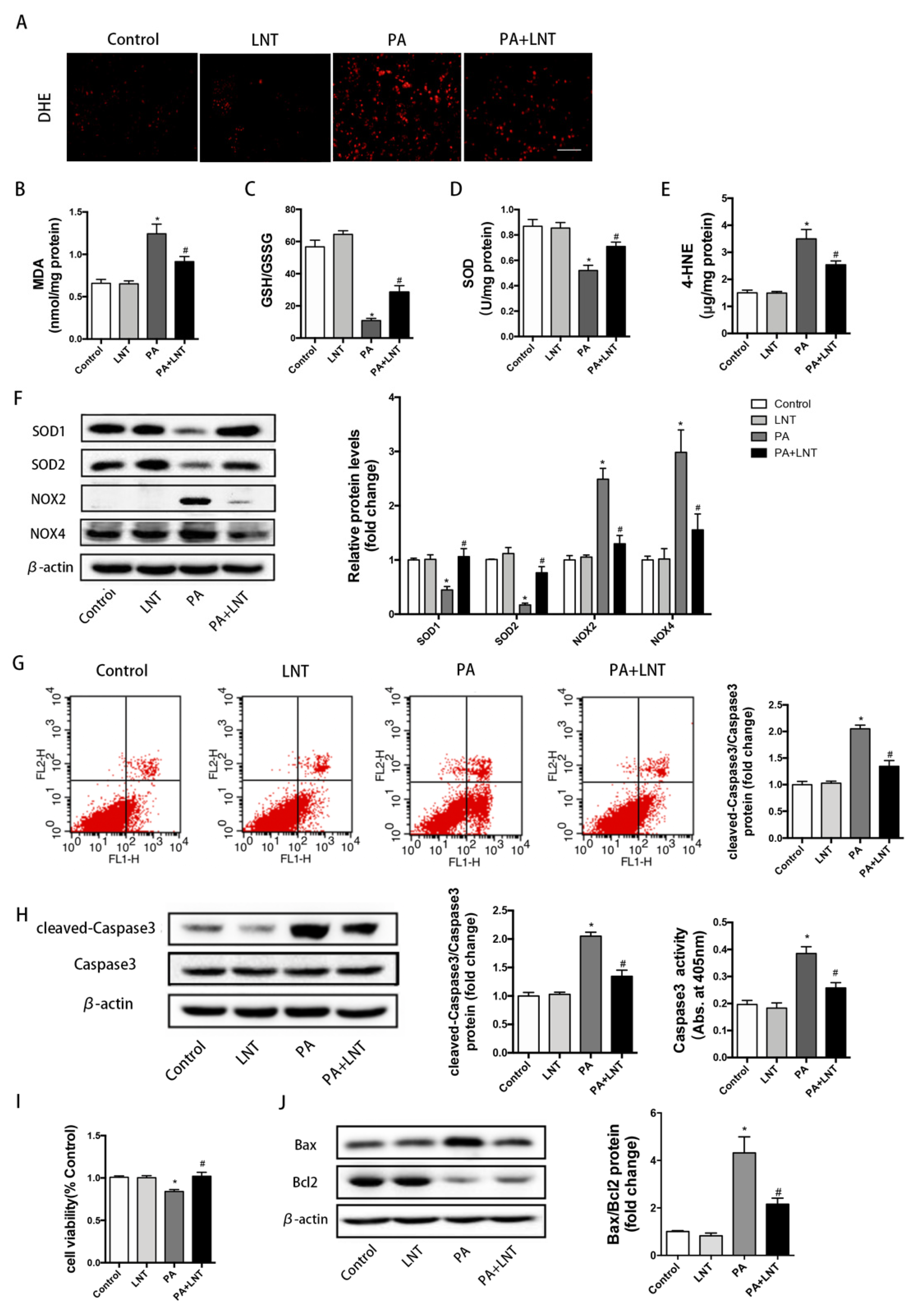
2.4. LNT Improved Oxidative Stress and Apoptosis in PA-Induced AML12 Cells
2.5. PPARα Knockdown Abolished the Protective Effects of LNT on Lipid Deposition in PA-Induced AML12 Cells
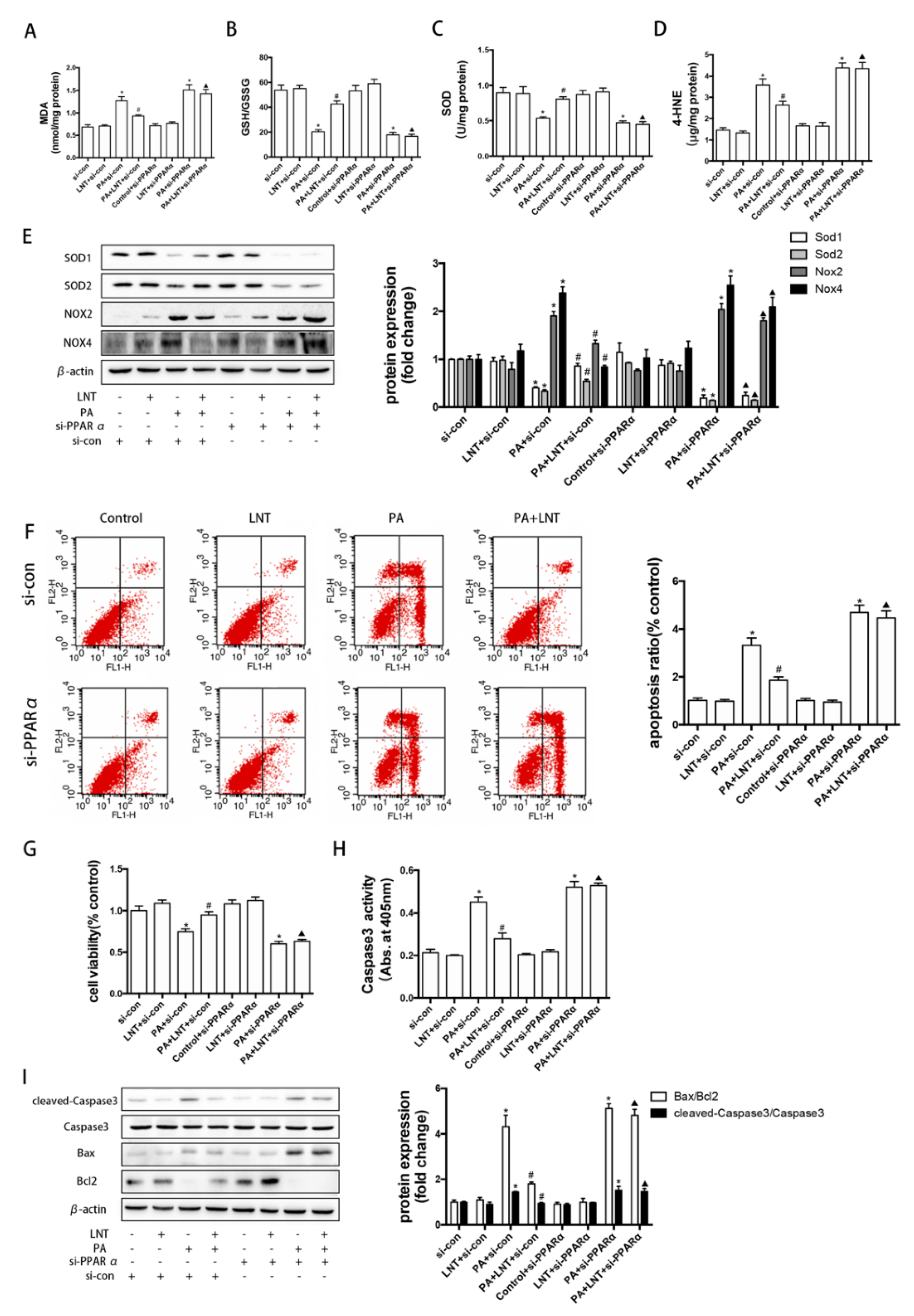
2.6. PPARα Knockdown Prevented the Effects of LNT on Oxidative Stress and Apoptosis in PA-Induced AML12 Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture and Treatments
4.3. Biochemical Parameters
4.4. Western Blot Analysis
4.5. Histological Analysis
4.6. Flow Cytometry Analysis
4.7. Quantitative RT-PCR
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cariou, B.; Byrne, C.D.; Loomba, R.; Sanyal, A.J. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes. Metab. 2021, 23, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Leoni, S.; Alswat, K.A.; Fouad, Y. History of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 5888. [Google Scholar] [CrossRef]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111, 154170. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Neuschwander-Tetri, B.A. The metabolic basis of nonalcoholic steatohepatitis. Endocrinol. Diabetes Metab. 2020, 3, e00112. [Google Scholar] [CrossRef] [PubMed]
- Orabi, D.; Berger, N.A.; Brown, J.M. Abnormal Metabolism in the Progression of Nonalcoholic Fatty Liver Disease to Hepatocellular Carcinoma: Mechanistic Insights to Chemoprevention. Cancers 2021, 13, 3473. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Iuculano, F.; Pallini, G.; Fargion, S.; Fracanzani, A.L. Nutrients, Genetic Factors, and Their Interaction in Non-Alcoholic Fatty Liver Disease and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 8761. [Google Scholar] [CrossRef] [PubMed]
- Carneros, D.; Lopez-Lluch, G.; Bustos, M. Physiopathology of Lifestyle Interventions in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 3472. [Google Scholar] [CrossRef]
- Parra-Vargas, M.; Rodriguez-Echevarria, R.; Jimenez-Chillaron, J.C. Nutritional Approaches for the Management of Nonalcoholic Fatty Liver Disease: An Evidence-Based Review. Nutrients 2020, 12, 3860. [Google Scholar] [CrossRef]
- Marchisello, S.; Di Pino, A.; Scicali, R.; Urbano, F.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Pathophysiological, Molecular and Therapeutic Issues of Nonalcoholic Fatty Liver Disease: An Overview. Int. J. Mol. Sci. 2019, 20, 1948. [Google Scholar] [CrossRef] [Green Version]
- Spahis, S.; Delvin, E.; Borys, J.M.; Levy, E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid. Redox. Signal. 2017, 26, 519–541. [Google Scholar] [CrossRef]
- Wong, V.W.; Adams, L.A.; de Ledinghen, V.; Wong, G.L.; Sookoian, S. Noninvasive biomarkers in NAFLD and NASH —Current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell Longev. 2020, 2020, 1617805. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Zein, N.N.; Yerian, L.M.; Lopez, A.R.; McCullough, A.J.; Feldstein, A.E. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 2006, 44, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Leaderer, B.P.; Feldstein, A.E.; Santoro, N.; McKay, L.A.; Caprio, S.; McConnell, R. Traffic-related air pollution associations with cytokeratin-18, a marker of hepatocellular apoptosis, in an overweight and obese paediatric population. Pediatr. Obes. 2018, 13, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Tang, J.; Chen, J.; Zhang, X. Polysaccharides in Lentinus edodes: Isolation, structure, immunomodulating activity and future prospective. Crit. Rev. Food Sci. Nutr. 2014, 54, 474–487. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. Beta-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Jiao, F.; Li, D.; Yang, S.; Zhang, J.; Zhang, C.; Jia, L. Inhibition effects of polysaccharides on HBV replication and cell proliferation from Lentinus edodes waste material. Microb. Pathog. 2018, 123, 461–466. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Kuai, J.; Fan, P.; Wang, X.; Zhou, P.; Yang, D.; Zheng, X.; Liu, X.; Wu, Q.; et al. Lentinan inhibits tumor angiogenesis via interferon gamma and in a T cell independent manner. J. Exp. Clin. Cancer Res. 2018, 37, 260. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Wang, J.; Zhang, Y.; Shao, Y.; Li, X.; Guo, Y.; Dong, W.; Wang, L.; Chen, F.; Han, X. Lentinan protects against pancreatic beta-cell failure in chronic ethanol consumption-induced diabetic mice via enhancing beta-cell antioxidant capacity. J. Cell Mol. Med. 2021, 25, 6161–6173. [Google Scholar] [CrossRef]
- Kabir, Y.; Yamaguchi, M.; Kimura, S. Effect of Shiitake (Lentinus edodes) and Maitake (Grjfola frondosa) Mushrooms on Blood Pressure and Plasma Lipids of Spontaneously Hypertensive Rats. J. Nutr. Sci. Vitaminol. 1987, 33, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Reyna-Villasmil, N.; Bermúdez-Pirela, V.; Mengual-Moreno, E.; Arias, N.; Cano-Ponce, C.; Leal-Gonzalez, E.; Souki, A.; Inglett, G.E.; Israili, Z.H.; Hernández-Hernández, R.; et al. Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am. J. Ther. 2007, 14, 203–212. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Physiological effects of different types of beta-glucan. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2007, 151, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Dixon, E.D.; Nardo, A.D.; Claudel, T.; Trauner, M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes 2021, 12, 645. [Google Scholar] [CrossRef]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.; Farrell, G.; Hall, P.; Robertson, G.; Leclercq, I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 2004, 39, 1286–1296. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Yoo, S.H.; Henderson, L.E.; Gonzalez, F.J.; Woodcroft, K.J.; Song, B.J. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J. Nutr. Biochem. 2011, 141, 603–610. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.-R.; Zheng, Q.; Dong, J.; Jiang, J. Effects of fenofibrate on hepatocyte apoptosis in nonalcoholic fatty liver. Zhonghua Gan Zang Bing Za Zhi 2015, 23, 688–693. [Google Scholar] [CrossRef]
- Cui, Y.; Chang, R.; Zhang, T.; Zhou, X.; Wang, Q.; Gao, H.; Hou, L.; Loor, J.J.; Xu, C. Chinese Herbal Formula (CHF03) Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) Through Inhibiting Lipogenesis and Anti-Oxidation Mechanisms. Front. Pharmacol. 2019, 10, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Gu, J.; Qi, J.; Zeng, X.N.; Ji, J.; Chen, Z.Z.; Sun, X.L. Lentinan exerts synergistic apoptotic effects with paclitaxel in A549 cells via activating ROS-TXNIP-NLRP3 inflammasome. J. Cell Mol. Med. 2015, 19, 1949–1955. [Google Scholar] [CrossRef]
- De Groot, A.P.; Luyken, R.; Pikaar, N.A. Cholesterol-lowering effect of rolled oats. Lancet 1963, 2, 303–304. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.J. Dietary Fibre from Whole Grains and Their Benefits on Metabolic Health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Motta, F.; Gershwin, M.E.; Selmi, C. Mushrooms and immunity. J. Autoimmun. 2021, 117, 102576. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, K.H.; Choi, H.J.; Lee, D.S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 2005, 27, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, J.M.; Benjachareowong, S.; Chou, E.; Ruderman, N.B.; Ido, Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes-roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes 2005, 54, 1838–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zi, Y.; Jiang, B.; He, C.; Liu, L. Lentinan inhibits oxidative stress and inflammatory cytokine production induced by benzo(a)pyrene in human keratinocytes. J. Cosmet. Dermatol. 2020, 19, 502–507. [Google Scholar] [CrossRef]
- Shojaie, L.; Iorga, A.; Dara, L. Cell Death in Liver Diseases: A Review. Int. J. Mol. Sci. 2020, 21, 9682. [Google Scholar] [CrossRef]
- Thapaliya, S.; Wree, A.; Povero, D.; Inzaugarat, M.E.; Berk, M.; Dixon, L.; Papouchado, B.G.; Feldstein, A.E. Caspase 3 inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig. Dis. Sci. 2014, 59, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Kupfahl, C.; Geginat, G.; Hof, H. Lentinan has a stimulatory effect on innate and adaptive immunity against murine Listeria monocytogenes infection. Int. Immunopharmacol. 2006, 6, 686–696. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.; Shan, W.; Shi, L.; Chang, X.; Zhu, Y.; Chen, F.; Han, X. Lentinan protects pancreatic beta cells from STZ-induced damage. J. Cell Mol. Med. 2016, 20, 1803–1812. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [Green Version]
- Tokoro, M.; Gotoh, K.; Kudo, Y.; Hirashita, Y.; Iwao, M.; Arakawa, M.; Endo, M.; Oribe, J.; Masaki, T.; Honda, K.; et al. alpha-Tocopherol suppresses hepatic steatosis by increasing CPT-1 expression in a mouse model of diet-induced nonalcoholic fatty liver disease. Obes. Sci. Pract. 2021, 7, 91–99. [Google Scholar] [CrossRef]
- Ohashi, K.; Munetsuna, E.; Yamada, H.; Ando, Y.; Yamazaki, M.; Taromaru, N.; Nagura, A.; Ishikawa, H.; Suzuki, K.; Teradaira, R.; et al. High fructose consumption induces DNA methylation at PPARalpha and CPT1A promoter regions in the rat liver. Biochem. Biophys. Res. Commun. 2015, 468, 185–189. [Google Scholar] [CrossRef]
- De Filippis, B.; Giancristofaro, A.; Ammazzalorso, A.; D’Angelo, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Petruzzelli, M.; Amoroso, R. Discovery of gemfibrozil analogues that activate PPARalpha and enhance the expression of gene CPT1A involved in fatty acids catabolism. Eur. J. Med. Chem. 2011, 46, 5218–5224. [Google Scholar] [CrossRef]
- Stefanovic-Racic, M.; Perdomo, G.; Mantell, B.S.; Sipula, I.J.; Brown, N.F.; O’Doherty, R.M. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E969–E977. [Google Scholar] [CrossRef] [Green Version]
- Nyman, L.R.; Tian, L.; Hamm, D.A.; Schoeb, T.R.; Gower, B.A.; Nagy, T.R.; Wood, P.A. Long term effects of high fat or high carbohydrate diets on glucose tolerance in mice with heterozygous carnitine palmitoyltransferase-1a (CPT-1a) deficiency: Diet influences on CPT1a deficient mice. Nutr. Diabetes 2011, 1, e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, J.C.; McLachlan, E.M.; Zeller, R. Transparency in Research involving Animals: The Basel Declaration and new principles for reporting research in BJP manuscripts. Br. J. Pharmacol. 2015, 172, 2427–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, P.; Zhao, G.; Xu, G.; Gruzdev, A.; Zeldin, D.C.; Wang, D.W. Cytochrome P450 epoxygenase CYP2J2 attenuates nephropathy in streptozotocin-induced diabetic mice. Prostaglandins Other Lipid Mediat. 2011, 96, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Tian, M.; Wang, F.; Zhang, Z.; Du, T.; Wang, W.; Yang, Y.; Li, X.; Chen, G.; Xiao, L.; et al. Amlodipine induces vasodilation via Akt2/Sp1-activated miR-21 in smooth muscle cells. Br. J. Pharmacol. 2019, 176, 2306–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, X.L.; Guo, R.X.; Qiao, Y.H.; Zhang, X.Y.; Chen, Z.H. Flow cytometer analysis of cell apoptosis of endometrial carcinoma with Wnt10b. J. Biol. Regul. Homeost. Agents 2016, 30, 547–552. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, T.; Fang, Q.; Zhang, Z.; Zhu, C.; Xu, R.; Chen, G.; Wang, Y. Lentinan Protects against Nonalcoholic Fatty Liver Disease by Reducing Oxidative Stress and Apoptosis via the PPARα Pathway. Metabolites 2022, 12, 55. https://doi.org/10.3390/metabo12010055
Du T, Fang Q, Zhang Z, Zhu C, Xu R, Chen G, Wang Y. Lentinan Protects against Nonalcoholic Fatty Liver Disease by Reducing Oxidative Stress and Apoptosis via the PPARα Pathway. Metabolites. 2022; 12(1):55. https://doi.org/10.3390/metabo12010055
Chicago/Turabian StyleDu, Tingyi, Qin Fang, Zhihao Zhang, Chuanmeng Zhu, Renfan Xu, Guangzhi Chen, and Yan Wang. 2022. "Lentinan Protects against Nonalcoholic Fatty Liver Disease by Reducing Oxidative Stress and Apoptosis via the PPARα Pathway" Metabolites 12, no. 1: 55. https://doi.org/10.3390/metabo12010055
APA StyleDu, T., Fang, Q., Zhang, Z., Zhu, C., Xu, R., Chen, G., & Wang, Y. (2022). Lentinan Protects against Nonalcoholic Fatty Liver Disease by Reducing Oxidative Stress and Apoptosis via the PPARα Pathway. Metabolites, 12(1), 55. https://doi.org/10.3390/metabo12010055






