l-Lactate: Food for Thoughts, Memory and Behavior
Abstract
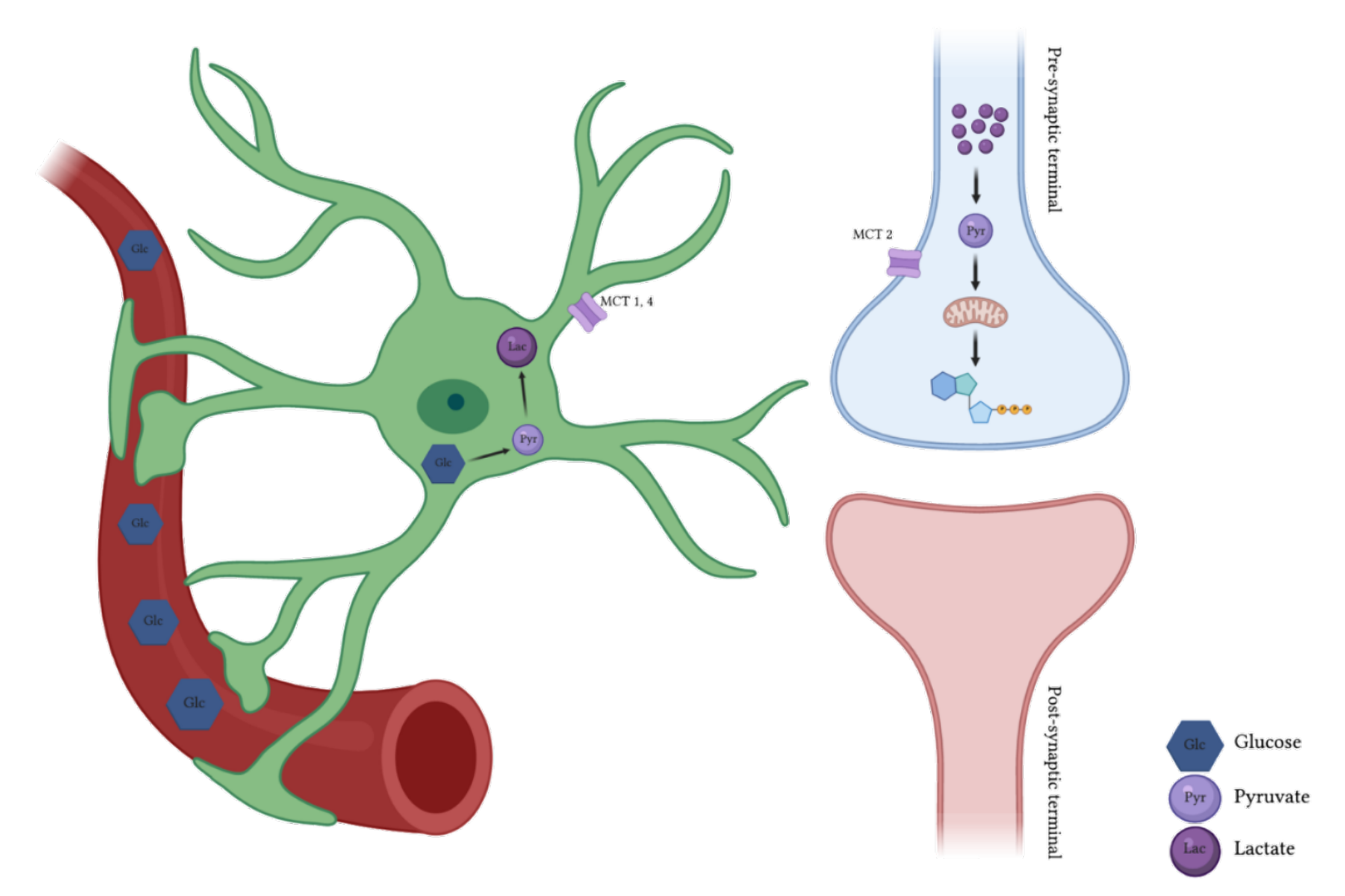
1. Brain Energy Metabolism
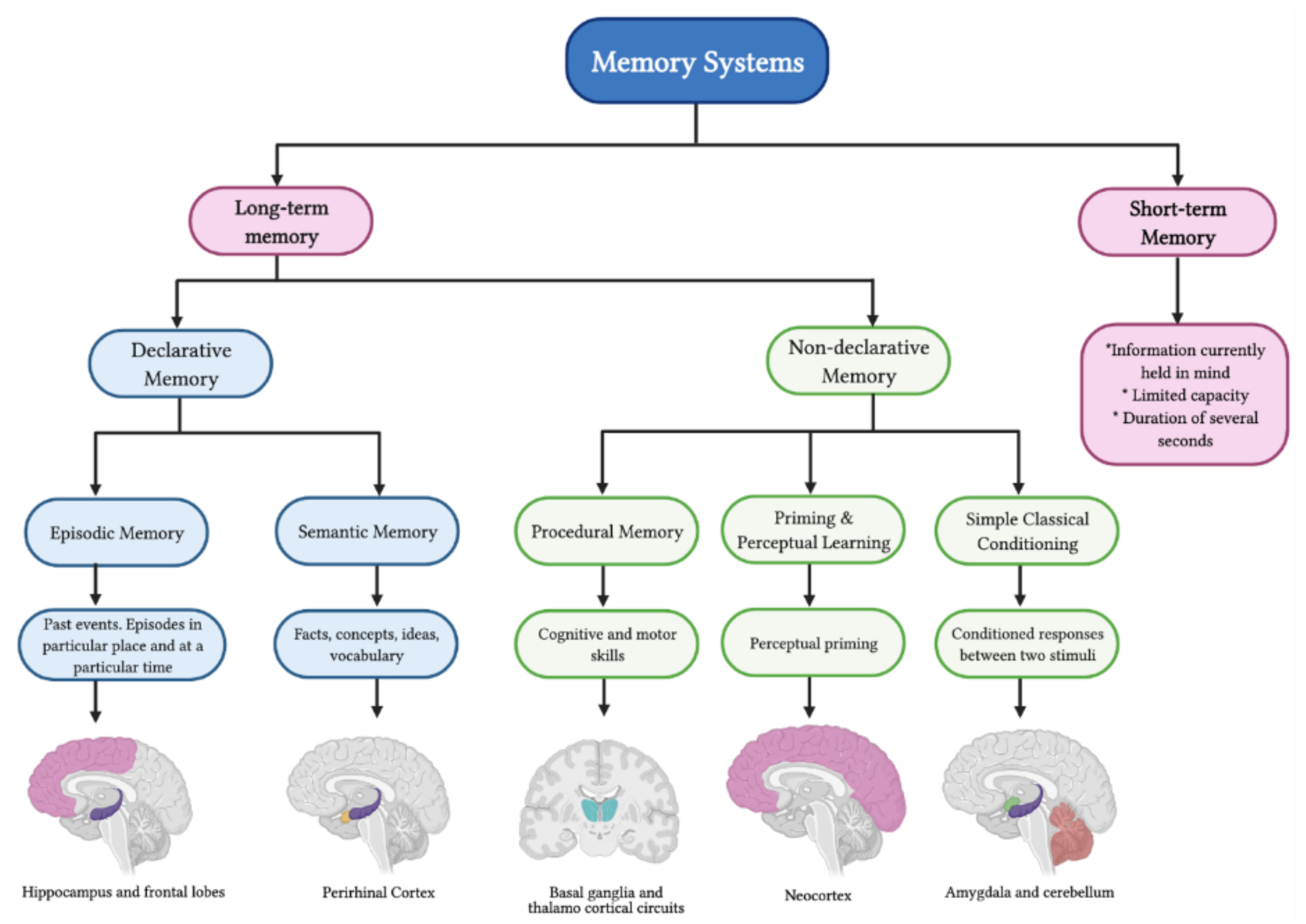
2. Memory Systems
Molecular Mechanisms behind Memory
3. Lactate: A Key Molecule for Memory
The Role of L-Lactate in Disease
4. Behavioral Perspective
4.1. Spatial Memory
4.2. Object Recognition Memory
4.3. Fear Conditioned Memory
4.4. Drug-Associated Memories
5. Morphological Changes Associated with Memory Consolidation: Role of Lactate
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Calì, C.; Agus, M.; Kare, K.; Boges, D.J.; Lehväslaiho, H.; Hadwiger, M.; Magistretti, P.J. 3D cellular reconstruction of cortical glia and parenchymal morphometric analysis from serial block-face electron microscopy of juvenile rat. Prog. Neurobiol. 2019, 183, 101696. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Magistretti, P.J. Brain Energy Metabolism. In Fundamental Neuroscience, 3rd ed.; Squire, L.R., Berg, D., Bloom, F.E., Du Lac, S., Ghosh, A., Spitzer, N.C., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 261–284. [Google Scholar] [CrossRef]
- Nelson, S.R.; Schulz, D.W.; Passonneau, J.V.; Lowry, O.H. Control of glycogen levels in brain. J. Neurochem. 1968, 15, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. Brain glycogen re-awakened. J. Neurochem. 2004, 89, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Agus, M.; Boges, D.; Gagnon, N.; Magistretti, P.J.; Hadwiger, M.; Calí, C. GLAM: Glycogen-derived Lactate Absorption Map for visual analysis of dense and sparse surface reconstructions of rodent brain structures on desktop systems and virtual environments. Comput. Graph. 2018, 74, 85–98. [Google Scholar] [CrossRef]
- Calì, C.; Baghabra, J.; Boges, D.J.; Holst, G.R.; Kreshuk, A.; Hamprecht, F.A.; Srinivasan, M.; Lehväslaiho, H.; Magistretti, P.J. Three-dimensional immersive virtual reality for studying cellular compartments in 3D models from EM preparations of neural tissues. J. Comp. Neurol. 2016, 524, 23–38. [Google Scholar] [CrossRef]
- Vezzoli, E.; Calì, C.; De Roo, M.; Ponzoni, L.; Sogne, E.; Gagnon, N.; Francolini, M.; Braida, D.; Sala, M.; Muller, D.; et al. Ultrastructural evidence for a role of astrocytes and glycogen-derived lactate in learning-dependent synaptic stabilization. Cere.l Cortex 2020, 30, 2114–2127. [Google Scholar] [CrossRef]
- Swanson, R.A.; Sagar, S.M.; Sharp, F.R. Regional brain glycogen stores and metabolism during complete global ischaemia. Neurol. Res. 1989, 11, 24–28. [Google Scholar] [CrossRef]
- Gruetter, R. Glycogen: The forgotten cerebral energy store. J. Neurosci. Res. 2003, 74, 179–183. [Google Scholar] [CrossRef]
- Obel, L.F.; Müller, M.S.; Walls, A.B.; Sickmann, H.M.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A. Brain glycogen—New perspectives on its metabolic function and regulation at the subcellular level. Front. Neuroenergetics 2012, 4, 1–15. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. Astrocytes couple synaptic activity to glucose utilization in the brain. Physiology 1999, 14, 177–182. [Google Scholar] [CrossRef]
- Magistretti, P.J. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006, 209, 2304–2311. [Google Scholar] [CrossRef]
- Coggan, J.S.; Cali, C.; Keller, D.; Agus, M.; Boges, D.; Abdellah, M.; Kare, K.; Lehväslaiho, H.; Eilemann, S.; Jolivet, R.; et al. A process for digitizing and simulating biologically realistic oligocellular networks demonstrated for the neuro-glio-vascular ensemble. Front. Neurosci. 2018, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Calì, C.; Kare, K.; Agus, M.; Veloz Castillo, M.F.; Boges, D.; Hadwiger, M.; Magistretti, P. A method for 3D reconstruction and virtual reality analysis of glial and neuronal cells. JoVE J. Vis. Exp. 2019, e59444. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.G.; Rothman, D.L. The ‘glycogen shunt’ in exercising muscle: A role for glycogen in muscle energetics and fatigue. Proc. Natl. Acad. Sci. USA 2001, 98, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.B.; Heimbürger, C.M.; Bouman, S.D.; Schousboe, A.; Waagepetersen, H.S. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience 2009, 158, 284–292. [Google Scholar] [CrossRef]
- Shulman, R.G.; Hyder, F.; Rothman, D.L. Cerebral energetics and the glycogen shunt: Neurochemical basis of functional imaging. Proc. Natl. Acad. Sci. USA 2001, 98, 6417–6422. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef]
- Steinman, M.Q.; Gao, V.; Alberini, C.M. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front. Integr. Neurosci. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Gibbs, M.E.; Hutchinson, D.; Hertz, L. Astrocytic involvement in learning and memory consolidation. Neurosci. Biobehav. Rev. 2008, 32, 927–944. [Google Scholar] [CrossRef]
- Bezzi, P.; Volterra, A. Astrocytes: Powering memory. Cell 2011, 144, 644–645. [Google Scholar] [CrossRef][Green Version]
- Squire, L.R. Definitions: From Synapses to Behavior. In Memory and Brain; Oxford University Press Inc.: New York, NY, USA, 1987; pp. 3–9. [Google Scholar]
- Baars, B.J.; Gage, N.M. Learning and Memory. In Cognition, Brain, and Consciousness; Elsevier: Amsterdam, The Netherlands, 2010; pp. 304–343. [Google Scholar] [CrossRef]
- Lucas, J.A. Memory, Overview. In Encyclopedia of the Human Brain; Elsevier: Amsterdam, The Netherlands, 2002; pp. 817–833. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Keane, M.T. Learning Ans Memory. In Cognitive Psychology: A Student’s Handbook, 5th ed.; Psychology Press: New York, NY, USA, 2005; pp. 189–228. [Google Scholar]
- Squire, L.R. Short-Term and Long-Term Memory Processes. In Memory and Brain; Oxford University Press Inc.: New York, NY, USA, 1987; pp. 134–150. Available online: https://ebookcentral.proquest.com/lib/kaust-ebooks/detail.action?docID=272279 (accessed on 28 February 2021).
- Ward, J. The Remembering Brain. In The Student’s Guide to Cognitive Neuroscience, 1st ed.; Psychology Press: New York, NY, USA, 2006; pp. 176–201. [Google Scholar]
- Kropotov, J.D. Memory Systems. In Quantitative EEG, Event-Related Potentials and Neurotherapy; Elsevier: Amsterdam, The Netherlands, 2009; pp. 310–324. [Google Scholar] [CrossRef]
- Squire, L.R. Divisions of Long-Term Memory. In Memory and Brain; Oxford University Press Inc.: New York, NY, USA, 1987; pp. 151–174. Available online: https://ebookcentral.proquest.com/lib/kaust-ebooks/detail.action?docID=272279 (accessed on 28 February 2021).
- Squire, L.R. Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 2004, 82, 171–177. [Google Scholar] [CrossRef]
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessières, B.; Gao, V. Astrocyte glycogen and lactate: New insights into learning and memory mechanisms. Glia 2018, 66, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Kinsbourne, M. Brain mechanisms and memory. Hum. Neurobiol. 1987, 6, 81–92. [Google Scholar] [CrossRef]
- Tulving, E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Eichenbaum, H. How does the brain organize memories? Science 1997, 277, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Memory systems in the brain. Annu. Rev. Psychol. 2000, 51, 599–630. [Google Scholar] [CrossRef]
- Radvansky, G.; Tamplin, A. Types of Memory. In Encyclopedia of Human Behavior, 2nd ed.; Ramachandran, V.S., Ed.; Elsevier Science & Technology: Berkeley, CA, USA, 2012; pp. 585–592. Available online: http://waraqa.org/wp-content/uploads/2021/04/Encyclopedia-of-Human-Behavior-Second-Edition.pdf (accessed on 28 February 2021).
- Zhang, Y.; Han, K.; Worth, R.; Liu, Z. Connecting concepts in the brain by mapping cortical representations of semantic relations. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex 2009, 19, 2767–2796. [Google Scholar] [CrossRef]
- Zichlin, M. Procedural Memory. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 2033–2034. [Google Scholar] [CrossRef]
- Rueda-Orozco, P.E.; Montes-Rodriguez, C.J.; Soria-Gomez, E.; Méndez-Díaz, M.; Prospéro-García, O. Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology 2008, 55, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Humphreys, G.W. Working memory, perceptual priming, and the perception of hierarchical forms: Opposite effects of priming and working memory without memory refreshing. Atten. Percept. Psychophys. 2010, 72, 1533–1555. [Google Scholar] [CrossRef][Green Version]
- Woltz, D.J. Perceptual and conceptual priming in a semantic reprocessing task. Mem. Cogn. 1996, 24, 429–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schreurs, B.G.; Alkon, D.L. Imaging learning and memory: Classical conditioning. Anat. Rec. 2001, 265, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Robleto, K. Brain mechanisms of extinction of the classically conditioned eyeblink response. Learn. Mem. 2004, 11, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, R.P.; Steinmetz, J.E. Neuroscience and learning: Lessons from studying the involvement of a region of cerebellar cortex in eyeblink classical conditioning. J. Exp. Anal. Behav. 2005, 84, 631–652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gruart, A.; Delgado-García, J.M. Activity-dependent changes of the hippocampal CA3-CA1 synapse during the acquisition of associative learning in conscious mice. Genes Brain Behav. 2007, 6, 24–31. [Google Scholar] [CrossRef]
- Gruart, A.; Leal-Campanario, R.; López-Ramos, J.C.; Delgado-García, J.M. Functional basis of associative learning and their relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 2015, 124, 3–18. [Google Scholar] [CrossRef]
- Fontinha, B.M.; Delgado-García, J.M.; Madroñal, N.; Ribeiro, J.A.; Sebastião, A.M.; Gruart, A. Adenosine A(2A) receptor modulation of hippocampal CA3-CA1 synapse plasticity during associative learning in behaving mice. Neuropsychopharmacology 2009, 34, 1865–1874. [Google Scholar] [CrossRef]
- Ortiz, O.; Delgado-García, J.M.; Espadas, I.; Bahí, A.; Trullas, R.; Dreyer, J.L.; Gruart, A.; Moratalla, R. Associative learning and CA3-CA1 synaptic plasticity are impaired in D 1R Null, Drd1a-/- mice and in hippocampal SiRNA silenced Drd1a mice. J. Neurosci. 2010, 30, 12288–12300. [Google Scholar] [CrossRef] [PubMed]
- Myhrer, T. Neurotransmitter systems involved in learning and memory in the rat: A meta-analysis based on studies of four behavioral tasks. Brain Res. Rev. 2003, 41, 268–287. [Google Scholar] [CrossRef]
- Meneses, A. Neurotransmitters and Memory: Cholinergic, Glutamatergic, GaBAergic, Dopaminergic, Serotonergic, Signaling, and Memory. In Identification of Neural Markers Accompanying Memory; Elsevier: Amsterdam, The Netherlands, 2014; pp. 5–45. [Google Scholar] [CrossRef]
- Squire, L.R. Amnesia and the Functional Organization of Memory. In Memory and Brain; Oxford University Press Inc.: New York, NY, USA, 1986; pp. 202–223. [Google Scholar]
- Bramham, C.R. Control of synaptic consolidation in the dentate gyrus: Mechanisms, functions, and therapeutic implications. Prog. Brain Res. 2007, 163, 453–471. [Google Scholar] [CrossRef]
- Plath, N.; Ohana, O.; Dammermann, B.; Errington, M.L.; Schmitz, D.; Gross, C.; Mao, X.; Engelsberg, A.; Mahlke, C.; Welzl, H.; et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 2006, 52, 437–444. [Google Scholar] [CrossRef]
- Vazdarjanova, A.; Ramirez-Amaya, V.; Insel, N.; Plummer, T.K.; Rosi, S.; Chowdhury, S.; Mikhael, D.; Worley, P.F.; Guzowski, J.F.; Barnes, C.A. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 2006, 498, 317–329. [Google Scholar] [CrossRef] [PubMed]
- El-Boustani, S.; Ip, J.P.K.; Breton-Provencher, V.; Knott, G.W.; Okuno, H.; Bito, H.; Sur, M. Locally coordinated synaptic plasticity of visual cortex neurons in vivo. Science 2018, 360, 1349–1354. [Google Scholar] [CrossRef]
- Roesler, R. Molecular mechanisms controlling protein synthesis in memory reconsolidation. Neurobiol. Learn. Mem. 2017, 142, 30–40. [Google Scholar] [CrossRef]
- Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009, 89, 121–145. [Google Scholar] [CrossRef]
- Alberini, C.M.; Kandel, E.R. The regulation of transcription in memory consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021741. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory: CAMP, PKA, CRE, CREB-1, CREB-2, and CPEB. Mol. Brain 2012, 5, 1–12. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C. NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Front. Mol. Neurosci. 2015, 8, 1–11. [Google Scholar] [CrossRef]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is plasticity of synapses the mechanism of long-term memory storage? Npj Sci. Learn. 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Tang, S.J.; Reis, G.; Kang, H.; Gingras, A.C.; Sonenberg, N.; Schuman, E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Orsi, S.A.; Moore, A.N. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-mammalian target of rapamycin pathway. J. Neurosci. 2006, 26, 8048–8056. [Google Scholar] [CrossRef] [PubMed]
- Jobim, P.F.C.; Pedroso, T.R.; Werenicz, A.; Christoff, R.R.; Maurmann, N.; Reolon, G.K.; Schröder, N.; Roesler, R. Impairment of object recognition memory by rapamycin inhibition of mTOR in the amygdala or hippocampus around the time of learning or reactivation. Behav. Brain Res. 2012, 228, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Katche, C.; Slipczuk, L.N.; Igaz, L.M.; Cammarota, M.; Izquierdo, I.; Medina, J.H. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol. Learn. Mem. 2007, 87, 303–307. [Google Scholar] [CrossRef]
- Funahashi, H.; Yada, T.; Suzuki, R.; Shioda, S. Distribution, function, and properties of leptin receptors in the brain. Int. Rev. Cytol. 2003, 224, 1–27. [Google Scholar] [CrossRef]
- Harvey, J.; Solovyova, N.; Irving, A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006, 45, 369–378. [Google Scholar] [CrossRef]
- Irving, A.J.; Harvey, J. Leptin regulation of hippocampal synaptic function in health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130155. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, V.; Contreras, A.; Merino, B.; Plaza, A.; Lorenzo, M.P.; García-Cáceres, C.; García, A.; Chowen, J.A.; Ruiz-Gayo, M.; Del Olmo, N.; et al. Specific deletion of the astrocyte leptin receptor induces changes in hippocampus glutamate metabolism, synaptic transmission and plasticity. Neuroscience 2020, 447, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake stimulates Na+,K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J. Neurochem. 1997, 69, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Pellerin, L.; Rothman, D.L.; Shulman, R.G. Energy on demand. Science 1999, 283, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. Edited by Darrell Brann. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef]
- Korol, D.L.; Gardner, R.S.; Tunur, T.; Gold, P.E. Involvement of lactate transport in two object recognition tasks that require either the hippocampus or striatum. Behav. Neurosci. 2019, 133, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Duran, J.; Saez, I.; Gruart, A.; Guinovart, J.J.; Delgado-García, J.M. Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J. Cereb. Blood Flow Metab. 2013, 33, 550–556. [Google Scholar] [CrossRef]
- Herrera-López, G.; Griego, E.; Galván, E.J. Lactate induces synapse-specific potentiation on CA3 pyramidal cells of rat hippocampus. PLoS ONE 2020, 15, e0242309. [Google Scholar] [CrossRef]
- Bingul, D.; Kalra, K.; Murata, E.M.; Belser, A.; Dash, M.B. Persistent changes in extracellular lactate dynamics following synaptic potentiation. Neurobiol. Learn. Mem. 2020, 175, 107314. [Google Scholar] [CrossRef] [PubMed]
- Mächler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; Von Faber-Castell, A.; Kaelin, V.; Zuend, M.; Martiín, A.S.; Romero-Gómez, I.; et al. In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 2016, 23, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Netzahualcoyotzi, C.; Pellerin, L. Neuronal and astroglial monocarboxylate transporters play key but distinct roles in hippocampus-dependent learning and memory formation. Prog. Neurobiol. 2020, 19, 101888. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Morrison, J.H. Noradrenaline- and vasoactive intestinal peptide-containing neuronal systems in neocortex: Functional convergence with contrasting morphology. Neuroscience 1988, 24, 367–378. [Google Scholar] [CrossRef]
- Zuend, M.; Saab, A.S.; Wyss, M.T.; Ferrari, K.D.; Hösli, L.; Looser, Z.J.; Stobart, J.L.; Duran, J.; Guinovart, J.J.; Barros, L.F.; et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2020, 2, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Velebit, J.; Vardjan, N.; Zorec, R.; Kreft, M. Noradrenaline-induced l-lactate production requires d-glucose entry and transit through the glycogen shunt in single-cultured rat astrocytes. J. Neurosci. Res. 2021, 99, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Gao, V.; Suzuki, A.; Magistretti, P.J.; Lengacher, S.; Pollonini, G.; Steinman, M.Q.; Alberini, C.M. Astrocytic Β2- adrenergic receptors mediate hippocampal long- term memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8526–8531. [Google Scholar] [CrossRef] [PubMed]
- Flavell, S.W.; Greenberg, M.E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 2008, 31, 563–590. [Google Scholar] [CrossRef]
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef]
- Margineanu, M.B.; Mahmood, H.; Fiumelli, H.; Magistretti, P.J. L-lactate regulates the expression of synaptic plasticity and neuroprotection genes in cortical neurons: A transcriptome analysis. Front. Mol. Neurosci. 2018, 11, 1–17. [Google Scholar] [CrossRef]
- Powell, C.L.; Davidson, A.R.; Brown, A.M. Universal glia to neurone lactate transfer in the nervous system: Physiological functions and pathological consequences. Biosensors 2020, 10, 183. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Zhang, X.; Alshakhshir, N.; Zhao, L. Glycolytic metabolism, brain resilience, and Alzheimer’s disease. Front. Neurosci. 2021, 15, 476. [Google Scholar] [CrossRef] [PubMed]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef]
- Ryu, W.-I.; Bormann, M.K.; Shen, M.; Kim, D.; Forester, B.; Park, Y.; So, J.; Seo, H.; Sonntag, K.-C.; Cohen, B.M. Brain cells derived from Alzheimer’s disease patients have multiple specific innate abnormalities in energy metabolism. Mol. Psychiatry 2021, 1–13. [Google Scholar] [CrossRef]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s disease: Biomarkers, treatment, and risk factors. Front. Neurosci. 2018, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, T.; Wang, W.; Xiang, Y.; Huang, Q.; Xie, C.; Zhao, L.; Zheng, H.; Yang, Y.; Gao, H. Brain-region specific metabolic abnormalities in Parkinson’s disease and levodopa-induced dyskinesia. Front. Aging Neurosci. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Tefera, T.W.; Borges, K. Metabolic dysfunctions in amyotrophic lateral sclerosis pathogenesis and potential metabolic treatments. Front. Neurosci. 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tefera, T.W.; Steyn, F.J.; Ngo, S.T.; Borges, K. CNS glucose metabolism in amyotrophic lateral sclerosis: A therapeutic target? Cell Biosci. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Dogan, A.E.; Yuksel, C.; Du, F.; Chouinard, V.-A.; Öngür, D. Brain lactate and PH in schizophrenia and bipolar disorder: A systematic review of findings from magnetic resonance studies. Neuropsychopharmacology 2018, 43, 1681–1690. [Google Scholar] [CrossRef]
- Rowland, L.M.; Pradhan, S.; Korenic, S.; Wijtenburg, S.A.; Hong, L.E.; Edden, R.A.; Barker, P.B. Elevated brain lactate in schizophrenia: A 7 T magnetic resonance spectroscopy study. Transl. Psychiatry 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Kuang, H.; Duong, A.; Jeong, H.; Zachos, K.; Andreazza, A.C. Lactate in bipolar disorder: A systematic review and meta-analysis. Psychiatry Clin. Neurosci. 2018, 72, 546–555. [Google Scholar] [CrossRef]
- Sullivan, C.R.; Mielnik, C.A.; Funk, A.; O’Donovan, S.M.; Bentea, E.; Pletnikov, M.; Ramsey, A.J.; Wen, Z.; Rowland, L.M.; McCullumsmith, R.E. Measurement of lactate levels in postmortem brain, IPSCs, and animal models of schizophrenia. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.-M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.-L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2016, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.F.; Setlow, B.; Wagner, E.K.; McGaugh, J.L. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genesArc, c-fos, and zif268. J. Neurosci. 2001, 21, 5089–5098. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Ding, R.; Tan, Y.; Du, A.; Wen, G.; Ren, X.; Yao, H.; Ren, W.; Liu, H.; Wang, X.; Yu, H.; et al. Redistribution of monocarboxylate 1 and 4 in hippocampus and spatial memory impairment induced by long-term ketamine administration. Front. Behav. Neurosci. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Harris, R.A.; Lone, A.; Lim, H.; Martinez, F.; Frame, A.K.; Scholl, T.J.; Cumming, R.C. Aerobic glycolysis is required for spatial memory acquisition but not memory retrieval in mice. ENeuro 2019, 6. [Google Scholar] [CrossRef]
- Radiske, A.; Rossato, J.I.; Gonzalez, M.C.; Köhler, C.A.; Bevilaqua, L.R.; Cammarota, M. BDNF controls object recognition memory reconsolidation. Neurobiol. Learn. Mem. 2017, 142, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Vaccari Cardoso, B.; Shevelkin, A.V.; Terrillion, S.; Mychko, O.; Mosienko, V.; Kasparov, S.; Pletnikov, M.V.; Teschemacher, A.G. Reducing L-lactate release from hippocampal astrocytes by intracellular oxidation increases novelty induced activity in mice. Glia 2021, 69, 1241–1250. [Google Scholar] [CrossRef]
- Hott, S.C.; Gomes, F.V.; Fabri, D.R.S.; Reis, D.G.; Crestani, C.C.; Côrrea, F.M.A.; Resstel, L.B.M. Both A1- and Β1-adrenoceptors in the bed nucleus of the stria terminalis are involved in the expression of conditioned contextual fear. Br. J. Pharmacol. 2012, 167, 207–221. [Google Scholar] [CrossRef]
- Giustino, T.F.; Maren, S. Noradrenergic modulation of fear conditioning and extinction. Front. Behav. Neurosci. 2018, 12, 1–20. [Google Scholar] [CrossRef]
- Oe, Y.; Wang, X.; Patriarchi, T.; Konno, A.; Ozawa, K.; Yahagi, K.; Hirai, H.; Tsuboi, T.; Kitaguchi, T.; Tian, L.; et al. Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Descalzi, G.; Gao, V.; Steinman, M.Q.; Suzuki, A.; Alberini, C.M. Lactate from astrocytes fuels learning-induced MRNA translation in excitatory and inhibitory neurons. Commun. Biol. 2019, 2, 247. [Google Scholar] [CrossRef] [PubMed]
- Boury-Jamot, B.; Halfon, O.; Magistretti, P.J.; Boutrel, B. Lactate release from astrocytes to neurons contributes to cocaine memory formation. BioEssays 2016, 38, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Boutrel, B.; Magistretti, P.J. A role for lactate in the consolidation of drug-related associative memories. Biol. Psychiatry 2016, 79, 875–877. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Y.; Meng, S.; Luo, Y.; Liang, J.; Li, J.; Ai, S.; Sun, C.; Shen, H.-W.; Zhu, W.; et al. Inhibition of lactate transport erases drug memory and prevents drug relapse. Biol. Psychiatry. 2016, 79, 928–939. [Google Scholar] [CrossRef]
- Boury-Jamot, B.; Carrard, A.; Martin, J.L.; Halfon, O.; Magistretti, P.J.; Boutrel, B. Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry 2016, 21, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xue, Y.-X.; Ding, Z.-B.; Xue, L.-F.; Xu, C.-M.; Lu, L. Glycogen Synthase Kinase 3β in the Baso...Ocaine Reward Memory_Enhanced Reader.Pdf. J. Neurochem. 2011, 118, 113–125. [Google Scholar] [CrossRef]
- Jian, M.; Luo, Y.X.; Xue, Y.X.; Han, Y.; Shi, H.S.; Liu, J.F.; Yan, W.; Wu, P.; Meng, S.-Q.; Deng, J.-H.; et al. EIF2α dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. J. Neurosci. 2014, 34, 10010–10021. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. The Central Role of Astrocytes in Neuroenergetics. In Neuroglia, 2nd ed.; Kettenmann, H., Ransom, B.R., Eds.; Oxford University Press: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Agus, M.; Calì, C.; Al-Awami, A.; Gobbetti, E.; Magistretti, P.; Hadwiger, M. Interactive volumetric visual analysis of glycogen-derived energy absorption in nanometric brain structures. Comput. Graph. Forum 2019, 38, 427–439. [Google Scholar] [CrossRef]
- Boges, D.J.; Agus, M.; Magistretti, P.J.; Calì, C. Forget About Electron Micrographs: A Novel Guide for Using 3D Models for Quantitative Analysis of Dense Reconstructions. In Neuromethods; Humana Press: New York, NY, USA, 2020; Volume 155, pp. 263–304. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloz Castillo, M.F.; Magistretti, P.J.; Calì, C. l-Lactate: Food for Thoughts, Memory and Behavior. Metabolites 2021, 11, 548. https://doi.org/10.3390/metabo11080548
Veloz Castillo MF, Magistretti PJ, Calì C. l-Lactate: Food for Thoughts, Memory and Behavior. Metabolites. 2021; 11(8):548. https://doi.org/10.3390/metabo11080548
Chicago/Turabian StyleVeloz Castillo, María Fernanda, Pierre J. Magistretti, and Corrado Calì. 2021. "l-Lactate: Food for Thoughts, Memory and Behavior" Metabolites 11, no. 8: 548. https://doi.org/10.3390/metabo11080548
APA StyleVeloz Castillo, M. F., Magistretti, P. J., & Calì, C. (2021). l-Lactate: Food for Thoughts, Memory and Behavior. Metabolites, 11(8), 548. https://doi.org/10.3390/metabo11080548







