Facilitating Imaging Mass Spectrometry of Microbial Specialized Metabolites with METASPACE
Abstract
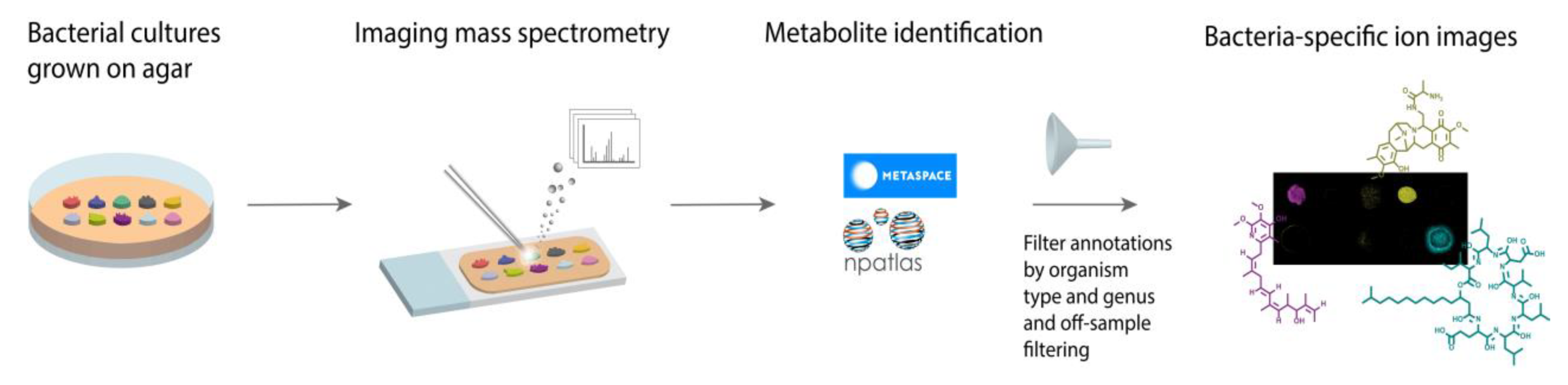
:1. Introduction
2. Results and Discussion
- (1)
- Lack of metabolite annotation due to inappropriate culture conditions. For example, the growth conditions used here may not be conducive to specific metabolite production [21]. In certain cases co-cultivation of multiple organisms may be required for metabolite elicitation [21,22]. Conversely, the presence of multiple organisms growing in close proximity may alter or even abolish metabolite production and therefore lead to lack of metabolite annotation by METASPACE. Or simply the microbes were not incubated for the correct duration to observe specific metabolite production. Insufficient culturing time may lead to metabolite analogs or incomplete metabolites altogether.
- (2)
- Lack of metabolite annotation due to technical MS aspects. Most importantly are sample preparation steps, specifically matrix application. Different matrices increase or decrease metabolite ionization. In order to maximize the number of metabolites observed, one would have to prepare multiple samples with multiple MALDI matrices and acquire data in both MS polarities. Even then, there could be metabolites that are produced by the microbes but these metabolites could be below instrument detection limits. In addition, potentially annotatable molecules that are present within NPA may not be observed if these molecules fall outside of the user defined MS settings. Less likely, but still theoretically plausible, are the events of uncommon adduct formation, which would not be taken into consideration by METASPACE. At the moment, METASPACE takes into account the most common MS adducts observed: +H, +Na, and +K, but METASPACE does have the ability to generate other adducts from chemical formulas such as [M]+ adducts, M+metal adducts, or M+adduct-neutral loss.
- (3)
- Lack of metabolite annotation due to METASPACE. The most important criteria for metabolite annotation is appropriate database selection in METASPACE. For example, if experimental data containing microbial samples were annotated against the more common databases on METASPACE, such as the Human Metabolome Database (HMDB), then the majority of the annotations would be false positives. Likewise if experimental data containing mammalian cell culture samples were annotated against microbial databases, such as NPA or PAMDB, the annotations here would similarly consist of false positives. Furthermore, database curation issues may also contribute to lack of annotation, for instance, if a bacteria is a known producer of a metabolite, but this entry has not been added to the database, then this annotation will be overlooked until the database is updated. Finally, if one were so inclined, a large database such as NPA could be filtered so that only metabolite entries of a single organism would be curated and then uploaded to METASPACE. This would be an extreme example of targeted imaging MS analysis.
3. Materials and Methods
3.1. Microbial Cultures of Actinomycets, Pseudomonads, and Bacillus
| Time (hours) | 0 | 48 | 72 |
| Action | Inoculate and incubate liquid cultures for:
| Inoculate and incubate agar cultures for:
| Inoculate and incubate liquid cultures for:
|
| Time (hours) | 120 | 144 | 168 |
| Action | Inoculate and incubate liquid cultures for:
| Inoculate and incubate agar cultures for:
| Photograph samples, cut out samples and transfer to glass slide, dry sample overnight |
3.2. Imaging MS of Microbial Cultures
3.3. Database Curation and Molecular Annotation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandrov, T. Spatial metabolomics and imaging mass spectrometry in the age of artificial intelligence. Annu. Rev. Biomed. Data Sci. 2020, 3, 61–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, J.; Ryan, K.S. Introducing the Parvome: Bioactive compounds in the microbial world. ACS Chem. Biol. 2011, 7, 252–259. [Google Scholar] [CrossRef]
- Davies, J. Specialized microbial metabolites: Functions and origins. J. Antibiot. 2013, 66, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Spraker, J.E.; Luu, G.T.; Sanchez, L.M. Imaging mass spectrometry for natural products discovery: A review of ionization methods. Nat. Prod. Rep. 2019, 37, 150–162. [Google Scholar] [CrossRef]
- Stasulli, N.; Shank, E.A. Profiling the metabolic signals involved in chemical communication between microbes using imaging mass spectrometry. FEMS Microbiol. Rev. 2016, 40, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Dunham, S.; Ellis, J.F.; Li, B.; Sweedler, J.V. Mass spectrometry imaging of complex microbial communities. Accounts Chem. Res. 2017, 50, 96–104. [Google Scholar] [CrossRef]
- Shank, E.A. Considering the lives of microbes in microbial communities. mSystems 2018, 3, e00155-17. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.D.; Melnik, A.V.; Koyama, N.; Lu, X.; Schorn, M.; Fang, J.; Aguinaldo, K.; Lincecum, T.L., Jr.; Ghequire, M.G.K.; Carrion, V.J.; et al. Indexing the Pseudomonas specialized metabolome enabled the discovery of poaeamide B and the bananamides. Nat. Microbiol. 2016, 2, 16197. [Google Scholar] [CrossRef] [Green Version]
- Farha, M.A.; Brown, E.D. Strategies for target identification of antimicrobial natural products. Nat. Prod. Rep. 2016, 33, 668–680. [Google Scholar] [CrossRef]
- Palmer, A.; Phapale, P.; Chernyavsky, I.; Lavigne, R.; Fay, D.; Tarasov, A.; Kovalev, V.; Fuchser, J.; Nikolenko, S.; Pineau, C.; et al. FDR-controlled metabolite annotation for high-resolution imaging mass spectrometry. Nat. Methods 2016, 14, 57–60. [Google Scholar] [CrossRef]
- Alexandrov, T.; Ovchinnikova, K.; Palmer, A.; Kovalev, V.; Tarasov, A.; Stuart, L.; Nigmetzianov, R.; Fay, D. A community-populated knowledge base of spatial metabolomes in health and disease. bioRxiv 2019, 539478. [Google Scholar] [CrossRef] [Green Version]
- Kooijman, P.C.; Nagornov, K.O.; Kozhinov, A.N.; Kilgour, D.P.A.; Tsybin, Y.; Heeren, R.M.A.; Ellis, S.R. Increased throughput and ultra-high mass resolution in DESI FT-ICR MS imaging through new-generation external data acquisition system and advanced data processing approaches. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajed, T.; Marcu, A.; Ramirez, M.; Pon, A.; Guo, A.C.; Knox, C.; Wilson, M.; Grant, J.R.; Djoumbou, Y.; Wishart, D.S. ECMDB 2.0: A richer resource for understanding the biochemistry of E. coli. Nucleic Acids Res. 2015, 44, D495–D501. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Brewer, L.K.; Jones, J.; Nguyen, A.T.; Marcu, A.; Wishart, D.S.; Oglesby-Sherrouse, A.G.; Kane, M.A.; Wilks, A. PAMDB: A comprehensive Pseudomonas aeruginosa metabolome database. Nucleic Acids Res. 2017, 46, D575–D580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzat-to-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Santen, J.A.; Jacob, G.; Singh, A.L.; Aniebok, V.; Balunas, M.J.; Bunsko, D.; Neto, F.C.; Castaño-Espriu, L.; Chang, C.; Clark, T.N.; et al. The natural products atlas: An open access knowledge base for microbial natural products discovery. ACS Cent. Sci. 2019, 5, 1824–1833. [Google Scholar] [CrossRef] [Green Version]
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef] [PubMed]
- Behie, S.W.; Bonet, B.; Zacharia, V.M.; McClung, D.J.; Traxler, M.F. Molecules to ecosystems: Actinomycete natural products in situ. Front. Microbiol. 2017, 7, 2149. [Google Scholar] [CrossRef] [Green Version]
- Pishchany, G.; Kolter, R. On the possible ecological roles of antimicrobials. Mol. Microbiol. 2020, 113, 580–587. [Google Scholar] [CrossRef]
- Ovchinnikova, K.; Kovalev, V.; Stuart, L.; Alexandrov, T. OffsampleAI: Artificial intelligence approach to recognize off-sample mass spectrometry images. BMC Bioinform. 2020, 21, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Ochi, K. Insights into microbial cryptic gene activation and strain improvement: Principle, application and technical aspects. J. Antibiot. 2017, 70, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Race, A.; Styles, I.; Bunch, J. Inclusive sharing of mass spectrometry imaging data requires a converter for all. J. Proteom. 2012, 75, 5111–5112. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, G.; Garrard, K.P.; Barry, J.; Muddiman, D.C. MSiReader: An open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J. Am. Soc. Mass Spectrom. 2013, 24, 718–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhart, M.; Nazari, M.; Garrard, K.P.; Muddiman, D.C. MSiReader v1.0: Evolving open-source mass spectrometry imaging software for targeted and untargeted analyses. J. Am. Soc. Mass Spectrom. 2018, 29, 8–16. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.D.; Saharuka, V.; Kovalev, V.; Stuart, L.; Del Prete, M.; Lubowiecka, K.; De Mot, R.; Venturi, V.; Alexandrov, T. Facilitating Imaging Mass Spectrometry of Microbial Specialized Metabolites with METASPACE. Metabolites 2021, 11, 477. https://doi.org/10.3390/metabo11080477
Nguyen DD, Saharuka V, Kovalev V, Stuart L, Del Prete M, Lubowiecka K, De Mot R, Venturi V, Alexandrov T. Facilitating Imaging Mass Spectrometry of Microbial Specialized Metabolites with METASPACE. Metabolites. 2021; 11(8):477. https://doi.org/10.3390/metabo11080477
Chicago/Turabian StyleNguyen, Don D., Veronika Saharuka, Vitaly Kovalev, Lachlan Stuart, Massimo Del Prete, Kinga Lubowiecka, René De Mot, Vittorio Venturi, and Theodore Alexandrov. 2021. "Facilitating Imaging Mass Spectrometry of Microbial Specialized Metabolites with METASPACE" Metabolites 11, no. 8: 477. https://doi.org/10.3390/metabo11080477
APA StyleNguyen, D. D., Saharuka, V., Kovalev, V., Stuart, L., Del Prete, M., Lubowiecka, K., De Mot, R., Venturi, V., & Alexandrov, T. (2021). Facilitating Imaging Mass Spectrometry of Microbial Specialized Metabolites with METASPACE. Metabolites, 11(8), 477. https://doi.org/10.3390/metabo11080477





