Comparative Metabolite Profiling of Traditional and Commercial Vinegars in Korea
Abstract
1. Introduction
2. Results and Discussion
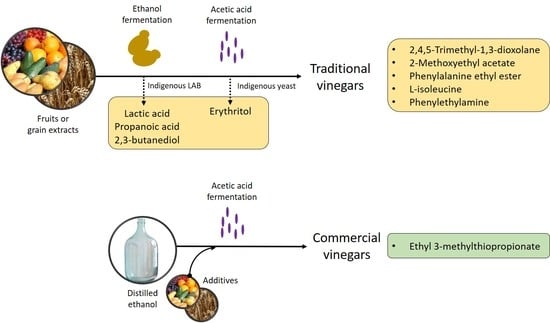
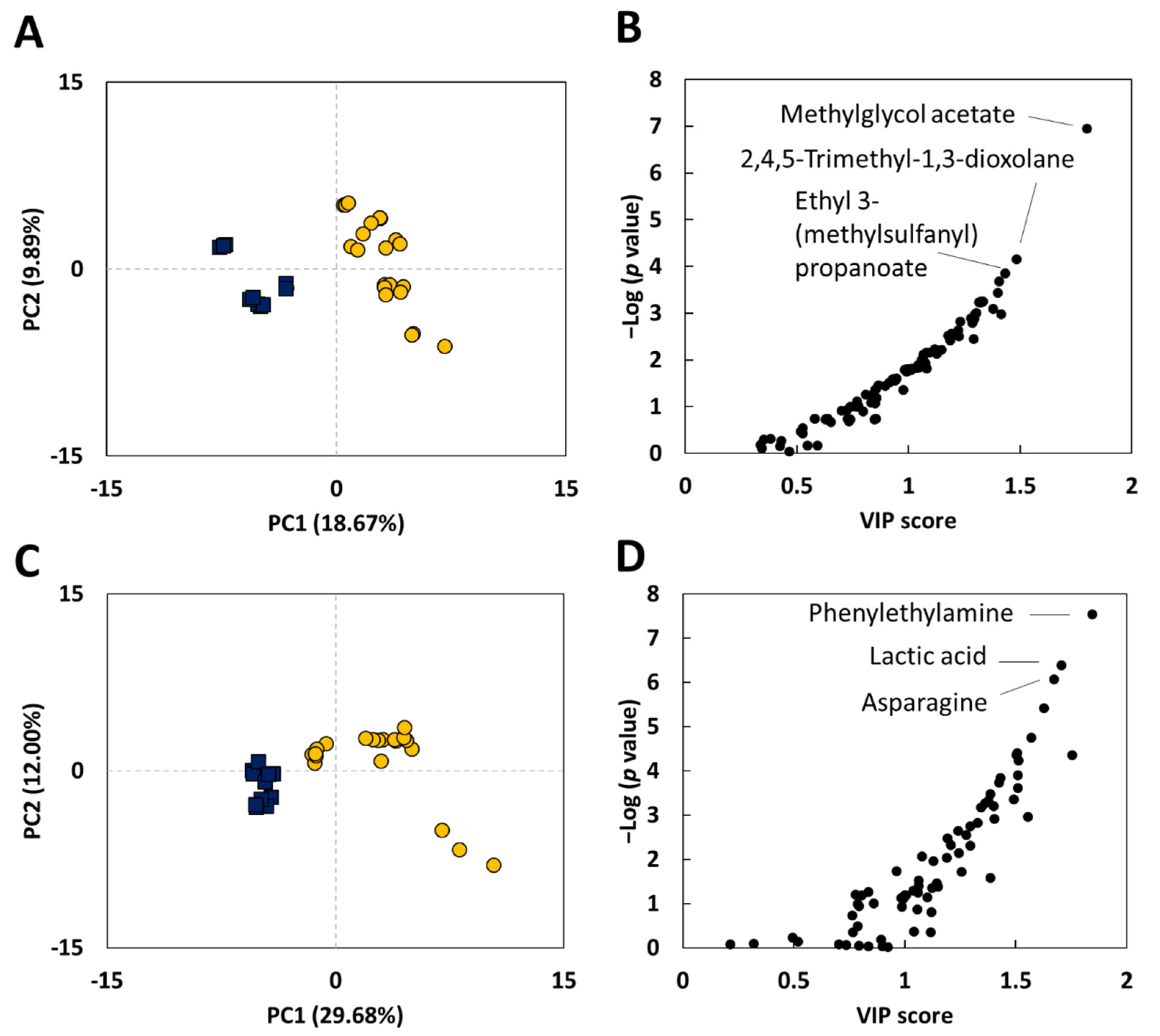
2.1. Metabolomic Differences between Traditional Vinegars and Commercial Vinegars
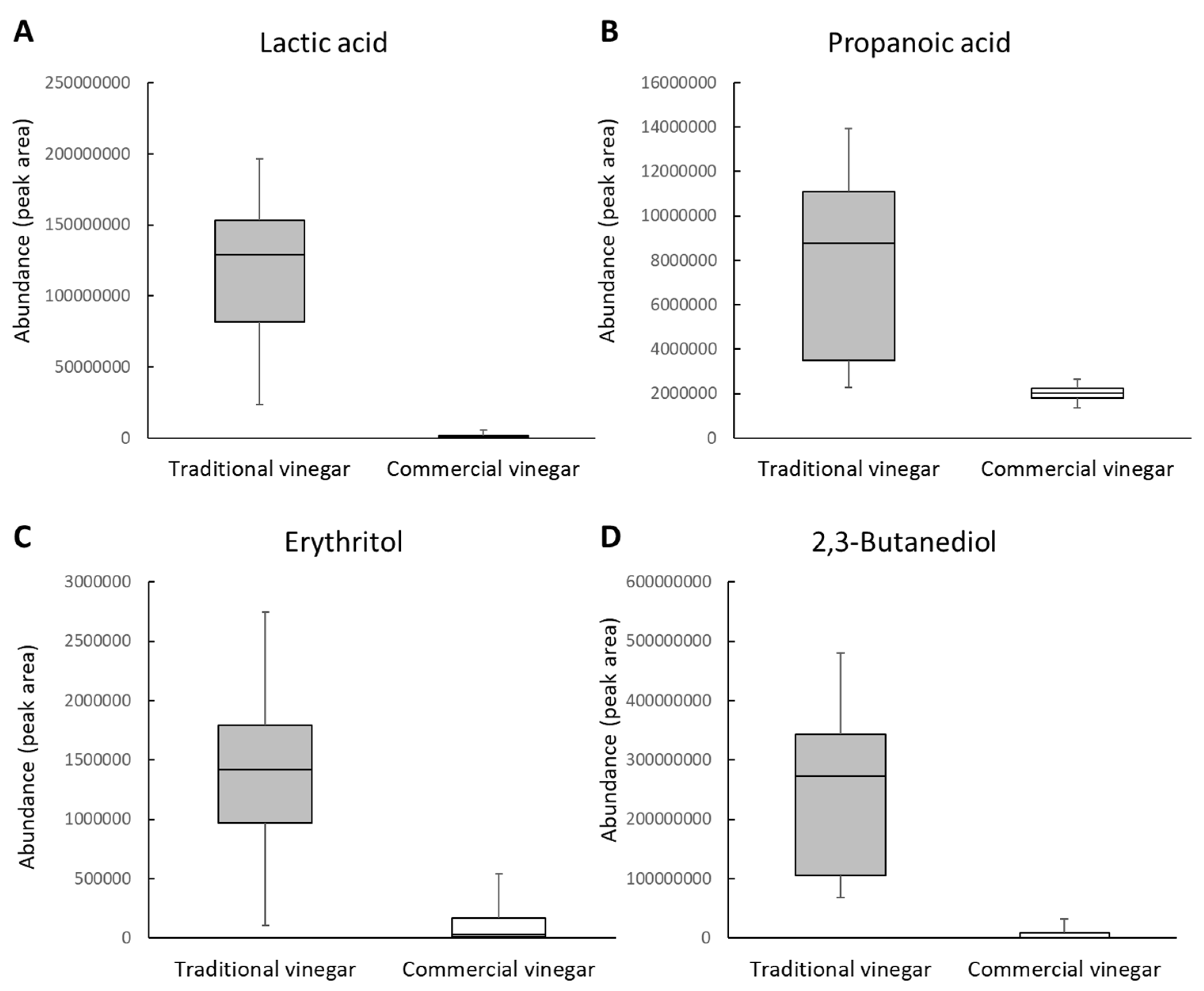
2.2. TV-Only Compounds
2.3. CV-Only Compounds
2.4. Common Vinegar Compounds
3. Materials and Methods
3.1. Raw Material Preparation
3.2. High-Performance Liquid Chromatography (HPLC) Analysis
3.3. Sample Derivatization for Analysis
3.4. Metabolite Analysis Using Gas Chromatography–Mass Spectrometry (GC/MS)
3.5. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Salbe, A.D.; Johnston, C.S.; Buyukbese, M.A.; Tsitouras, P.D.; Harman, S.M. Vinegar lacks antiglycemic action on enteral carbohydrate absorption in human subjects. Nutr. Res. 2009, 29, 846–849. [Google Scholar] [CrossRef]
- Budak, N.H.; Kumbul Doguc, D.; Savas, C.M.; Seydim, A.C.; Kok Tas, T.; Ciris, M.I.; Guzel-Seydim, Z.B. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J. Agric. Food Chem. 2011, 59, 6638–6644. [Google Scholar] [CrossRef]
- Petsiou, E.I.; Mitrou, P.I.; Raptis, S.A.; Dimitriadis, G.D. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr. Rev. 2014, 72, 651–661. [Google Scholar] [CrossRef]
- Pazuch, C.M.; Siepmann, F.B.; Canan, C.; Colla, E. Vinegar: Functional aspects. Científica 2015, 43, 302–308. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Kim, E.-J.; Cho, K.-M.; Kwon, S.J.; Seo, S.-H.; Park, S.-E.; Son, H.-S. Factors affecting vinegar metabolites during two-stage fermentation through metabolomics study. LWT 2021, 135, 110081. [Google Scholar] [CrossRef]
- Zhao, G.; Kuang, G.; Li, J.; Hadiatullah, H.; Chen, Z.; Wang, X.; Yao, Y.; Pan, Z.-H.; Wang, Y. Characterization of aldehydes and hydroxy acids as the main contribution to the traditional Chinese rose vinegar by flavor and taste analyses. Food Res. Int. 2020, 129, 108879. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Sun, B.; Zhou, C.; Li, M.; Chen, F. Effects of different brewing processes on the volatile flavor profiles of Chinese vinegar determined by HS-SPME-AEDA with GC-MS and GC-O. LWT 2020, 133, 109969. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Zheng, F.; Sun, B.; Chen, F. Comparison of Aroma Profiles of Traditional and Modern Zhenjiang Aromatic Vinegars and Their Changes During the Vinegar Aging by SPME-GC-MS and GC-O. Food Anal. Methods 2019, 12, 544–557. [Google Scholar] [CrossRef]
- Pinu, F.R.; de Carvalho-Silva, S.; Trovatti Uetanabaro, A.P.; Villas-Boas, S.G. Vinegar Metabolomics: An Explorative Study of Commercial Balsamic Vinegars Using Gas Chromatography-Mass Spectrometry. Metabolites 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Inaoka, T.; Nakamura, T.; Kimura, K.; Sekiyama, Y.; Tomita, S. Nuclear magnetic resonance- and gas chromatography/mass spectrometry-based metabolomic characterization of water-soluble and volatile compound profiles in cabbage vinegar. J. Biosci. Bioeng. 2018, 126, 53–62. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar functions on health: Constituents, sources, and formation mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Li, S.; Li, P.; Feng, F.; Luo, L.-X. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biotechnol. 2015, 99, 4997–5024. [Google Scholar] [CrossRef]
- Yetiman, A.E.; Kesmen, Z. Identification of acetic acid bacteria in traditionally produced vinegar and mother of vinegar by using different molecular techniques. Int. J. Food Microbiol. 2015, 204, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Liu, Z.; Song, Z.; Wang, C.; Liu, Y.; Gan, J.; Ma, X.; Lu, A. Application of a strategy based on metabolomics guided promoting blood circulation bioactivity compounds screening of vinegar. Chem. Cent. J. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Callejón, R.M.; Morales, M.L.; García-Parrilla, M.C. A survey of biogenic amines in vinegars. Food Chem. 2013, 141, 2713–2719. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.K.; Lee, M.Y.; Kim, H.Y.; Lee, S.; Yeo, S.H.; Baek, S.Y.; Lee, C.H. Comparison of traditional and commercial vinegars based on metabolite profiling and antioxidant activity. J. Microbiol. Biotechnol. 2015, 25, 217–226. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeong, D.; Lee, Y.; Hahn, D.; Nam, J.O.; Lee, W.Y.; Hong, D.H.; Kim, S.R.; Ha, Y.S. Development and Metabolite Profiling of Elephant Garlic Vinegar. J. Microbiol. Biotechnol. 2018, 28, 50–58. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.; Xing, J.; Chao, J.; Qin, X.; Li, Z. Comparison of two types of vinegar with different aging times by NMR-based metabolomic approach. J. Food Biochem. 2019, 43, e12835. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Lim, H.-J.; Kim, M.-S.; Kim, D.S.; Hwang, C.E.; Nam, S.H.; Joo, O.S.; Lee, B.W.; Kim, J.K.; Shin, E.-C. Time course effects of fermentation on fatty acid and volatile compound profiles of Cheonggukjang using new soybean cultivars. J. Food Drug Anal. 2017, 25, 637–653. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, X.; Ji, K.; Guo, K.; Li, Y.; Wu, C.; Xu, G. Characterization of flavor compounds in Chinese liquor Moutai by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Anal. Chim. Acta 2007, 597, 340–348. [Google Scholar] [CrossRef]
- Aloys, N. Volatile compounds in Ikivunde and Inyange, two Burundian cassava products. Glob. Adv. Res. J. Food Sci. Technol. 2012, 1, 1–7. [Google Scholar]
- Herraiz, T.; Ough, C.S. Identification and determination of amino acid ethyl esters in wines by capillary gas chromatography-mass spectrometry. J. Agric. Food Chem. 1992, 40, 1015–1021. [Google Scholar] [CrossRef]
- Herraiz, T.; Ough, C.S. Formation of Ethyl Esters of Amino Acids by Yeasts during the Alcoholic Fermentation of Grape Juice. Am. J. Enol. Vitic. 1993, 44, 41–48. [Google Scholar]
- Ferreira, I.M.; Guido, L.F. Impact of Wort Amino Acids on Beer Flavour: A Review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Kang, Y.P.; Park, J.H.; Lee, J.; Kwon, S.W. Determination of biogenic amines in Bokbunja (Rubus coreanus Miq.) wines using a novel ultra-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry. Food Chem. 2012, 132, 1185–1190. [Google Scholar] [CrossRef]
- Song, N.-E.; Cho, H.-S.; Baik, S.-H. Bacteria isolated from Korean black raspberry vinegar with low biogenic amine production in wine. Braz. J. Microbiol. 2016, 47, 452–460. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, J.; Li, Q.; Luo, S.; Zhang, J.; Huang, M.; Chen, F.; Zheng, F.; Sun, X.; Li, H. Characterization of key aroma compounds in Meilanchun sesame flavor style baijiu by application of aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission/addition experiments. RSC Adv. 2018, 8, 23757–23767. [Google Scholar] [CrossRef]
- Montero-Calderón, M.; Rojas-Graü, M.A.; Martín-Belloso, O. Aroma Profile and Volatiles Odor Activity along Gold Cultivar Pineapple Flesh. J. Food Sci. 2010, 75, S506–S512. [Google Scholar] [CrossRef]
- Song, N.-E.; Jeong, D.-Y.; Baik, S.-H. Application of indigenous Saccharomyces cerevisiae to improve the black raspberry (Rubus coreanus Miquel) vinegar fermentation process and its microbiological and physicochemical analysis. Food Sci. Biotechnol. 2018, 28, 481–489. [Google Scholar] [CrossRef]
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 2012, 30, 289–297. [Google Scholar] [CrossRef]
- Rather, I.A.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K.; Bhat, E.A.; Lim, J.; Paek, W.K.; Park, Y.-H. Probiotic Lactobacillus sakei proBio-65 Extract Ameliorates the Severity of Imiquimod Induced Psoriasis-Like Skin Inflammation in a Mouse Model. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Carly, F.; Fickers, P. Erythritol production by yeasts: A snapshot of current knowledge. Yeast 2018, 35, 455–463. [Google Scholar] [CrossRef]
- Ye, S.; Park, H.; Kwak, Y.; Jeong, D.; Kim, S.R. Isolation of a New Yeast Strain Producing Erythritol. KSBB J. 2019, 34, 25–30. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid bacteria: Physiology and carbon sources oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kim, S.R. Metabolic Changes Induced by Deletion of Transcriptional Regulator GCR2 in Xylose-Fermenting Saccharomyces cerevisiae. Microorganisms 2020, 8, 1499. [Google Scholar] [CrossRef]
- Chung, N.; Jo, Y.; Joe, M.-H.; Jeong, M.-H.; Jeong, Y.-J.; Kwon, J.-H. Rice vinegars of different origins: Discriminative characteristics based on solid-phase microextraction and gas chromatography with mass spectrometry, an electronic nose, electronic tongue and sensory evaluation. J. Inst. Brew. 2017, 123, 159–166. [Google Scholar] [CrossRef]
- Cho, S.W.; Ko, H.J.; Park, T.H. Identification of a Lung Cancer Biomarker Using a Cancer Cell Line and Screening of Olfactory Receptors for Biomarker Detection. Biotechnol. Bioprocess Eng. 2021, 26, 55–62. [Google Scholar] [CrossRef]
- Yun, E.J.; Oh, E.J.; Liu, J.-J.; Yu, S.; Kim, D.H.; Kwak, S.; Kim, K.H.; Jin, Y.-S. Promiscuous activities of heterologous enzymes lead to unintended metabolic rerouting in Saccharomyces cerevisiae engineered to assimilate various sugars from renewable biomass. Biotechnol. Biofuels 2018, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, M.P.; Moxley, J.F.; Tong, L.V.; Walther, J.L.; Jensen, K.L.; Stephanopoulos, G.N. Systematic Identification of Conserved Metabolites in GC/MS Data for Metabolomics and Biomarker Discovery. Anal. Chem. 2007, 79, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.I.; Bhagabati, N.K.; Braisted, J.C.; Liang, W.; Sharov, V.; Howe, E.A.; Li, J.; Thiagarajan, M.; White, J.A.; Quackenbush, J. TM4 Microarray Software Suite. Methods Enzymol. 2006, 411, 134–193. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Budczies, J.; Weichert, W.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Niesporek, S.; Noske, A.; Buckendahl, A.; Dietel, M.; et al. Metabolite profiling of human colon carcinoma—Deregulation of TCA cycle and amino acid turnover. Mol. Cancer 2008, 7, 72. [Google Scholar] [CrossRef]

| Traditional Vinegars (*) | Geographical Origin in Korea | Raw Materials | Acetate (g/L) | Ethanol (g/L) | Glucose + Fructose (g/L) |
|---|---|---|---|---|---|
| TV1 (101) | Yeongdong, Chungbuk | Persimmon | 37.6 ± 0.2 | 13.7 ± 0.2 | 0 |
| TV2 (746) | Icheon, Gyeonggi | Persimmon | 62.4 ± 1.6 | 1.7 ± 0.1 | 0.3 ± 0.03 |
| TV3 (163) | Jinju, Gyeongnam | Persimmon | 50.3 ± 0.1 | 2.5 ± 0.1 | 0 |
| TV4 (553) | Wanju, Jeonbuk | Persimmon | 35.2 ± 0.1 | 13.1 ± 0.3 | 0 |
| TV5 (135) | Jeongeup, Jeonbuk | Persimmon | 41.1 ± 1.7 | 12.7 ± 0.7 | 46.5 ± 2.2 |
| TV6 (794) | Yeongcheon, Gyeongbuk | Brown rice | 42.8 ± 1.5 | 0.4 ± 0.5 | 30.7 ± 1.1 |
| TV7 (378) | Yecheon, Gyeongbuk | Multigrains | 53.4 ± 0.7 | 4.6 ± 0.1 | 2.2 ± 0.3 |
| Commercial Vinegars | Manufacturer | Raw Materials | Acetate (g/L) | Ethanol (g/L) | Glucose + Fructose (g/L) |
| CV1 | Ottogi | Apple | 64.5 ± 2.0 | 1.4 ± 0.03 | 11.3 ± 0.4 |
| CV2 | Ottogi | plum | 63.5 ± 1.2 | 1.4 ± 0.1 | 4.1 ± 0.01 |
| CV3 | Daesang | Apple | 51.6 ± 0.4 | 1.0 ± 0.04 | 8.8 ± 0.1 |
| CV4 | Ottogi | Brown rice | 65.9 ± 1.1 | 1.5 ± 0.1 | 3.1 ± 0.1 |
| Total Metabolites | Significantly Different Metabolites * | High in Traditional Vinegars a | High in Commercial Vinegars b | |
|---|---|---|---|---|
| Volatile | 102 | 5 | 4 (3) | 1 (1) |
| Nonvolatile | 78 | 15 | 14 (2) | 1 (0) |
| Total | 180 | 20 | 18 (5) | 2 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.; Kim, J.-W.; Gu, B.; Kim, S.; Kim, H.; Kim, W.-C.; Lee, M.-R.; Kim, S.-R. Comparative Metabolite Profiling of Traditional and Commercial Vinegars in Korea. Metabolites 2021, 11, 478. https://doi.org/10.3390/metabo11080478
Shin M, Kim J-W, Gu B, Kim S, Kim H, Kim W-C, Lee M-R, Kim S-R. Comparative Metabolite Profiling of Traditional and Commercial Vinegars in Korea. Metabolites. 2021; 11(8):478. https://doi.org/10.3390/metabo11080478
Chicago/Turabian StyleShin, Minhye, Jeong-Won Kim, Bonbin Gu, Sooah Kim, Hojin Kim, Won-Chan Kim, Mee-Ryung Lee, and Soo-Rin Kim. 2021. "Comparative Metabolite Profiling of Traditional and Commercial Vinegars in Korea" Metabolites 11, no. 8: 478. https://doi.org/10.3390/metabo11080478
APA StyleShin, M., Kim, J.-W., Gu, B., Kim, S., Kim, H., Kim, W.-C., Lee, M.-R., & Kim, S.-R. (2021). Comparative Metabolite Profiling of Traditional and Commercial Vinegars in Korea. Metabolites, 11(8), 478. https://doi.org/10.3390/metabo11080478