Effect of Aloysia citrodora Essential Oil on Biochemicals, Antioxidant Characteristics, and Shelf Life of Strawberry Fruit during Storage
Abstract
1. Introduction
2. Results and Discussion
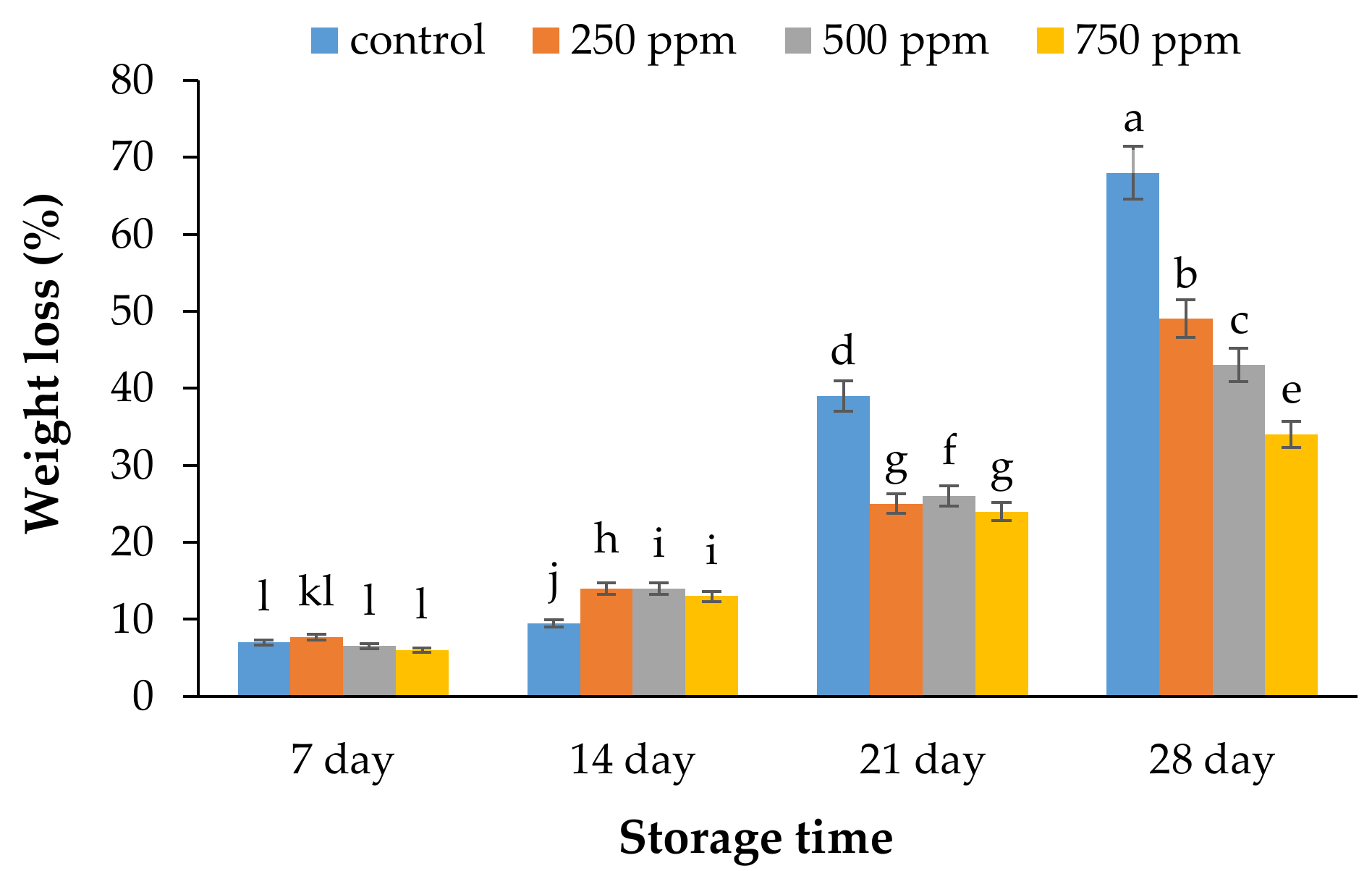
2.1. Weight Loss
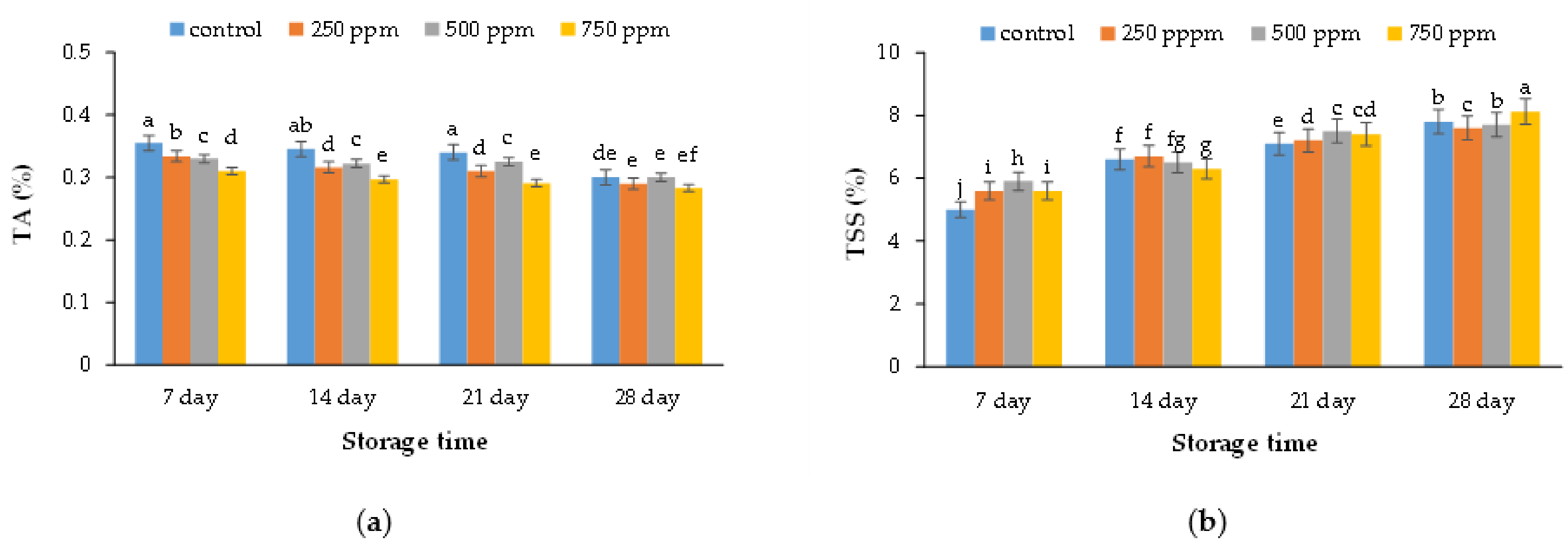
2.2. Chemical Characteristics (Titratable Acidity and Total Soluble Solid)
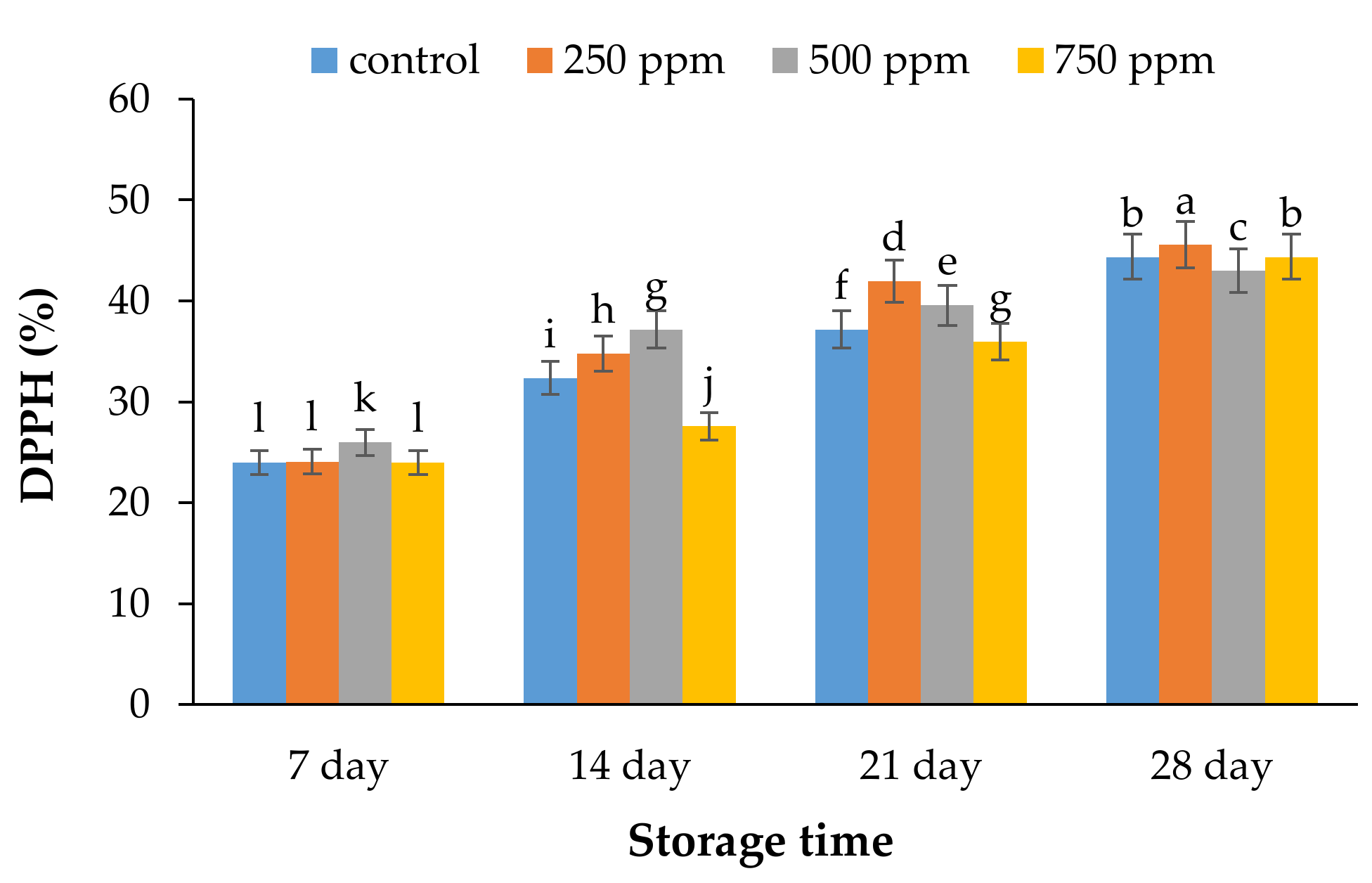
2.3. Antioxidant Activity by DPPH Assay
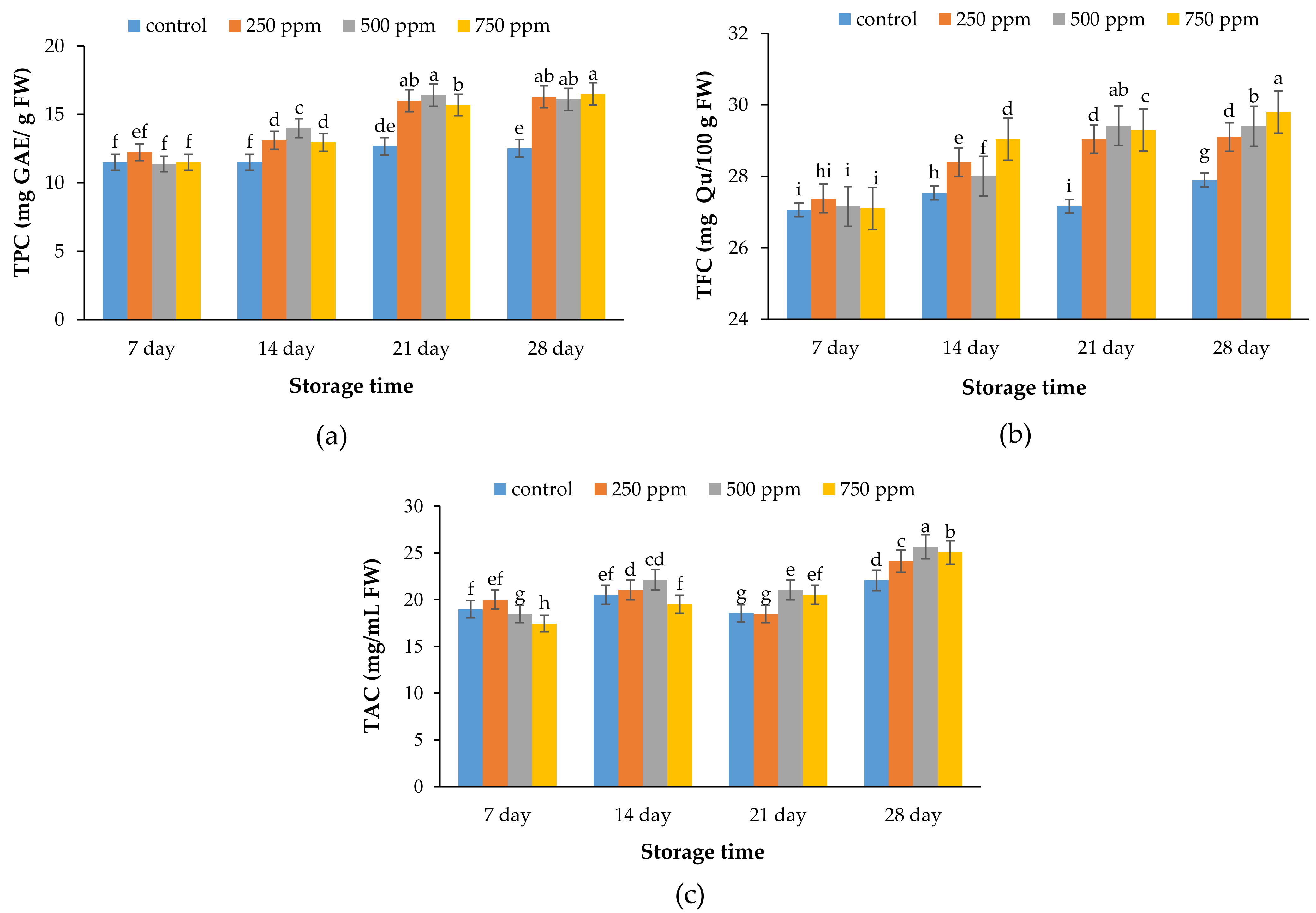
2.4. Total Phenolic Content (TPC)
2.5. Total Flavonoid Content (TFC)
2.6. Total Anthocyanin Content (TAC)
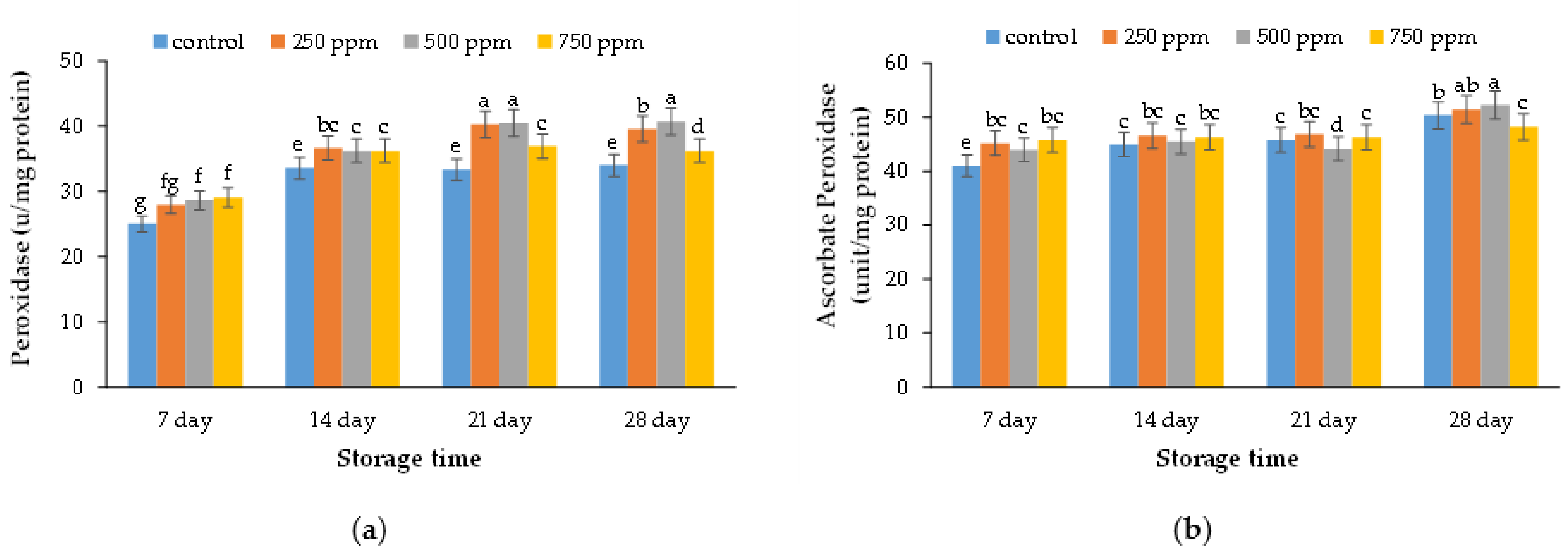
2.7. Peroxidase Enzyme Activity
2.8. Ascorbate Peroxidase Enzyme Activity
3. Materials and Methods
3.1. Fruits
3.2. Preparation of ACEOs and Treatments
3.3. Weight Loss
3.4. Chemical Characteristics (Titratable Acidity and Total Soluble Solid)
3.5. Antioxidant Activity by DPPH
3.6. Total Phenolic Content (TPC)
3.7. Total Flavonoid Content (TFC)
3.8. Total Anthocyanin Content (TAC)
3.9. Peroxidase Enzyme Activity
3.10. Ascorbate Peroxidase Enzyme Activity
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kafkas, E.; Koşar, M.; Paydaş, S.; Kafkas, S.; Başer, K.H.C. Quality characteristics of strawberry genotypes at different maturation stages. Food Chem. 2007, 100, 1229–1236. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Cianciosi, D.; Ansary, J.; Chen, X.; Zhang, D.; Gil, E.; Forbes-Hernández, T. The roles of strawberry and honey phytochemicals on human health: A possible clue on the molecular mechanisms involved in the prevention of oxidative stress and inflammation. Phytomedicine 2020. [Google Scholar] [CrossRef] [PubMed]
- Khripach, V.; Zhabinskii, V.; De Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Ishkeh, S.R.; Shirzad, H.; Asghari, M.R.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Effect of Chitosan Nanoemulsion on Enhancing the Phytochemical Contents, Health-Promoting Components, and Shelf Life of Raspberry (Rubus sanctus Schreber). Appl. Sci. 2021, 11, 2224. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Mo, E.K.; Sung, C.K. Phenylethyl alcohol (PEA) application slows fungal growth and maintains aroma in strawberry. Postharvest Biol. Technol. 2007, 45, 234–239. [Google Scholar] [CrossRef]
- Inselberg, H.; do Nascimento Nunes, M.C. Using Cannabidiol as a potential postharvest treatment to maintain quality and extend the shelf life of strawberries. Postharvest Biol. Technol. 2021, 173, 111416. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkordi, M.; Jafarzadehe, S.H.; Mousavi Khaneghah, A. Packaging of beef fillet with active chitosan film incorporated with ε-polylysine: An assessment of quality indices and shelf life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Ayaseh, A.; Alirezalu, K.; Nemati, Z.; Pateiro, M.; Lorenzo, J.M. Effect of Chitosan Coating Incorporated with Artemisia fragrans Essential Oil on Fresh Chicken Meat during Refrigerated Storage. Polymers 2021, 13, 716. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Hamdi, M.; Restuccia, C. Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int. J. Food Microbiol. 2014, 170, 21–28. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef]
- Kim, N.-S.; Lee, D.-S. Headspace solid-phase microextraction for characterization of fragrances of lemon verbena (Aloysia triphylla) by gas chromatography-mass spectrometry. J. Sep. Sci. 2004, 27, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Portmann, E.; Nigro, M.M.L.; Reides, C.G.; Llesuy, S.; Ricco, R.A.; Wagner, M.L.; Gurni, A.A.; Carballo, M.A. Aqueous extracts of Lippia turbinata and Aloysia citriodora (Verbenaceae): Assessment of antioxidant capacity and DNA damage. Int. J. Toxicol. 2012, 31, 192–202. [Google Scholar] [CrossRef]
- Temkin-Gorodeiski, N.; Shapiro, B.; Grinberg, S.; Rosenberger, I.; Fallik, E. Post harvest treatments to control eggplant deterioration during storage. J. Hortic. Sci. 1993, 68, 689–693. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Alkalai, S.; Yekutieli, O.; Wiseblum, A.; Regev, R.; Beres, H.; Bar-Lev, E. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharvest Biol. Technol. 1999, 15, 25–32. [Google Scholar] [CrossRef]
- Klein, I.; Strime, M.; Fanberstein, L.; Mani, Y. Irrigation and fertigation effects on phosphorus and potassium nutrition of wine grapes. Vitis 2000, 39, 55–62. [Google Scholar] [CrossRef]
- Shojaie, B.; Mostajeran, A.; Ghanadian, M. Flavonoid dynamic responses to different drought conditions: Amount, type, and localization of flavonols in roots and shoots of Arabidopsis thaliana L. Turkish J. Biol. 2016, 40, 612–622. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Gao, P.; Zhu, Z.; Zhang, P. Effects of chitosan-glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr. Polym. 2013, 95, 371–378. [Google Scholar] [CrossRef]
- Amal, S.H.A.; El-Mogy, M.M.; Aboul-Anean, H.E.; Alsanius, B.W. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of Edible Coatings to Preserve Quality of Lightly (and Slightly) Processed Products. Crit. Rev. Food Sci. Nutr. 1995, 35, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.F.; Li, J.C.; Zhang, S.L. Change in the organic acid content and releted metabolic enzyme activities in developing Xinping pear fruit. Afr. J. Agric. Res. 2011, 6, 3560–3566. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- HongBo, S.; ZongSuo, L.; MingAn, S.; ShiMeng, S.; ZanMin, H. Investigation on dynamic changes of photosynthetic characteristics of 10 wheat (Triticum aestivum L.) genotypes during two vegetative-growth stages at water deficits. Colloids Surfaces B Biointerfaces 2005, 43, 221–227. [Google Scholar] [CrossRef]
- Neri, F.; Mari, M.; Brigati, S.; Bertolini, P. Fungicidal activity of plant volatile compounds for controlling Monilinia laxa in stone fruit. Plant Dis. 2007, 91, 30–35. [Google Scholar] [CrossRef]
- Serrano, M.; Martinez-Romero, D.; Castillo, S.; Guille, F.; Valero, D. The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov. Food Sci. Emerg. Technol. 2005, 6, 115–123. [Google Scholar] [CrossRef]
- Prasad, K.; Stadelbacher, G.J. Effect of acetaldehyde vapor on postharvest decay and market quality of fresh strawberries. Phytopathology 1974, 64, 948–951. [Google Scholar] [CrossRef]
- Alirezalu, K.; Shafaghi Molan, H.; Yaghoubi, M.; Pateiro, P.; Lorenzo, J.M. ε-polylysine coating with stinging nettle extract for fresh beef preservation. Meat Sci. 2021, 176, 108474. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Hesari, J.; Yaghoubi, M.; Mousavi Khaneghah, A.; Alirezalu, A.; Pateiro, M.; Lorenzo, J.M. Combined effects of ε-polylysine and ε-polylysine nanoparticles with plant extracts on the shelf life and quality characteristics of nitrite-free frankfurter-type sausages. Meat Sci. 2021, 172, 108318. [Google Scholar] [CrossRef]
- Alirezalu, K.; Yaghoubi, M.; Nemati, Z.; Farmani, B.; Mousavi Khaneghah, A. Efficacy of stinging nettle extract in combination with ε-polylysine on the quality, safety, and shelf life of rainbow trout fillets. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Hatami Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef]
- Shameh, S.; Alirezalu, A.; Hosseini, B.; Maleki, R. Fruit phytochemical composition and color parameters of 21 accessions of five Rosa species grown in North West Iran. J. Sci. Food Agric. 2019, 99, 5740–5751. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Gniewosz, M.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Wȩglarz, Z. Antimicrobial and antioxidant properties of pullulan film containing sweet basil extract and an evaluation of coating effectiveness in the prolongation of the shelf life of apples stored in refrigeration conditions. Innov. Food Sci. Emerg. Technol. 2014, 23, 171–181. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Sivakumar, D.; Soundy, P.; Korsten, L. Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol. Technol. 2013, 81, 66–72. [Google Scholar] [CrossRef]
- Hussain, P.R.; Suradkar, P.P.; Wani, A.M.; Dar, M.A. Potential of carboxymethyl cellulose and γ-irradiation to maintain quality and control disease of peach fruit. Int. J. Biol. Macromol. 2016, 82, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Pateiro, M.; Domínguez, R.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Shpigelman, A.; Granato, D.; Franco, D. Berries extracts as natural antioxidants in meat products: A review. Food Res. Int. 2018, 106, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Gang, W.; Zhen-Kuan, W.; Yong-Xiang, W.; Li-Ye, C.; Hong-Bo, S. The mutual responses of higher plants to environment: Physiological and microbiological aspects. Colloids Surfaces B Biointerfaces 2007, 59, 113–119. [Google Scholar] [CrossRef]
- Dris, R.; Niskanen, R.; Jain, S.M. Crop Management and Postharvest Handling of Horticultural Products; Science Publishers: Enfield, UK, 2001. [Google Scholar]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseini Mehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Tripathi, P.; Dubey, N.K.; Banerji, R.; Chansouria, J.P.N. Evaluation of some essential oils as botanical fungitoxicants in management of post-harvest rotting of citrus fruits. World J. Microbiol. Biotechnol. 2004, 20, 317–321. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Pérez Alonso, J.J.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Bartosz, G. Oxidative stress in plants. Acta Physiol. Plant 1997, 19, 47–64. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneersel-Vam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Afshar Mohammadian, M.; Rezaei, S.H.; Ramezani, M.R. Investigation of the resistance of two olive cultivars to cold stress. J. Process Plant Funct. 2012, 2, 1–11. [Google Scholar]
- Hernández-Muñoz, P.; Almenar, E.; Del Valle, V.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef]
- Khosroshahi, M.R.Z.; Esna-Ashari, M.; Ershadi, A. Effect of exogenous putrescine on post-harvest life of strawberry (Fragaria ananassa Duch.) fruit, cultivar Selva. Sci. Hortic. 2007, 114, 27–32. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. High oxygen treatment increases antioxidant capacity and postharvest life of strawberry fruit. Food Technol. Biotechnol. 2007, 45, 166–173. [Google Scholar]
- Moshari-Nasirkandi, A.; Alirezalu, A.; Hachesu, M.A. Effect of lemon verbena bio-extract on phytochemical and antioxidant capacity of strawberry (Fragaria × ananassa Duch. cv. Sabrina) fruit during cold storage. Biocatal. Agric. Biotechnol. 2020, 25, 101613. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Shin, Y.; Liu, R.H.; Nock, J.F.; Holliday, D.; Watkins, C.B. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 2007, 45, 349–357. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Technol. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirzad, H.; Alirezalu, A.; Alirezalu, K.; Yaghoubi, M.; Ghorbani, B.; Pateiro, M.; Lorenzo, J.M. Effect of Aloysia citrodora Essential Oil on Biochemicals, Antioxidant Characteristics, and Shelf Life of Strawberry Fruit during Storage. Metabolites 2021, 11, 256. https://doi.org/10.3390/metabo11050256
Shirzad H, Alirezalu A, Alirezalu K, Yaghoubi M, Ghorbani B, Pateiro M, Lorenzo JM. Effect of Aloysia citrodora Essential Oil on Biochemicals, Antioxidant Characteristics, and Shelf Life of Strawberry Fruit during Storage. Metabolites. 2021; 11(5):256. https://doi.org/10.3390/metabo11050256
Chicago/Turabian StyleShirzad, Habib, Abolfazl Alirezalu, Kazem Alirezalu, Milad Yaghoubi, Bahareh Ghorbani, Mirian Pateiro, and José M. Lorenzo. 2021. "Effect of Aloysia citrodora Essential Oil on Biochemicals, Antioxidant Characteristics, and Shelf Life of Strawberry Fruit during Storage" Metabolites 11, no. 5: 256. https://doi.org/10.3390/metabo11050256
APA StyleShirzad, H., Alirezalu, A., Alirezalu, K., Yaghoubi, M., Ghorbani, B., Pateiro, M., & Lorenzo, J. M. (2021). Effect of Aloysia citrodora Essential Oil on Biochemicals, Antioxidant Characteristics, and Shelf Life of Strawberry Fruit during Storage. Metabolites, 11(5), 256. https://doi.org/10.3390/metabo11050256









