Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis
Abstract
1. Introduction
2. Thermogenic Adipose Tissue
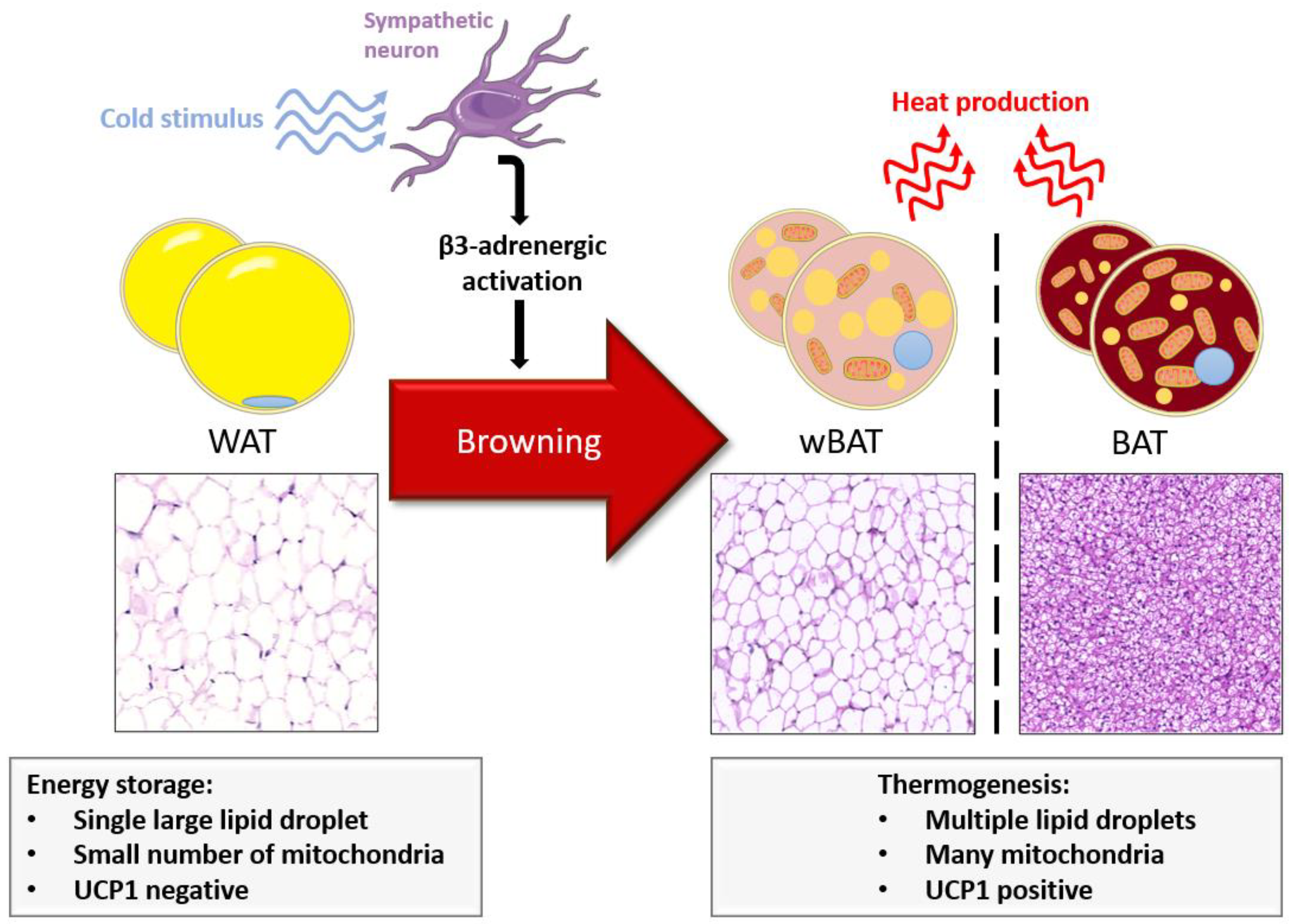
2.1. BAT and wBAT: Two Different Adipogenesis
2.2. Brown and Beige Cells Shared Gene Expression
| Adipogenic and Thermogenic Genes Expressed in BAT/wBAT | Related Protein | Key-Roles in BAT/wBAT Function | Ref. |
|---|---|---|---|
| Ucp1 | UCP1 |
| [12,40] |
| |||
| Ppar-γ | PPAR-γ |
| [24,25,29] |
| |||
| |||
| Ppar-α | PPAR-α |
| [40] |
| |||
| PGC1-α | PGC1-α |
| [30,31,35] |
| |||
| Foxc2 | FOXC2 |
| [32,33,35] |
| |||
| IRF4 | IRF4 |
| [39,41] |
| |||
| Prdm16 | PRDM16 |
| [24,26,32] |
| |||
| |||
| RB1 | pRb |
| [23,35,38] |
| |||
| |||
| Fgf21 | FGF21 |
| [42,43,44,45] |
| |||
| NFIA | NFIA |
| [36] |
| |||
| CEBP | CEBP |
| [48,49] |
| |||
| Elovl6 | ELOVL6 |
| [50] |
| |||
| Elovl3 | ELOVL3 |
| [50] |
| |||
| CIDEA | CIDEA |
| [46,47] |
| |||
| |||
| PRLR | PRLR |
| [27] |
| |||
| |||
| |||
| ADRB3 | β3-adrenergic receptor |
| [22,33,40,51,52,53] |
| DIO2 | DIO2 |
| [54,55] |
2.3. BAT and wBAT in Pathophysiological Conditions
2.4. Research on AT Browning
3. Atherosclerosis
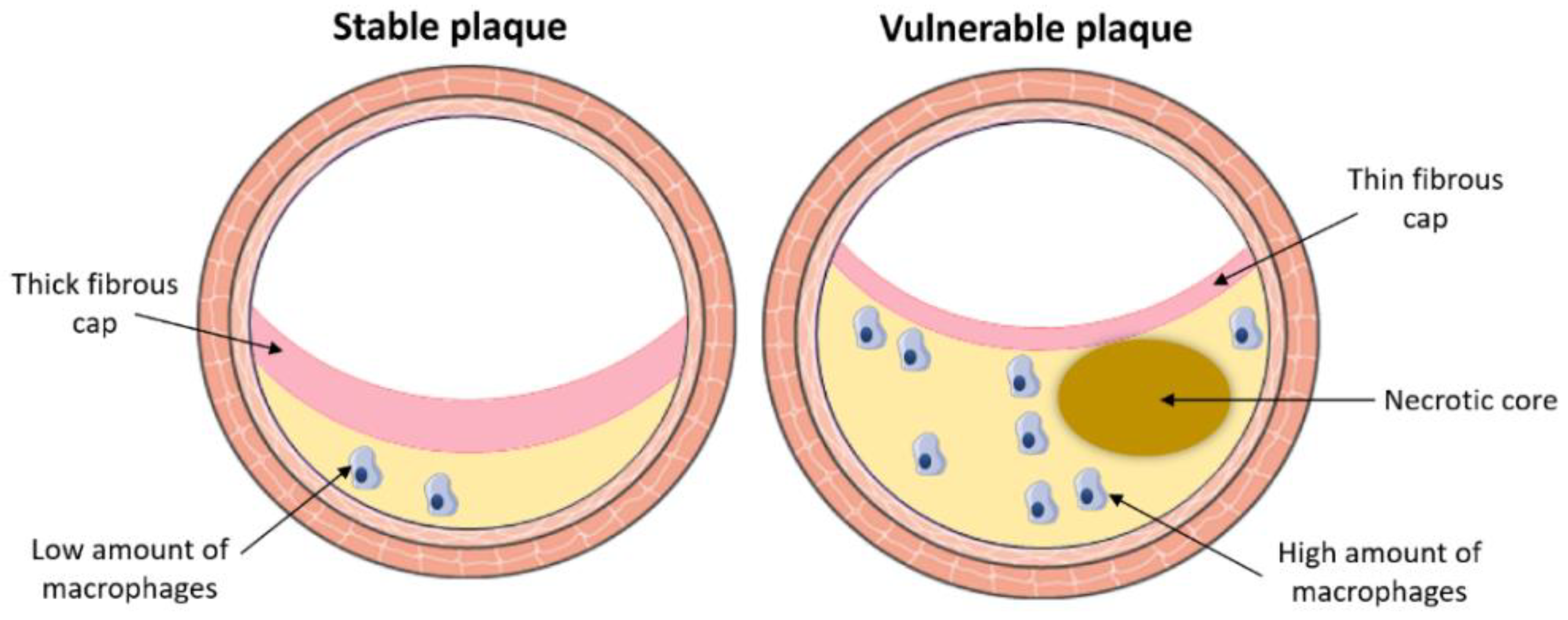
3.1. Pathological Process
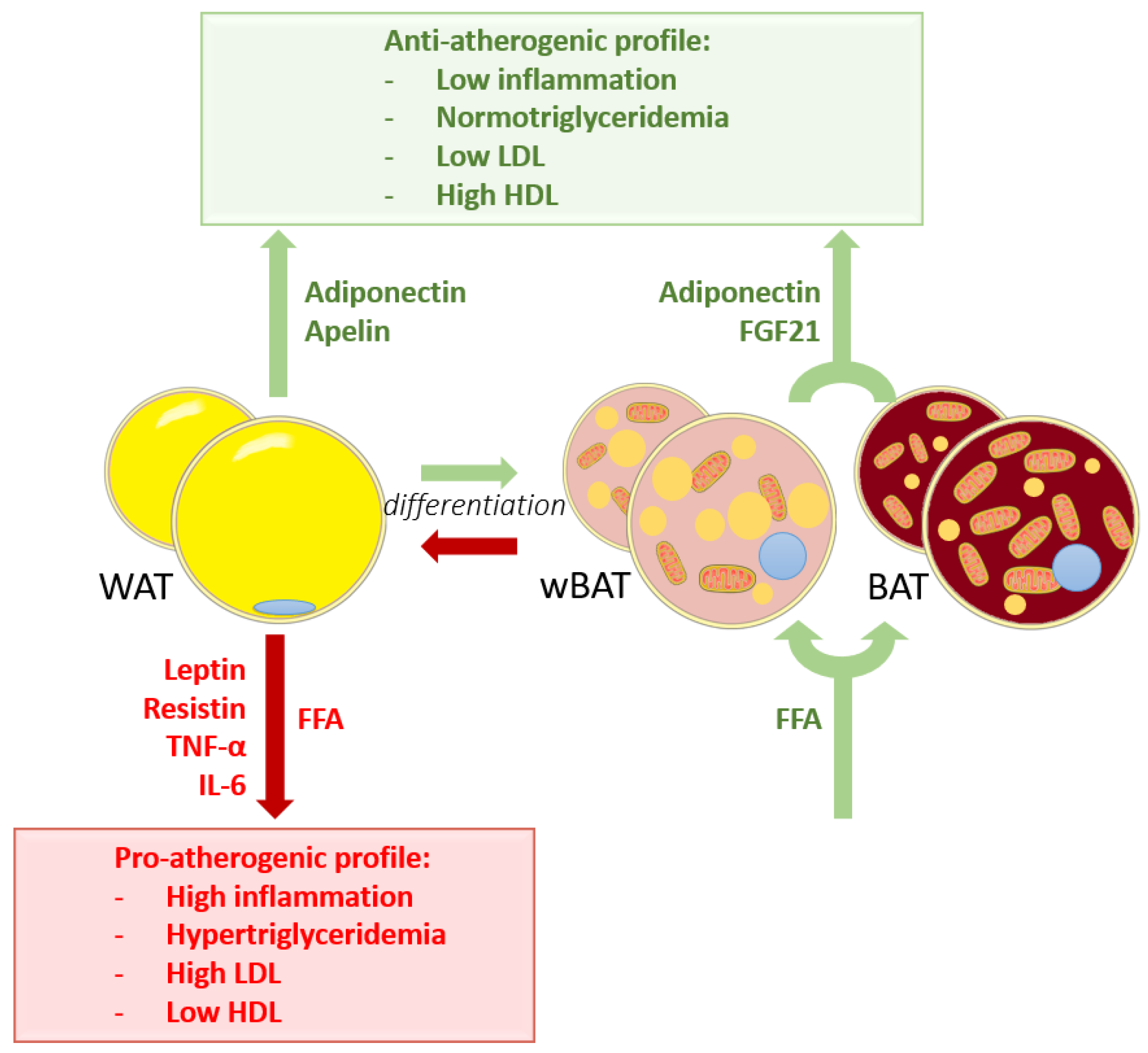
3.2. WAT Worsens Atherosclerosis
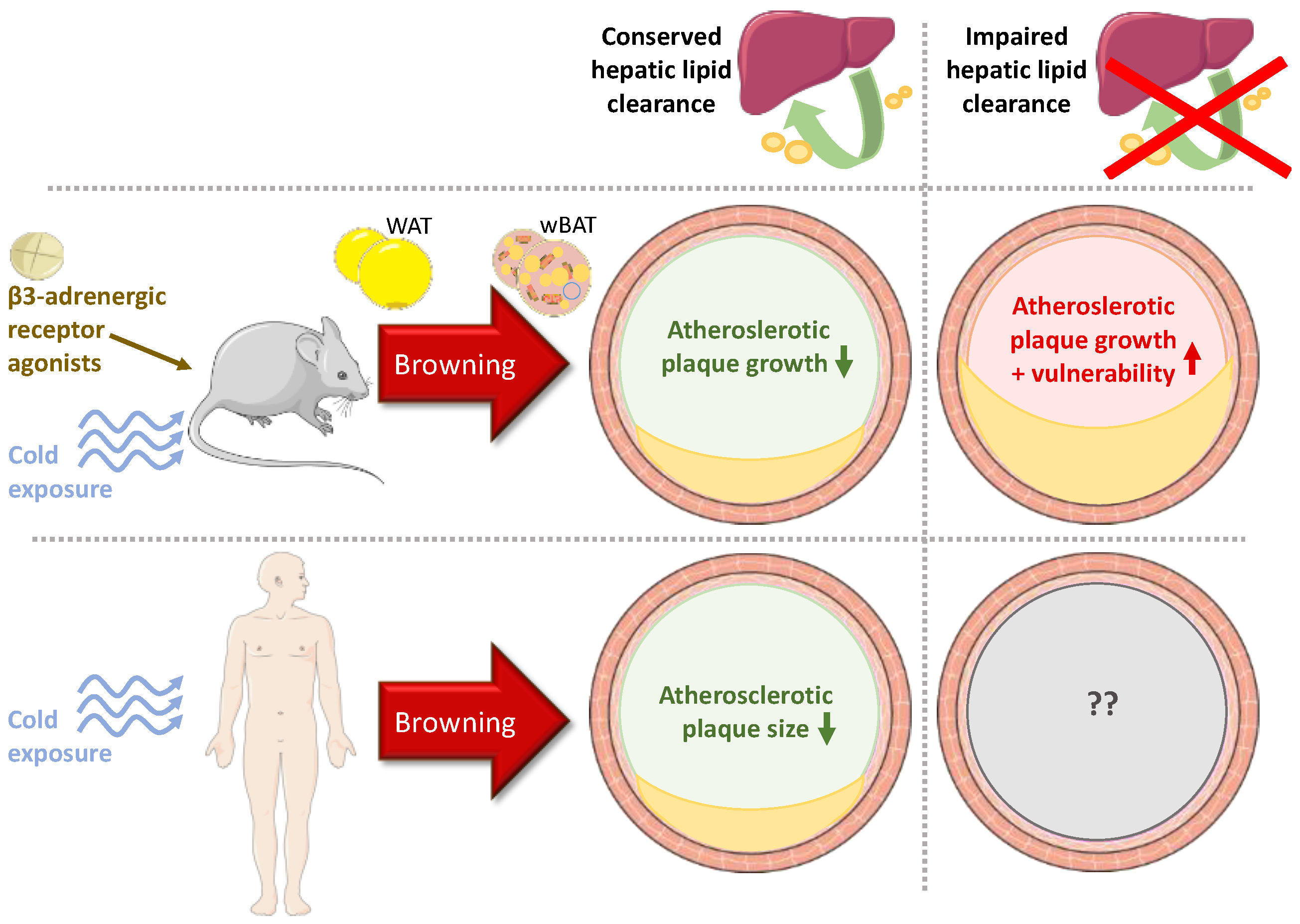
3.3. Influence of BAT on Atherosclerosis
3.4. Influence of wBAT on Atherosclerosis
4. Discussion and Conclusions
4.1. Experimental Studies
4.2. Clinical Studies
4.3. Browning vs. Established Atheroprotective Therapies
Funding
Conflicts of Interest
References
- Ottaviani, E.; Malagoli, D.; Franceschi, C. The evolution of the adipose tissue: A neglected enigma. Gen. Comp. Endocrinol. 2011, 174, 1–4. [Google Scholar] [CrossRef]
- Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moustaid-Moussa, N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J. Nutr. 2000, 130, 3110S–3115S. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Ruiz-Fernández, C.; Pino, J.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Lago, F.; Mobasheri, A.; Gualillo, O. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 2019, 165, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Mattu, H.S.; Randeva, H.S. Role of adipokines in cardiovascular disease. J. Endocrinol. 2013, 216, T17–T36. [Google Scholar] [CrossRef]
- Yiannikouris, F.; Gupte, M.; Putnam, K.; Cassis, L. Adipokines and blood pressure control. Curr. Opin. Nephrol. Hypertens. 2010, 19, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Potter, V.J. Inflammation and macrophage modulation in adipose tissues. Cell. Microbiol. 2014, 16, 1484–1492. [Google Scholar] [CrossRef]
- Cinti, S. Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. Metab. 2009, 297, E977–E986. [Google Scholar] [CrossRef]
- Enerbäck, S. Human Brown Adipose Tissue. Cell Metab. 2010, 11, 248–252. [Google Scholar] [CrossRef]
- Wehrli, N.E.; Bural, G.; Houseni, M.; Alkhawaldeh, K.; Alavi, A.; Torigian, D.A. Determination of Age-Related Changes in Structure and Function of Skin, Adipose Tissue, and Skeletal Muscle With Computed Tomography, Magnetic Resonance Imaging, and Positron Emission Tomography. Semin. Nucl. Med. 2007, 37, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Kahn, C.R. Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 143–149. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kameya, T.; Nakada, K.; Kawai, Y.; Saito, M. Brown Adipose Tissue, Whole-Body Energy Expenditure, and Thermogenesis in Healthy Adult Men. Obesity 2011, 19, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A.; Murano, I.; Zingaretti, M.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef]
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human adipose beiging in response to cold and mirabegron. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Orava, J.; Nuutila, P.; Lidell, M.E.; Oikonen, V.; Noponen, T.; Viljanen, T.; Scheinin, M.; Taittonen, M.; Niemi, T.; Enerbäck, S.; et al. Different Metabolic Responses of Human Brown Adipose Tissue to Activation by Cold and Insulin. Cell Metab. 2011, 14, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Wennmalm, K.; Larsson, O.; Walden, T.B.; Lassmann, T.; Petrovic, N.; Hamilton, D.L.; Gimeno, R.E.; Wahlestedt, C.; Baar, K.; et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA 2007, 104, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nat. Cell Biol. 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef]
- Auffret, J.; Viengchareun, S.; Carré, N.; Denis, R.G.P.; Magnan, C.; Marie, P.; Muscat, A.; Fève, B.; Lombès, M.; Binart, N. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J. 2012, 26, 3728–3737. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Carobbio, S.; Vidal-Puig, A. Adipose tissue plasticity: How fat depots respond differently to pathophysiological cues. Diabetologia 2016, 59, 1075–1088. [Google Scholar] [CrossRef]
- Barberá, M.J.; Schlüter, A.; Pedraza, N.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome Proliferator-activated Receptor α Activates Transcription of the Brown Fat Uncoupling Protein-1 Gene. J. Biol. Chem. 2001, 276, 1486–1493. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Cederberg, A.; Grønning, L.M.; Ahrén, B.; Taskén, K.; Carlsson, P.; Enerbäck, S. FOXC2 Is a Winged Helix Gene that Counteracts Obesity, Hypertriglyceridemia, and Diet-Induced Insulin Resistance. Cell 2001, 106, 563–573. [Google Scholar] [CrossRef]
- Lidell, M.E.; Seifert, E.L.; Westergren, R.; Heglind, M.; Gowing, A.; Sukonina, V.; Arani, Z.; Itkonen, P.; Wallin, S.; Westberg, F.; et al. The Adipocyte-Expressed Forkhead Transcription Factor Foxc2 Regulates Metabolism through Altered Mitochondrial Function. Diabetes 2011, 60, 427–435. [Google Scholar] [CrossRef]
- Wu, Z.; Boss, O. Targeting PGC-1α to control energy homeostasis. Expert Opin. Ther. Targets 2007, 11, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Tiraby, C.; Tavernier, G.; Lefort, C.; Larrouy, D.; Bouillaud, F.; Ricquier, D.; Langin, D. Acquirement of Brown Fat Cell Features by Human White Adipocytes. J. Biol. Chem. 2003, 278, 33370–33376. [Google Scholar] [CrossRef]
- Hansen, J.B.; Jorgensen, C.; Petersen, R.K.; Hallenborg, P.; De Matteis, R.; Boye, H.A.; Petrovic, N.; Enerback, S.; Nedergaard, J.; Cinti, S.; et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 4112–4117. [Google Scholar] [CrossRef]
- Hiraike, Y.; Waki, H.; Yuta, H.; Nakamura, M.; Miyake, K.; Nagano, G.; Nakaki, R.; Suzuki, K.; Kobayashi, H.; Yamamoto, S.; et al. NFIA co-localizes with PPARγ and transcriptionally controls the brown fat gene program. Nat. Cell Biol. 2017, 19, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Calo, E.; Quintero-Estades, J.A.; Danielian, P.S.; Nedelcu, S.; Berman, S.D.; Lees, J.A. Rb regulates fate choice and lineage commitment in vivo. Nat. Cell Biol. 2010, 466, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Banks, A.; Liu, T.; Kazak, L.; Rao, R.R.; Cohen, P.; Wang, X.; Yu, S.; Lo, J.C.; Tseng, Y.-H.; et al. IRF4 Is a Key Thermogenic Transcriptional Partner of PGC-1α. Cell 2014, 158, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Coulter, A.; Rim, J.S.; Koza, R.A.; Kozak, L.P. Transcriptional Synergy and the Regulation of Ucp1 during Brown Adipocyte Induction in White Fat Depots. Mol. Cell. Biol. 2005, 25, 8311–8322. [Google Scholar] [CrossRef]
- Eguchi, J.; Wang, X.; Yu, S.; Kershaw, E.E.; Chiu, P.C.; Dushay, J.; Estall, J.L.; Klein, U.; Maratos-Flier, E.; Rosen, E.D. Transcriptional Control of Adipose Lipid Handling by IRF4. Cell Metab. 2011, 13, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, P.; Sánchez, C.H.; Escalona-Garrido, C.; Pereira, L.; Contreras, C.; López, M.; Balsinde, J.; de Pablo, F.; Valverde, Á.M. Increased FGF21 in brown adipose tissue of tyrosine hydroxylase heterozygous mice: Implications for cold adaptation. J. Lipid Res. 2018, 59, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1 and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.J.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; Van Der Lans, A.A.; Cheng, C.C.; Adams, A.C.; Lichtenbelt, W.D.V.M.; Schrauwen, P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015, 5, 10275. [Google Scholar] [CrossRef] [PubMed]
- Jash, S.; Banerjee, S.; Lee, M.-J.; Farmer, S.R.; Puri, V. CIDEA Transcriptionally Regulates UCP1 for Britening and Thermogenesis in Human Fat Cells. iScience 2019, 20, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Patil, M.; Satyanarayana, A. Negative Regulators of Brown Adipose Tissue (BAT)-Mediated Thermogenesis. J. Cell. Physiol. 2014, 229, 1901–1907. [Google Scholar] [CrossRef]
- Park, J.H.; Ehur, W.; Lee, S.B. Intricate Transcriptional Networks of Classical Brown and Beige Fat Cells. Front. Endocrinol. 2015, 6, 124. [Google Scholar] [CrossRef]
- Steger, D.J.; Grant, G.R.; Schupp, M.; Tomaru, T.; Lefterova, M.I.; Schug, J.; Manduchi, E.; Stoeckert, C.J.; Lazar, M.A. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev. 2010, 24, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Virtue, S.; Bidault, G.; Dale, M.; Hagen, R.; Griffin, J.L.; Vidal-Puig, A. Brown Adipose Tissue Thermogenic Capacity Is Regulated by Elovl6. Cell Rep. 2015, 13, 2039–2047. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Jing, J.; Cui, X.; Shi, H.; Xue, B. Sympathetic nerve innervation is required for beigeing in white fat. Physiol. Rep. 2019, 7, e14031. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.-H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Kranz, M.; Klöting, N.; Kunath, A.; Steinhoff, K.; Rijntjes, E.; Köhrle, J.; Zeisig, V.; Hankir, M.; Gebhardt, C.; et al. Thyroid hormone status defines brown adipose tissue activity and browning of white adipose tissues in mice. Sci. Rep. 2016, 6, 38124. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Lichtenbelt, W.V.M.; Richard, D. Brown and beige adipose tissues: Phenotype and metabolic potential in mice and men. J. Appl. Physiol. 2018, 124, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Labbé, S.M.; Ecaron, A.; Elanfray, D.; Emonge-Roffarello, B.; Bartness, T.J.; Erichard, D. Hypothalamic control of brown adipose tissue thermogenesis. Front. Syst. Neurosci. 2015, 9, 150. [Google Scholar] [CrossRef]
- Bartness, T.J.; Ryu, V. Neural control of white, beige and brown adipocytes. Int. J. Obes. Suppl. 2015, 5, S35–S39. [Google Scholar] [CrossRef]
- Soeder, K.J.; Snedden, S.K.; Cao, W.; Della Rocca, G.J.; Daniel, K.W.; Luttrell, L.M.; Collins, S. The β3-Adrenergic Receptor Activates Mitogen-activated Protein Kinase in Adipocytes through a Gi-dependent Mechanism. J. Biol. Chem. 1999, 274, 12017–12022. [Google Scholar] [CrossRef]
- Cohade, C.; Mourtzikos, K.A.; Wahl, R.L. “USA-Fat”: Prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J. Nucl. Med. 2003, 44, 1267–1270. [Google Scholar]
- Wang, Q.; Zhang, M.; Xu, M.; Gu, W.; Xi, Y.; Qi, L.; Li, B.; Wang, W. Brown Adipose Tissue Activation Is Inversely Related to Central Obesity and Metabolic Parameters in Adult Human. PLoS ONE 2015, 10, e0123795. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.P.V.; Hernández-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Park, A.; Oh, K.-J.; Kim, W.K.; Bae, K.-H. The Role of Adipose Tissue Mitochondria: Regulation of Mitochondrial Function for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4924. [Google Scholar] [CrossRef]
- De Lorenzo, F.; Mukherjee, M.; Kadziola, Z.; Sherwood, R.; Kakkar, V.V. Central cooling effects in patients with hypercholesterolaemia. Clin. Sci. 1998, 95, 213–217. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Kim, J.I.; Jeon, Y.G.; Park, J.; Kim, J.B. Effects of Three Thiazolidinediones on Metabolic Regulation and Cold-Induced Thermogenesis. Mol. Cells 2018, 41, 900–908. [Google Scholar] [PubMed]
- Vegiopoulos, A.; Müller-Decker, K.; Strzoda, D.; Schmitt, I.; Chichelnitskiy, E.; Ostertag, A.; Diaz, M.B.; Rozman, J.; De Angelis, M.H.; Nüsing, R.M.; et al. Cyclooxygenase-2 Controls Energy Homeostasis in Mice by de Novo Recruitment of Brown Adipocytes. Science 2010, 328, 1158–1161. [Google Scholar] [CrossRef]
- Lundholm, K.; Daneryd, P.; Körner, U.; Hyltander, A.; Bosaeus, I. Evidence that long-term COX-treatment improves energy homeostasis and body composition in cancer patients with progressive cachexia. Int. J. Oncol. 2004, 24, 505–512. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nat. Cell Biol. 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in Exercise Training- and Cold-Induced UCP1 Expression in Subcutaneous White Adipose Tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Schéele, C.; Pedersen, B.K. Exercise and browning of white adipose tissue—A translational perspective. Curr. Opin. Pharmacol. 2020, 52, 18–24. [Google Scholar] [CrossRef]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to Brown Fat Phenotypic Switch Induced by Genetic and Environmental Activation of a Hypothalamic-Adipocyte Axis. Cell Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.-Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A Novel Role for Subcutaneous Adipose Tissue in Exercise-Induced Improvements in Glucose Homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Shirkhani, S.; Marandi, S.M.; Kazeminasab, F.; Esmaeili, M.; Ghaedi, K.; Esfarjani, F.; Shiralian-Esfahani, H.; Nasr-Esfahani, M.H. Comparative studies on the effects of high-fat diet, endurance training and obesity on Ucp1 expression in male C57BL/6 mice. Gene 2018, 676, 16–21. [Google Scholar] [CrossRef] [PubMed]
- McKie, G.L.; Medak, K.D.; Knuth, C.M.; Shamshoum, H.; Townsend, L.K.; Peppler, W.T.; Wright, D.C. Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice. J. Physiol. 2019, 597, 4581–4600. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.F.; Civelek, M.; Fang, Y.; Fleming, I. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc. Res. 2013, 99, 315–327. [Google Scholar] [CrossRef]
- Warboys, C.M.; Amini, N.; De Luca, A.; Evans, P.C. The role of blood flow in determining the sites of atherosclerotic plaques. F1000 Med. Rep. 2011, 3, 5. [Google Scholar] [CrossRef]
- Moore, K.J.; Tabas, I. Macrophages in the Pathogenesis of Atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Bentzon, J.F.; Daemen, M.; Falk, E.; Garcia-Garcia, H.M.; Herrmann, J.; Hoefer, I.; Jauhiainen, S.; Jukema, J.W.; Krams, R.; et al. Stabilization of atherosclerotic plaques: An update. Eur. Hear. J. 2013, 34, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Rudaz, A.; Rima, A.; Humair, J.P. Cardiovascular risk scores: Why, how and when to use them? Rev. Med. Suisse 2010, 6, 1809-12–1814-5. [Google Scholar] [PubMed]
- Rudaz, A.; Rima, A.; Humair, J.P. Scores de risque cardiovasculaire: Pourquoi, comment et quand les utiliser? Rev. Med. Suisse 2010, 6, 1809–1815. [Google Scholar]
- van Dam, A.D.; Boon, M.R.; Berbée, J.F.; Rensen, P.C.; van Harmelen, V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur. J. Pharmacol. 2017, 816, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Van Harmelen, V.; Lönnqvist, F.; Thörne, A.; Wennlund, A.; Large, V.; Reynisdottir, S.; Arner, P. Noradrenaline-induced lipolysis in isolated mesenteric, omental and subcutaneous adipocytes from obese subjects. Int. J. Obes. 1997, 21, 972–979. [Google Scholar] [CrossRef][Green Version]
- Ouwens, D.M.; Sell, H.; Greulich, S.; Eckel, J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 2010, 14, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Yudkin, J.S.; Eringa, E.; Stehouwer, C.D.A. “Vasocrine” signalling from perivascular fat: A mechanism linking insulin resistance to vascular disease. Lancet 2005, 365, 1817–1820. [Google Scholar] [CrossRef]
- Morel, S.; Kwak, B.; Rohner-Jeanrenaud, F.; Steffens, S.; Molica, F. Adipokines at the crossroad between obesity and cardiovascular disease. Thromb. Haemost. 2015, 113, 553–566. [Google Scholar] [CrossRef]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef]
- Otero, M.; Lago, R.; Gomez, R.; Dieguez, C.; Lago, F.; Goómez-Reino, J.; Gualillo, O. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology 2006, 45, 944–950. [Google Scholar] [CrossRef]
- Braga, V.; Medeiros, I.; Ribeiro, T.; França-Silva, M.; Botelho-Ono, M.; Guimarães, D. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: Implications in neurogenic hypertension. Braz. J. Med. Biol. Res. 2011, 44, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Ali, H.; El-Mottaleb, N.A.; Hamed, H.B.; Abd-Elsayed, A. Serum adiponectin and leptin as predictors of the presence and degree of coronary atherosclerosis. Coron. Artery Dis. 2011, 22, 264–269. [Google Scholar] [CrossRef]
- Beltowski, J. Leptin and atherosclerosis. Atherosclerosis 2006, 189, 47–60. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Chao, Y.-W.; Tsai, Y.-L.; Lien, C.-C.; Chang, C.-F.; Deng, M.-C.; Ho, L.-T.; Kwok, C.F.; Juan, C.-C. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J. Cell. Physiol. 2011, 226, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin Is an Inflammatory Marker of Atherosclerosis in Humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel Modulator for Endothelial Adhesion Molecules. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, J.; Tang, S.; Rye, K.-A.; Ong, K.L. The role of fibroblast growth factor 21 in atherosclerosis. Atherosclerosis 2017, 257, 259–265. [Google Scholar] [CrossRef]
- Shimada, K.; Miyazaki, T.; Daida, H. Adiponectin and atherosclerotic disease. Clin. Chim. Acta 2004, 344, 1–12. [Google Scholar] [CrossRef]
- Zhong, J.-C.; Zhang, Z.-Z.; Wang, W.; McKinnie, S.M.; Vederas, J.C.; Oudit, G.Y. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta (BBA)–Mol. Basis Dis. 2017, 1863, 1942–1950. [Google Scholar] [CrossRef]
- Weir, R.A.; Chong, K.S.; Dalzell, J.R.; Petrie, C.J.; Murphy, C.A.; Steedman, T.; Mark, P.B.; McDonagh, T.A.; Dargie, H.J.; McMurray, J.J. Plasma apelin concentration is depressed following acute myocardial infarction in man. Eur. J. Hear. Fail. 2009, 11, 551–558. [Google Scholar] [CrossRef]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Hear. J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Frishman, W.H. Inflammation and Atherosclerosis. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Finelli, C.; Sommella, L.; Gioia, S.; La Sala, N.; Tarantino, G. Should visceral fat be reduced to increase longevity? Ageing Res. Rev. 2013, 12, 996–1004. [Google Scholar] [CrossRef]
- Berbée, J.F.P.; Boon, M.R.; Khedoe, P.P.S.J.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef]
- Dong, M.; Yang, X.; Lim, S.; Cao, Z.; Honek, J.; Lu, H.; Zhang, C.; Seki, T.; Hosaka, K.; Wahlberg, E.; et al. Cold Exposure Promotes Atherosclerotic Plaque Growth and Instability via UCP1-Dependent Lipolysis. Cell Metab. 2013, 18, 118–129. [Google Scholar] [CrossRef]
- Sui, W.; Li, H.; Yang, Y.; Jing, X.; Xue, F.; Cheng, J.; Dong, M.; Zhang, M.; Pan, H.; Chen, Y.; et al. Bladder drug mirabegron exacerbates atherosclerosis through activation of brown fat-mediated lipolysis. Proc. Natl. Acad. Sci. USA 2019, 116, 10937–10942. [Google Scholar] [CrossRef]
- Bartelt, A.; John, C.; Schaltenberg, N.; Berbée, J.F.P.; Worthmann, A.; Cherradi, M.L.; Schlein, C.; Piepenburg, J.; Boon, M.R.; Rinninger, F.; et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 2017, 8, 15010. [Google Scholar] [CrossRef]
- Wang, G.-X.; Zhao, X.-Y.; Lin, J.D. The brown fat secretome: Metabolic functions beyond thermogenesis. Trends Endocrinol. Metab. 2015, 26, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. Novel Adipose Tissue Targets to Prevent and Treat Atherosclerosis. Organotypic Models Drug Dev. 2020, 1–22. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.-S.; Lindberg, R.A.; et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes 2008, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Kortelainen, M.L. Association between cardiac pathology and fat tissue distribution in an autopsy series of men without premortem evidence of cardiovascular disease. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1996, 20, 245–252. [Google Scholar]
- Takx, R.; Ishai, A.; Truong, Q.A.; MacNabb, M.H.; Scherrer-Crosbie, M.; Tawakol, A. Supraclavicular Brown adipose tissue FDG uptake and cardiovascular disease. J. Nucl. Med. 2016, 57, 1221–1225. [Google Scholar] [CrossRef]
- Raiko, J.; Orava, J.; Savisto, N.; Virtanen, K.A. High Brown Fat Activity Correlates With Cardiovascular Risk Factor Levels Cross-Sectionally and Subclinical Atherosclerosis at 5-Year Follow-Up. Arter. Thromb. Vasc. Biol. 2020, 40, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Holewijn, S.; Heijer, M.D.; Stalenhoef, A.F.H.; De Graaf, J. Non-invasive measurements of atherosclerosis (NIMA): Current evidence and future perspectives. Neth. J. Med. 2010, 68, 388–399. [Google Scholar] [PubMed]
- Labbé, S.M.; Caron, A.; Festuccia, W.T.; LeComte, R.; Richard, D. Interscapular brown adipose tissue denervation does not promote the oxidative activity of inguinal white adipose tissue in male mice. Am. J. Physiol. Metab. 2018, 315, E815–E824. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Feng, F.; Wu, T.; Luo, D.; Hu, C.; Sun, C. Foxc2 coordinates inflammation and browning of white adipose by leptin-STAT3-PRDM16 signal in mice. Int. J. Obes. 2018, 42, 252–259. [Google Scholar] [CrossRef]
- Worthmann, A.; John, C.; Rühlemann, M.C.; Baguhl, M.; Heinsen, F.-A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Alonso, R.; De Isla, L.P.; Muñiz-Grijalvo, O.; Diaz-Diaz, J.L.; Mata, P.; Foundation, M.S.F.H. Familial Hypercholesterolaemia Diagnosis and Management. Eur. Cardiol. Rev. 2018, 13, 14–20. [Google Scholar] [CrossRef]
- Ouellet, V.; Labbé, S.M.; Blondin, D.P.; Phoenix, S.; Guérin, B.; Haman, F.; Turcotte, E.E.; Richard, D.; Carpentier, A.C. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Investig. 2012, 122, 545–552. [Google Scholar] [CrossRef]
- Blondin, D.P.; Labbé, S.M.; Noll, C.; Kunach, M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Haman, F.; Richard, D.; Carpentier, A.C. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects With Type 2 Diabetes. Diabetes 2015, 64, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Labbé, S.M.; Phoenix, S.; Guérin, B.; Turcotte, É.E.; Richard, D.; Carpentier, A.C.; Haman, F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J. Physiol. 2015, 593, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Blanchard, P.-G.; Mathieu, P.; Deshaies, Y.; Richard, D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int. J. Cardiol. 2013, 167, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.-S.; et al. Hyperglycemia Promotes Myelopoiesis and Impairs the Resolution of Atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, A.; Schlein, C.; Berbée, J.F.P.; Rensen, P.C.N.; Heeren, J.; Bartelt, A. Effects of Pharmacological Thermogenic Adipocyte Activation on Metabolism and Atherosclerotic Plaque Regression. Nutrients 2019, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, R.Y.; Gao, R.; Mi, Q.; Dai, Q.; Zhu, F.; Xiao, J. Physical Exercise Is a Potential “Medicine” for Atherosclerosis. Adv. Exp. Med. Biol. 2017, 999, 269–286. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Brosteaux, C.; Ruiz, J.; Buclin, T.; Kuntzer, T.; Rodondi, N. Statins and muscular side-effects. Rev. Med. Suisse 2010, 6, 510–512. [Google Scholar]
- Zhou, E.; Li, Z.; Nakashima, H.; Choukoud, A.; Kooijman, S.; Berbée, J.F.; Rensen, P.C.; Wang, Y. Beneficial effects of brown fat activation on top of PCSK9 inhibition with alirocumab on dyslipidemia and atherosclerosis development in APOE*3-Leiden.CETP mice. Pharmacol. Res. 2021, 167, 105524. [Google Scholar] [CrossRef]
- Porter, C.; Chondronikola, M.; Sidossis, L.S. The Therapeutic Potential of Brown Adipocytes in Humans. Front. Endocrinol. 2015, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Verdejo, R.; Marlatt, K.L.; Ravussin, E.; Galgani, J.E. Contribution of brown adipose tissue to human energy metabolism. Mol. Asp. Med. 2019, 68, 82–89. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, C.L.; Molica, F.; Kwak, B.R. Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis. Metabolites 2021, 11, 319. https://doi.org/10.3390/metabo11050319
Roth CL, Molica F, Kwak BR. Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis. Metabolites. 2021; 11(5):319. https://doi.org/10.3390/metabo11050319
Chicago/Turabian StyleRoth, Christel L., Filippo Molica, and Brenda R. Kwak. 2021. "Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis" Metabolites 11, no. 5: 319. https://doi.org/10.3390/metabo11050319
APA StyleRoth, C. L., Molica, F., & Kwak, B. R. (2021). Browning of White Adipose Tissue as a Therapeutic Tool in the Fight against Atherosclerosis. Metabolites, 11(5), 319. https://doi.org/10.3390/metabo11050319






