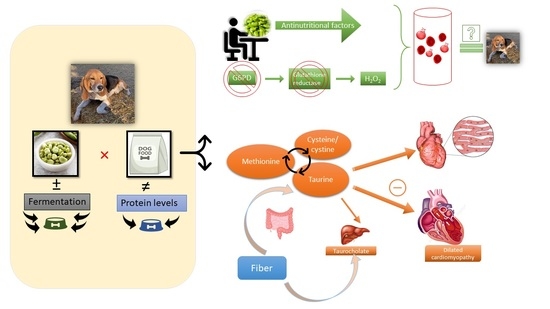
The Effects of Fermentation of Low or High Tannin Fava Bean-Based Diets on Glucose Response, Cardiovascular Function, and Fecal Bile Acid Excretion during a 28-Day Feeding Period in Dogs: Comparison with Commercial Diets with Normal vs. High Protein
Abstract
1. Introduction
2. Results
2.1. Body Weight, Meal Portion, and Body Condition Score
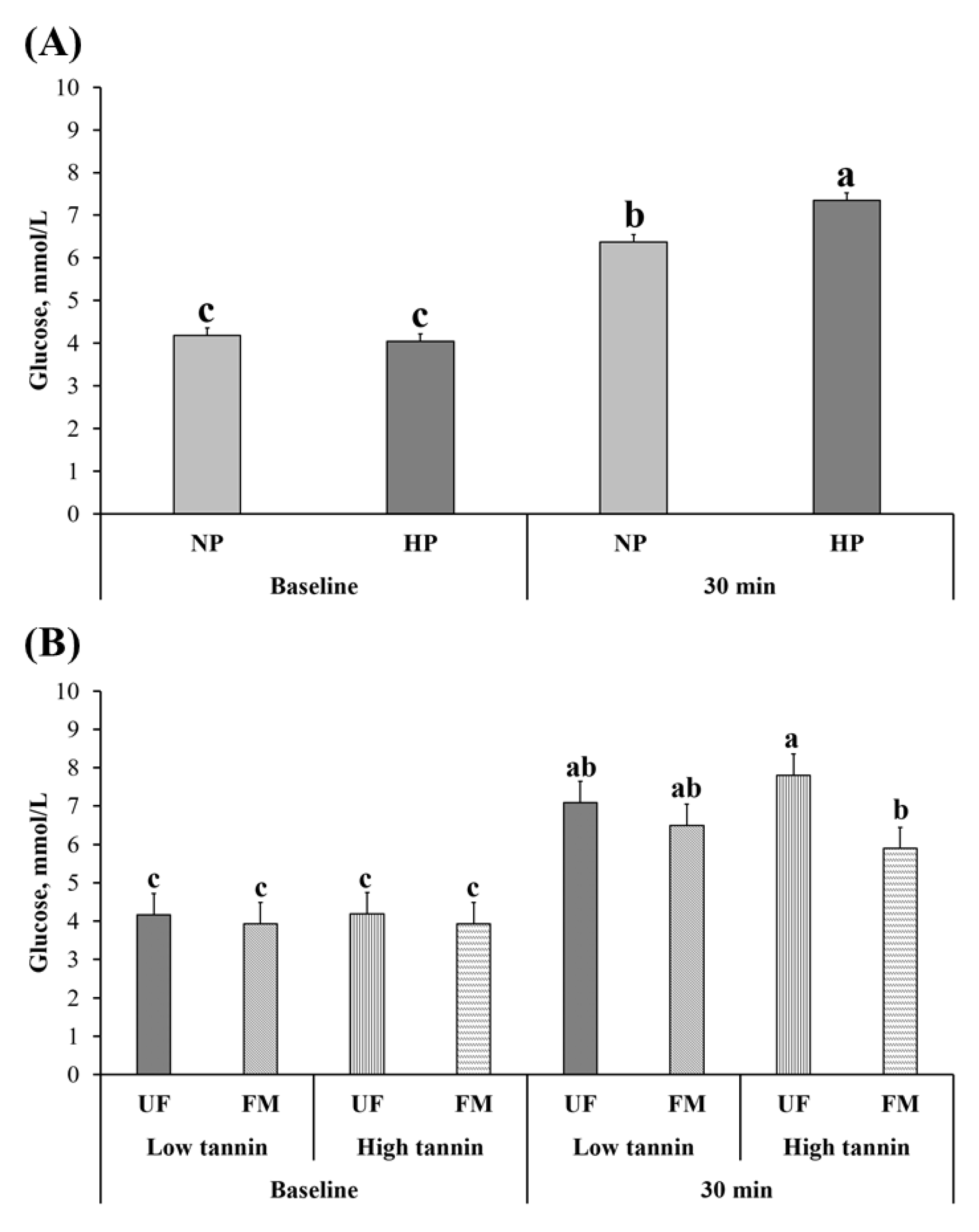
2.2. Glucose Response
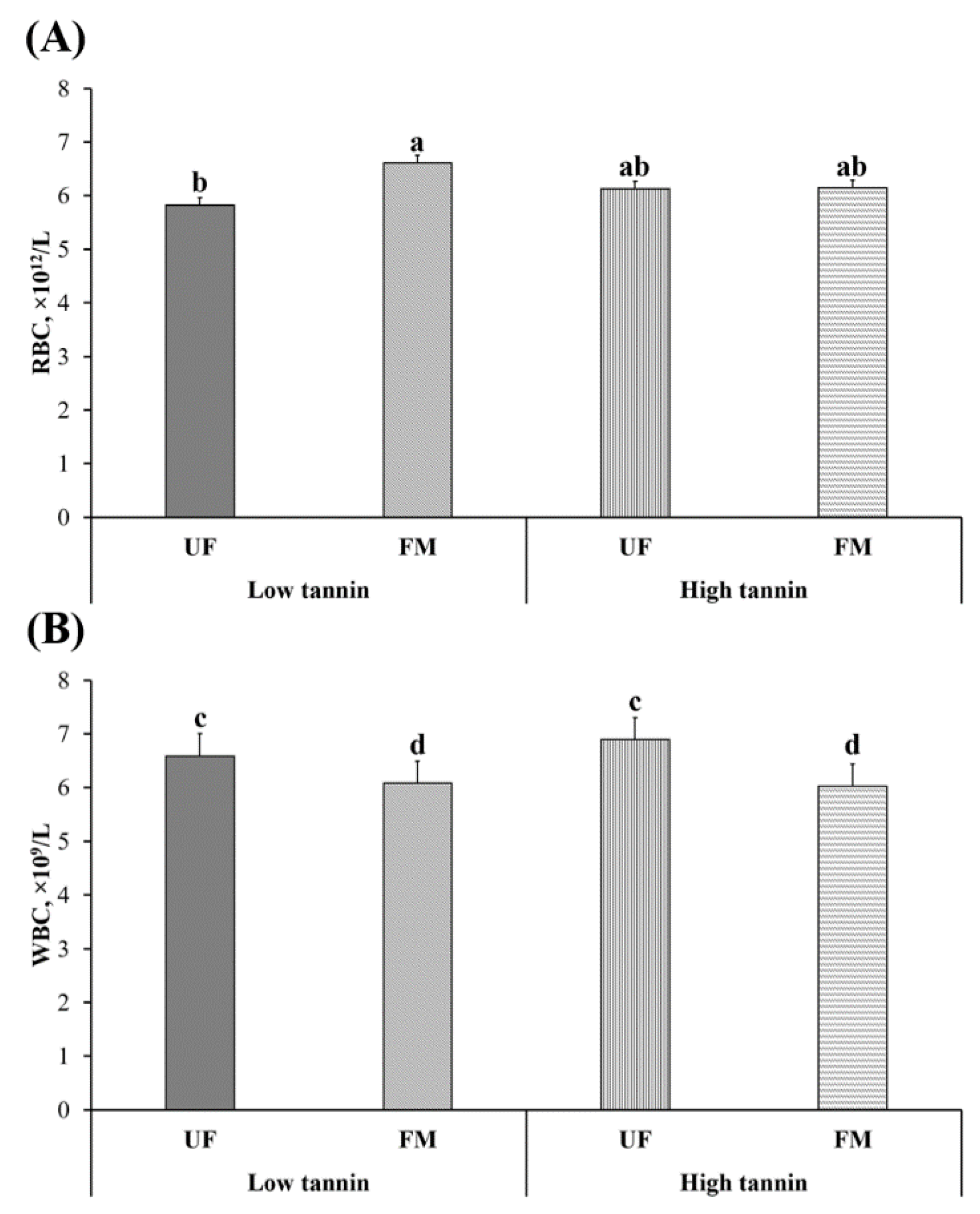
2.3. Red and White Blood Cell Count
2.4. Blood Parameters of Hepatic Function
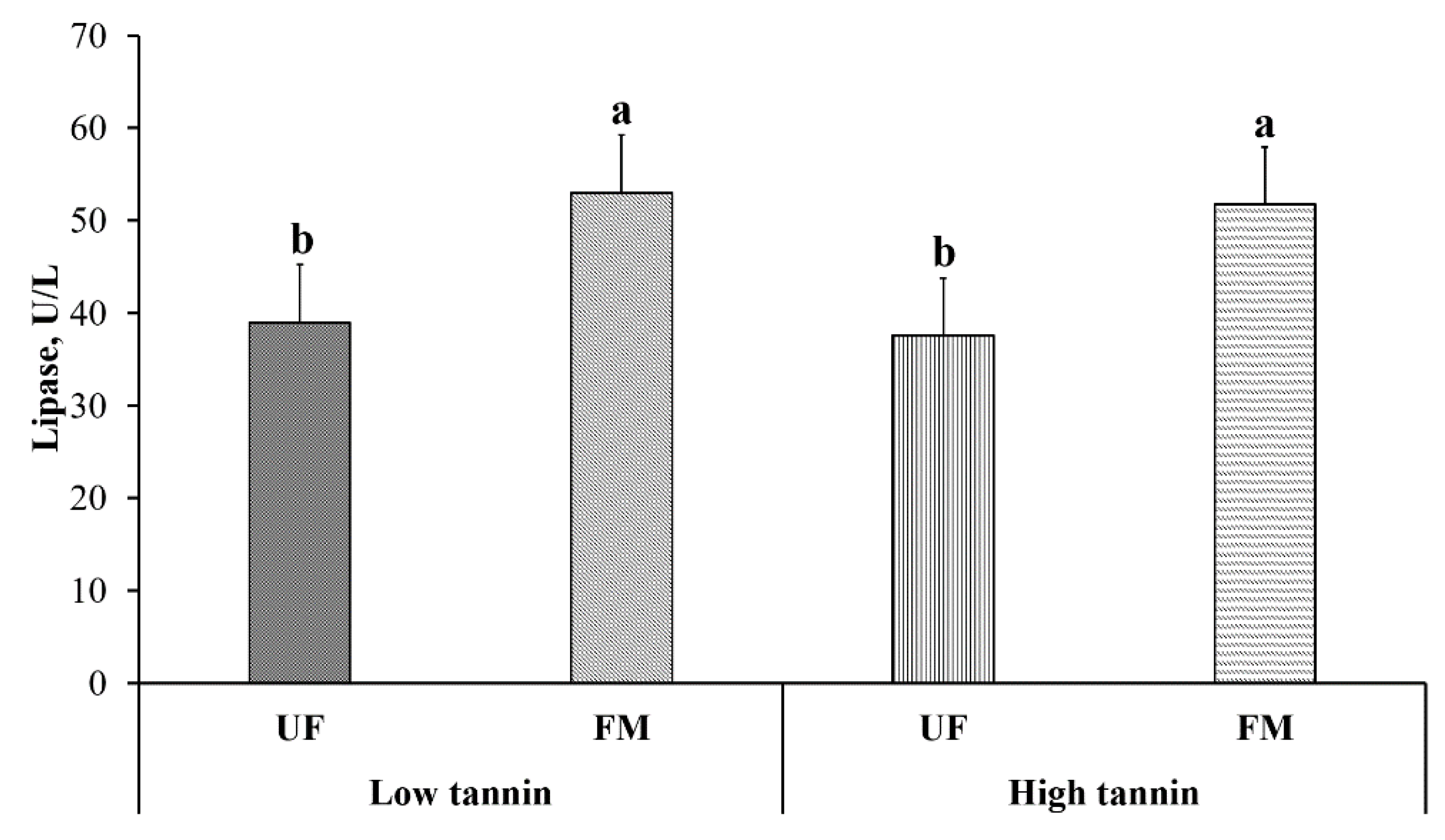
2.5. Blood Electrolytes and Enzymes
2.6. Cardiovascular Function
2.7. Digestibility
2.8. Plasma Amino Acid Levels
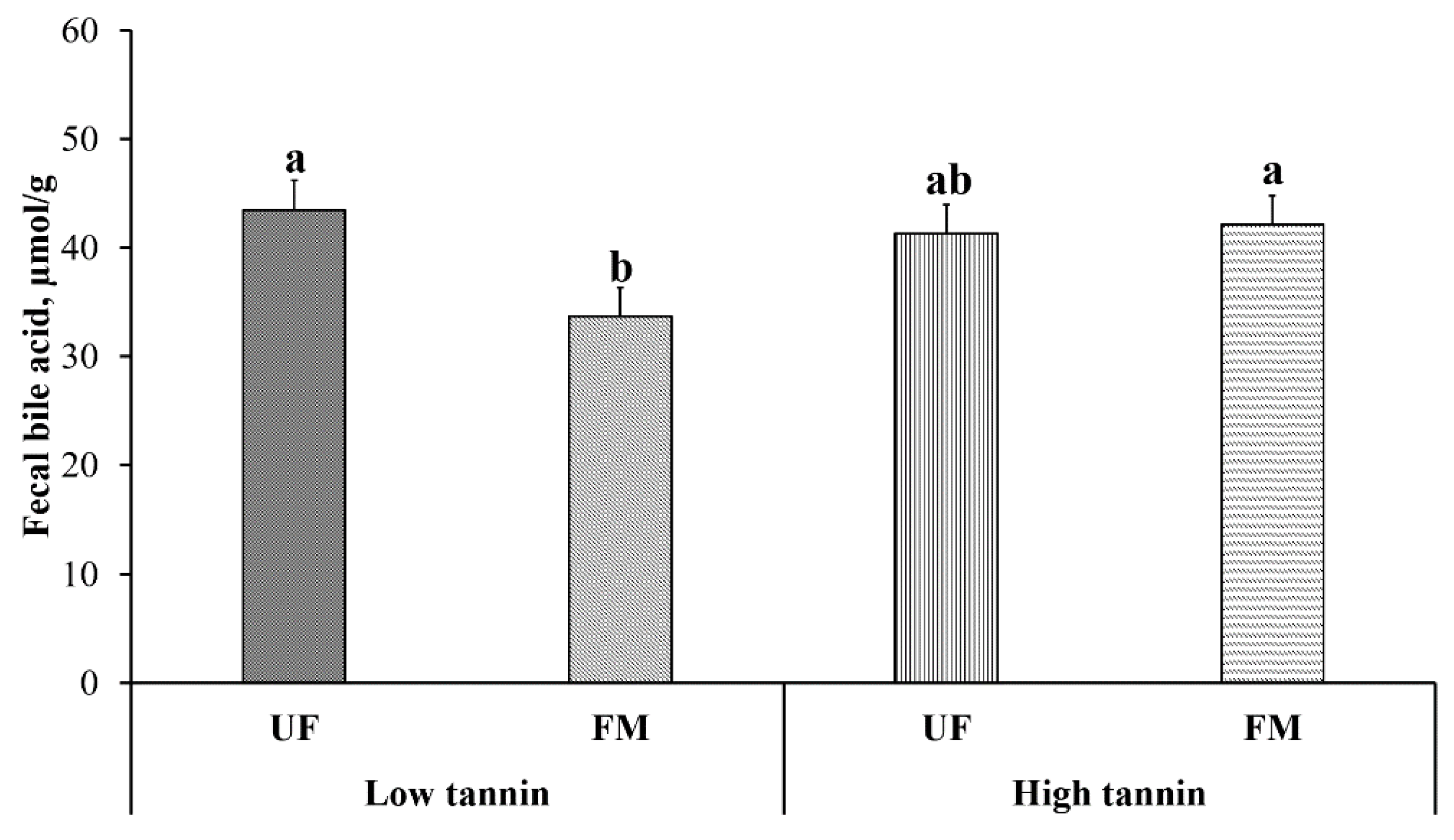
2.9. Fecal Bile Acid Content
3. Discussion
3.1. Toxicity from Fava Beans, Digestibility, Glucose Tolerance, and Anti-Nutritional Factors
3.2. High Dietary Protein Diet on Dog Health
3.3. Cardiac Function, Cystine, Methionine, and Taurine
4. Materials and Methods
4.1. Fava Bean Ingredients and Fermentation Protocol
4.2. Animals and Diets
4.3. Digestibility Protocol
4.4. Oral Glucose Response Test and Blood Analysis
4.5. Cardiac Function, Blood Pressure, and Vascular Health
4.6. Fecal Bile Acid Content
4.7. Statistical Analysis
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M.-N. Fava bean (Vicia faba L.) for food applications: From seed to ingredient processing and its effect on functional properties, antinutritional factors, flavor, and color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy. Available online: https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-investigation-potential-link-between-certain-diets-and-canine-dilated-cardiomyopathy (accessed on 13 September 2021).
- Dutton, E.; López-Alvarez, J. An update on canine cardiomyopathies—Is it all in the genes? J. Small Anim. Pract. 2018, 59, 455–464. [Google Scholar] [CrossRef]
- Fascetti, A.J.; Reed, J.R.; Rogers, Q.R.; Backus, R.C. Taurine deficiency in dogs with dilated cardiomyopathy: 12 Cases (1997–2001). J. Am. Vet. Med. Assoc. 2003, 223, 1137–1141. [Google Scholar] [CrossRef]
- Backus, R.C.; Ko, K.S.; Fascetti, A.J.; Kittleson, M.D.; Macdonald, K.A.; Maggs, D.J.; Berg, J.R.; Rogers, Q.R. Low plasma taurine concentration in newfoundland dogs is associated with low plasma methionine and cyst(e)ine concentrations and low taurine synthesis. J. Nutr. 2006, 136, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.J.; Berg, H.M. Effect of taurine on sarcoplasmic reticulum function and force in skinned fast-twitch skeletal muscle fibres of the rat. J. Physiol. 2002, 538, 185–194. [Google Scholar] [CrossRef]
- Mansilla, W.D.; Marinangeli, C.P.F.; Ekenstedt, K.J.; Larsen, J.A.; Aldrich, G.; Columbus, D.A.; Weber, L.; Abood, S.K.; Shoveller, A.K. Special topic: The association between pulse ingredients and canine dilated cardiomyopathy: Addressing the knowledge gaps before establishing causation. J. Anim. Sci. 2019, 97, 983–997. [Google Scholar] [CrossRef]
- Kim, S.W.; Rogers, Q.R.; Morris, J.G. Dietary antibiotics decrease taurine loss in cats fed a canned heat-processed diet. J. Nutr. 1996, 126, 509–515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.W.; Rogers, Q.R.; Morris, J.G. Maillard reaction products in purified diets induce taurine depletion in cats which is reversed by antibiotics. J. Nutr. 1996, 126, 195–201. [Google Scholar] [CrossRef]
- O’Máille, E.R.; Richards, T.G.; Short, A.H. Acute taurine depletion and maximal rates of hepatic conjugation and secretion of cholic acid in the dog. J. Physiol. 1965, 180, 67–79. [Google Scholar]
- Story, J.A.; Kritchevsky, D. Bile acid metabolism and fiber. Am. J. Clin. Nutr. 1978, 31, S199–S202. [Google Scholar] [CrossRef]
- Liener, I.E. Antinutritional Factors in Legume Seeds: State of the Art. In Recent Advances of Research in Antinutritional Factors in Legume Seeds, Proceedings of the International Workshop on Antinutritional Factors (ANF) in Legume Seeds, Wageningen, The Netherlands, 23–25 November 1988; PUDOC: Wageningen, The Netherland, 1989. [Google Scholar]
- Santana, F.M.C.; Pinto, T.; Fialho, A.M.; Sá-Correia, I.; Empis, J.M.A. Bacterial removal of quinolizidine alkaloids and other carbon sources from a lupinus albus aqueous extract. J. Agric. Food Chem. 2002, 50, 2318–2323. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of sourdough fermentation and pseudo-cereals and leguminous flours for the making of a functional bread enriched of gamma-aminobutyric acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef]
- Curiel, J.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C. Exploitation of the nutritional and functional characteristics of traditional italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hébert, A.; Forquin-Gomez, M.-P.; Roux, A.; Aubert, J.; Junot, C.; Heilier, J.-F.; Landaud, S.; Bonnarme, P.; Beckerich, J.-M. New insights into sulfur metabolism in yeasts as revealed by studies of yarrowia lipolytica. Appl. Environ. Microbiol. 2013, 79, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.G.; Morris, T.; Quilliam, C.; Rodrigues, L.A.; Loewen, M.E.; Weber, L.P. The effect of fermentation of high- or low-tannin fava bean on glucose tolerance, body weight, cardiovascular function, and blood parameters in dogs after 7 days of feeding: Comparison with commercial diets with normal vs. high protein. Front. Vet. Sci. 2021, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D. Development and validation of body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Association of American Feed Control Officials. Official Publication; AAFCO: Champaign, IL, USA, 2017. [Google Scholar]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef]
- Brigham, M.P.; Stein, W.H.; Moore, S. The concentrations of cysteine and cystine in human blood plasma. J. Clin. Investig. 1960, 39, 1633–1638. [Google Scholar] [CrossRef]
- Ketnawa, S.; Ogawa, Y. Evaluation of protein digestibility of fermented soybeans and changes in biochemical characteristics of digested fractions. J. Funct. Foods 2019, 52, 640–647. [Google Scholar] [CrossRef]
- Westergaard, H. Bile acid malabsorption. Curr. Treat. Options Gastroenterol. 2007, 10, 28–33. [Google Scholar] [CrossRef]
- Nyman, M.; Schweizer, T.F.; Tyrén, S.; Reimann, S.; Asp, N.-G. Fermentation of vegetable fiber in the intestinal tract of rats and effects on fecal bulking and bile acid excretion. J. Nutr. 1990, 120, 459–466. [Google Scholar] [CrossRef]
- Bush, B.M. Interpretation of Laboratory Results for Small Animal Clinicians, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1991. [Google Scholar]
- Çalışkantürk Karataş, S.; Günay, D.; Sayar, S. In vitro evaluation of whole faba bean and its seed coat as a potential source of functional food components. Food Chem. 2017, 230, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Faba Bean Variety Report 2015/16. Available online: https://saskpulse.com/files/general/151026_Faba_bean_variety_report2.pdf (accessed on 13 September 2021).
- Wei, X. Effects of Short-Term Germination and Autoclaving on Selected Compounds in Faba Bean and Faba Bean Applications in Low-Fat Pork Bologna. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2019. [Google Scholar]
- Bunglavan, S.; Dutta, N. Use of tannins as organic protectants of proteins in digestion of ruminants. J. Livest. Sci. 2013, 4, 67–77. [Google Scholar]
- Addisu, S. Effect of dietary tannin source feeds on ruminal fermentation and production of cattle: A review. Online J. Anim. Feed Res. 2016, 6, 42–56. [Google Scholar]
- Burkholder, W.J.; Lees, G.E.; LeBlanc, A.K.; Slater, M.R.; Bauer, J.E.; Kashtan, C.E.; McCracken, B.A.; Hannah, S.S. Diet modulates proteinuria in heterozygous female dogs with X-linked hereditary nephropathy. J. Vet. Intern. Med. 2004, 18, 165–175. [Google Scholar] [CrossRef]
- Fisher, K.D.; Scheffler, T.L.; Kasten, S.C.; Reinholt, B.M.; Eyk, G.R.; van Escobar, J.; Scheffler, J.M.; Gerrard, D.E. Energy dense, protein restricted diet increases adiposity and perturbs metabolism in young, genetically lean Pigs. PLoS ONE 2013, 8, e72320. [Google Scholar] [CrossRef]
- Willard, M.D.; Twedt, D.C. Gastrointestinal, pancreatic, and hepatic disorders. In Small Animal Clinical Diagnosis by Laboratory Methods, 5th ed.; Willard, M., Ed.; Saunders: Philadelphia, PA, USA, 2012; pp. 191–225. [Google Scholar]
- Lawrence, Y.A.; Dangott, L.J.; Rodrigues-Hoffmann, A.; Steiner, J.M.; Suchodolski, J.S.; Lidbury, J.A. Proteomic analysis of liver tissue from dogs with chronic hepatitis. PLoS ONE 2018, 13, e0208394. [Google Scholar] [CrossRef] [PubMed]
- Lucena, R.; Novales, M.; Blanco, B.; Hernández, E.; Ginel, P.J. Effect of probiotic enterococcus faecium SF68 on liver function in healthy dogs. J. Vet. Intern. Med. 2019, 33, 2628–2634. [Google Scholar] [CrossRef]
- Rodrigues, J.C.L.; Rohan, S.; Ghosh Dastidar, A.; Harries, I.; Lawton, C.B.; Ratcliffe, L.E.; Burchell, A.E.; Hart, E.C.; Hamilton, M.C.K.; Paton, J.F.R.; et al. Hypertensive heart disease versus hypertrophic cardiomyopathy: Multi-parametric cardiovascular magnetic resonance discriminators when end-diastolic wall thickness ≥ 15 Mm. Eur. Radiol. 2017, 27, 1125–1135. [Google Scholar] [CrossRef]
- Oyama, M.A.; Sisson, D.D. Cardiac troponin-I concentration in dogs with cardiac disease. J. Vet. Intern. Med. 2004, 18, 831–839. [Google Scholar] [CrossRef]
- Ljungvall, I.; Höglund, K.; Tidholm, A.; Olsen, L.H.; Borgarelli, M.; Venge, P.; Häggström, J. Cardiac troponin I is associated with severity of myxomatous mitral valve disease, age, and C-reactive protein in dogs. J. Vet. Intern. Med. 2010, 24, 153–159. [Google Scholar] [CrossRef]
- Berdeaux, A.; Ghaleh, B.; Dubois-Randé, J.L.; Vigué, B.; Drieu La Rochelle, C.; Hittinger, L.; Giudicelli, J.F. Role of vascular endothelium in exercise-induced dilation of large epicardial coronary arteries in conscious dogs. Circulation 1994, 89, 2799–2808. [Google Scholar] [CrossRef]
- Tuttle, J.L.; Nachreiner, R.D.; Bhuller, A.S.; Condict, K.W.; Connors, B.A.; Herring, B.P.; Dalsing, M.C.; Unthank, J.L. Shear level influences resistance artery remodeling: Wall dimensions, cell density, and ENOS expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1380–H1389. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Bielli, A.; Scioli, M.G.; Mazzaglia, D.; Doldo, E.; Orlandi, A. Antioxidants and vascular health. Life Sci. 2015, 143, 209–216. [Google Scholar] [CrossRef]
- Borgarelli, M.; Santilli, R.A.; Chiavegato, D.; D’Agnolo, G.; Zanatta, R.; Mannelli, A.; Tarducci, A. Prognostic indicators for dogs with dilated cardiomyopathy. J. Vet. Intern. Med. 2006, 20, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Fuchida, A.; Suzuki, S.; Motoki, H.; Kanzaki, Y.; Maruyama, T.; Hasizume, N.; Kozuka, A.; Yahikozawa, K.; Kuwahara, K. Prognostic significance of diastolic blood pressure in patients with heart failure with preserved ejection fraction. Heart Vessels 2021, 36, 1159–1165. [Google Scholar] [CrossRef]
- Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; de Leon, A.C.; Faxon, D.P.; Freed, M.D.; Gaasch, W.H.; Lytle, B.W.; Nishimura, R.A.; O’Gara Patrick, T.; et al. ACC/AHA 2006 practice guidelines for the management of patients with valvular heart disease: Executive summary. J. Am. Coll. Cardiol. 2006, 48, 598–675. [Google Scholar] [CrossRef]
- Vahanian, A.; Baumgartner, H.; Bax, J.; Butchart, E.; Dion, R.; Filippatos, G.; Flachskampf, F.; Hall, R.; Iung, B.; Kasprzak, J.; et al. Guidelines on the management of valvular heart disease: The task force on the management of valvular heart disease of the european society of cardiology. Eur. Heart J. 2007, 28, 230–268. [Google Scholar] [PubMed]
- Adin, D.; DeFrancesco, T.C.; Keene, B.; Tou, S.; Meurs, K.; Atkins, C.; Aona, B.; Kurtz, K.; Barron, L.; Saker, K. Echocardiographic phenotype of canine dilated cardiomyopathy differs based on diet type. J. Vet. Cardiol. 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Dukes-McEwan, J.; Borgarelli, M.; Tidholm, A.; Vollmar, A.C.; Häggström, J.; ESVC Taskforce for Canine Dilated Cardiomyopathy. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J. Vet. Cardiol. 2003, 5, 7–19. [Google Scholar] [CrossRef]
- Harrison, M.; Thomas, G.; Gilham, M.; Gray, K.; Colyer, A.; Allaway, D. Short-term determination and long-term evaluation of the dietary methionine requirement in adult dogs. Br. J. Nutr. 2020, 123, 1333–1344. [Google Scholar] [CrossRef]
- Pezzali, J.G.; Acuff, H.L.; Henry, W.; Alexander, C.; Swanson, K.S.; Aldrich, C.G. Effects of different carbohydrate sources on taurine status in healthy beagle dogs. J. Anim. Sci. 2020, 98, skaa010. [Google Scholar] [CrossRef] [PubMed]
- Delaney, S.J.; Kass, P.H.; Rogers, Q.R.; Fascetti, A.J. Plasma and whole blood taurine in normal dogs of varying size fed commercially prepared food. J. Anim. Physiol. Anim. Nutr. 2003, 87, 236–244. [Google Scholar] [CrossRef]
- Curso Almeida, P. Effects of Pea Starch Yeast Fermentation on Glycemic Index, Palatability, Metabolic Status and Intestinal Health of Dogs and Cats fed a Pea based Diet. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2020. [Google Scholar]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Rockville, MD, USA, 1970. [Google Scholar]
- Zhang, F.; Adeola, O. Techniques for evaluating digestibility of energy, amino acids, phosphorus, and calcium in feed ingredients for pigs. Anim. Nutr. 2017, 3, 344–352. [Google Scholar] [CrossRef]
- Tôrres, C.L.; Backus, R.C.; Fascetti, A.J.; Rogers, Q.R. Taurine status in normal dogs fed a commercial diet associated with taurine deficiency and dilated cardiomyopathy. J. Anim. Physiol. Anim. Nutr. 2003, 87, 359–372. [Google Scholar] [CrossRef]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef]
- Heinze, C.R.; Larsen, J.A.; Kass, P.H.; Fascetti, A.J. Plasma amino acid and whole blood taurine concentrations in cats eating commercially prepared diets. Am. J. Vet. Res. 2009, 70, 1374–1382. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Celermajer, D.S. Research methods in human cardiovascular pharmacology edited by Dr S. Maxwell and Prof. D. Webb Flow—Mediated dilatation. Br. J. Clin. Pharmacol. 2000, 50, 397–404. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Drew, M.D.; Huang, Q.; Silver, T.I.; Weber, L.P. Postprandial impairment of flow-mediated dilation and elevated methylglyoxal after simple but not complex carbohydrate consumption in dogs. Nutr. Res. 2012, 32, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Schwaegler, R.G.; Freeman, R.V.; Linefsky, J. Echocardiography Review Guide E-Book: Companion to the Textbook of Clinical Echocardiography, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am. Soc. Echocardiog. 2005, 18, 1440–1463. [Google Scholar]
- Cornell, C.C.; Kittleson, M.D.; Torre, P.D.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Int. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Association of American Feed Control Officials. Model Bill and Regulations; AAFCO: Lousville, KY, USA, 2019; pp. 107–232. [Google Scholar]
| Item | Commercial | Low Tannin | High Tannin | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NP | HP | SEM | UF | FM | UF | FM | SEM | P | FB | FM | |
| Body weight (kg) | 9.40 | 9.20 | 0.881 | 9.20 | 9.19 | 9.15 | 9.28 | 0.843 | 0.87 | 0.98 | 0.94 |
| Food portion (g/d) | 89.75 | 84.75 | 8.162 | 79.57 | 99.50 | 96.43 | 81.38 | 11.008 | 0.67 | 0.96 | 0.83 |
| BCS | 4.50 | 4.75 | 0.222 | 4.88 | 4.63 | 4.63 | 4.88 | 0.263 | 0.43 | 1.00 | 1.00 |
| Item | Reference | Commercial | Low Tannin | High Tannin | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NP | HP | SEM | UF | FM | UF | FM | SEM | P | FB | FM | FB*FM | ||
| Cholesterol (mmol/L) | 2.70–5.94 | 4.98 | 4.54 | 0.304 | 3.37 | 3.72 | 3.44 | 3.65 | 0.269 | 0.32 | 0.99 | 0.29 | 0.80 |
| TB (μmol/L) | 1.0–4.0 | 1.14 | 1.05 | 0.111 | 1.61 | 1.35 | 1.64 | 1.89 | 0.355 | 0.58 | 0.42 | 0.97 | 0.47 |
| DB (μmol/L) | 0.0–2.0 | 0.46 | 0.58 | 0.055 | 0.61 a,b | 0.63 a,b | 1.01 a | 0.51 b | 0.036 | 0.17 | 0.85 | 0.11 | 0.04 |
| IB (μmol/L) | 0.0–2.5 | 0.77 | 0.48 | 0.075 | 0.66 | 0.73 | 1.06 | 1.01 | 0.194 | 0.02 | 0.07 | 0.96 | 0.77 |
| ALP (U/L) | 9–90 | 37.63 | 30.57 | 4.865 | 76.63 | 65.50 | 64.75 | 52.86 | 10.732 | 0.34 | 0.24 | 0.26 | 0.97 |
| GGT (U/L) | 0–8 | 1.25 | 1.63 | 0.457 | 2.75 | 2.14 | 2.85 | 1.57 | 0.540 | 0.57 | 0.66 | 0.08 | 0.52 |
| ALT (U/L) | 19–59 | 20.00 | 19.50 | 1.336 | 20.43 | 25.38 | 24.50 | 26.25 | 1.969 | 0.80 | 0.23 | 0.09 | 0.43 |
| GLDH (U/L) | 0–7 | 2.43 | 3.38 | 0.357 | 3.14 | 2.63 | 3.13 | 2.86 | 0.301 | 0.07 | 0.71 | 0.19 | 0.67 |
| CK (U/L) | 51–418 | 152.75 | 146.43 | 20.526 | 142.86 | 182.25 | 160.12 | 171.71 | 29.031 | 0.82 | 0.90 | 0.37 | 0.63 |
| Total protein (g/L) | 55–71 | 50.63 | 47.57 | 4.908 | 54.50 | 55.13 | 54.38 | 54.63 | 1.334 | 0.65 | 0.81 | 0.74 | 0.88 |
| Albumin (A; g/L) | 32–42 | 32.00 | 34.50 | 1.395 | 33.25 | 34.00 | 33.75 | 33.63 | 1.167 | 0.23 | 0.95 | 0.79 | 0.71 |
| Globulin (G; g/L) | 20–34 | 18.63 | 18.63 | 0.718 | 21.25 | 21.13 | 20.38 | 21.00 | 0.774 | 0.99 | 0.52 | 0.74 | 0.63 |
| A: G | 1.06–1.82 | 1.74 | 1.88 | 0.116 | 1.59 | 1.63 | 1.65 | 1.61 | 0.088 | 0.42 | 0.78 | 0.97 | 0.64 |
| Urea (mmol/L) | 3.5–11.4 | 5.56 | 7.26 | 0.251 | 5.88 | 5.83 | 6.14 | 5.61 | 0.365 | 0.02 | 0.36 | 0.78 | 0.66 |
| Creatinine (mmol/L) | 41.121 | 60.00 | 67.38 | 5.031 | 61.75 | 55.29 | 64.13 | 62.38 | 6.033 | 0.65 | 0.36 | 0.22 | 0.19 |
| Item | Reference | Commercial | Low Tannin | High Tannin | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NP | HP | SEM | UF | FM | UF | FM | SEM | P | FB | FM | FB*FM | ||
| Na | 140–153 | 145.87 | 147.63 | 0.49 | 146.87 | 146.50 | 146.63 | 146.86 | 0.37 | 0.02 | 0.88 | 0.85 | 0.42 |
| K | 3.8–5.6 | 4.65 | 4.71 | 0.06 | 4.63 | 4.61 | 4.54 | 4.64 | 0.06 | 0.44 | 0.66 | 0.53 | 0.42 |
| Na:K | 28–38 | 31.25 | 31.38 | 0.37 | 31.63 | 31.75 | 32.25 | 31.75 | 0.41 | 0.81 | 0.45 | 0.65 | 0.45 |
| Cl | 105–120 | 114.00 | 111.38 | 0.42 | 109.75 | 94.43 | 109.50 | 109.25 | 6.73 | <0.01 | 0.30 | 0.27 | 0.28 |
| HCO3− | 15–25 | 18.50 | 18.75 | 0.48 | 19.38 | 18.38 | 17.29 | 19.13 | 1.05 | 0.71 | 0.53 | 0.69 | 0.19 |
| Anion gap | 12–26 | 18.38 | 22.50 | 0.57 | 22.63 | 24.00 | 19.86 | 22.88 | 1.27 | <0.01 | 0.14 | 0.09 | 0.53 |
| Ca | 1.91–3.03 | 2.42 | 2.90 | 0.35 | 2.48 | 2.49 | 2.46 | 2.49 | 0.03 | 0.33 | 0.77 | 0.38 | 0.84 |
| P | 0.63–2.41 | 1.14 | 1.24 | 0.04 | 1.43 a | 1.28 a,b | 1.23 b | 1.29 a,b | 0.05 | 0.08 | 0.08 | 0.43 | 0.05 |
| Mg | 0.70–1.16 | 0.74 | 0.80 | 0.02 | 1.14 | 1.45 | 1.00 | 1.28 | 0.41 | 0.01 | 0.70 | 0.48 | 0.97 |
| Item | Commercial | Low Tannin | High Tannin | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NP | HP | SEM | UF | FM | UF | FM | SEM | P | FB | FM | FB*FM | |
| EDV M-mode (mL/kg BW) | 1.72 | 2.39 | 0.246 | 1.99 | 1.98 | 1.21 | 1.95 | 0.25 | 0.09 | 0.72 | 0.59 | 0.62 |
| EDV Simpson’s rule (mL/kg BW) | 1.44 | 2.11 | 0.144 | 2.08 | 2.08 | 2.11 | 1.92 | 0.104 | <0.01 | 0.52 | 0.39 | 0.32 |
| ESV M-mode (mL/kg BW) | 0.55 | 0.45 | 0.062 | 0.45 | 0.57 | 0.46 | 0.66 | 0.104 | 0.25 | 0.91 | 0.08 | 0.92 |
| ESV Simpson’s rule (mL/kg BW) | 0.57 | 0.67 | 0.056 | 0.56 | 0.62 | 0.79 | 0.56 | 0.108 | 20 | 0.42 | 0.42 | 0.17 |
| LVIDd (cm/BW1/3) | 1.17 | 1.24 | 0.044 | 1.17 | 1.15 | 1.14 | 1.16 | 0.044 | 0.27 | 0.82 | 0.95 | 0.67 |
| LVIDs (cm/BW1/3) | 0.82 | 0.71 | 0.039 | 0.66 | 0.63 | 0.63 | 0.68 | 0.036 | 0.05 | 0.7 | 0.75 | 0.24 |
| SV M-mode (mL/kg BW) | 1.45 | 1.83 | 0.253 | 1.67 | 1.57 | 1.63 | 1.92 | 0.316 | 0.3 | 0.61 | 0.75 | 0.52 |
| SV Simpson’s mode (mL/kg BW) | 0.98 | 1.45 | 0.114 | 1.51 | 1.38 | 1.49 | 1.37 | 0.106 | 0.02 | 0.82 | 0.22 | 0.95 |
| CO M-mode (L/min BW) | 0.15 | 0.17 | 0.023 | 0.71 | 0.67 | 0.14 | 0.13 | 0.376 | 0.51 | 0.14 | 0.93 | 0.97 |
| CO Simpson’s rule (L/min BW) | 0.09 | 0.14 | 0.013 | 0.15 | 0.13 | 0.13 | 0.11 | 0.015 | 0.02 | 0.38 | 0.28 | 0.95 |
| EF M-mode (%) | 56.07 | 75.75 | 6.147 | 76.25 | 77 | 77.81 | 73.94 | 2.934 | 0.04 | 0.8 | 0.59 | 0.43 |
| EF Simpson’s rule (%) | 62.36 | 68.31 | 2.357 | 72.79 | 68 | 73.63 | 71 | 1.989 | 0.08 | 0.35 | 0.08 | 0.59 |
| HR (bpm) | 83.43 | 94.63 | 11.977 | 95.5 | 88.13 | 85 | 90.13 | 6.809 | 0.51 | 0.54 | 0.87 | 0.37 |
| DWT (cm) | 0.82 | 0.82 | 0.044 | 0.88 | 0.86 | 2.37 | 1.3 | 0.326 | 0.79 | 0.01 | 0.14 | 0.15 |
| SWT (cm) | 1.2 | 2.22 | 0.053 | 1.31 b | 1.33 b | 2.53 a | 1.34 b | 0.224 | 0.81 | 0.02 | 0.02 | 0.02 |
| E/A ratio | 0.18 | 0.19 | 0.014 | 0.15 | 0.16 | 0.18 | 0.16 | 0.242 | 0.63 | 0.69 | 0.68 | 0.65 |
| MV (E wave) (cm/s) | 71.61 | 69.31 | 12.067 | 80.53 | 69.16 | 67.93 | 77.2 | 10.55 | 0.9 | 0.83 | 0.92 | 0.33 |
| MV (A wave) (cm/s) | 23.15 | 37.03 | 4.306 | 40.39 | 36.46 | 31.49 | 29.59 | 3.651 | 0.05 | 0.04 | 0.45 | 0.79 |
| SBP (mmHg) | 136.4 | 127.25 | 7.128 | 117.19 b | 152.79 a | 132.31 a,b | 138.19 a,b | 10 | 0.38 | 0.97 | 0.16 | 0.05 |
| DBP (mmHg) | 75.73 | 60.5 | 3.948 | 67.19 | 81.96 | 66.31 | 75.58 | 6.016 | 0.01 | 0.55 | 0.05 | 0.65 |
| FMD (%) | 9.84 | 7.47 | 2.955 | 6.18 | 4.24 | 7.25 | 3.75 | 0.672 | 0.6 | 0.85 | 0.04 | 0.63 |
| NT-ProBNP (pg/mL) | 15.08 | 8.84 | 8.276 | 23.93 | 15.38 | 7.44 | 17.87 | 9.065 | 0.6 | 0.45 | 0.92 | 0.31 |
| Cardiac troponin I (pg/mL) | 5.23 | 2.98 | 2.655 | 8.14 | 4.94 | 7.86 | 17.11 | 9.352 | 0.55 | 0.53 | 0.75 | 0.51 |
| Item | Low Tannin | High Tannin | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| UF | FM | UF | FM | SEM | FB | FM | FB*FM | |
| Crude protein | 85.38 a,b | 86.63 a | 85.41 a,b | 84.20 b | 0.428 | 0.07 | 0.98 | 0.04 |
| Fat | 76.79 | 75.94 | 88.12 | 83.94 | 8.346 | 0.24 | 0.75 | 0.83 |
| Non-fiber carbohydrates | 96.23 | 97.05 | 96.46 | 96.74 | 0.298 | 0.88 | 0.07 | 0.36 |
| Gross energy | 91.10 b | 92.07 a | 91.37 a,b | 90.88 b | 0.321 | 0.15 | 0.46 | 0.03 |
| Methionine | 71.13 | 70.55 | 62.01 | 62.67 | 11.084 | 0.45 | 0.99 | 0.95 |
| Cysteine | 82.16 | 82.92 | 96.41 | 82.84 | 11.224 | 0.52 | 0.56 | 0.52 |
| Taurine | 81.32 | 91.85 | 91.00 | 90.35 | 6.514 | 0.51 | 0.43 | 0.37 |
| Item | Commercial | Low Tannin | High Tannin | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NP | HP | SEM | UF | FM | UF | FM | SEM | P | FB | FM | |
| Taurine | 105.38 | 91.25 | 10.712 | 84.71 | 73.57 | 77.43 | 78.75 | 12.652 | 0.36 | 0.93 | 0.70 |
| Cystine | 16.29 | 22.13 | 2.287 | 20.00 | 17.50 | 19.00 | 15.13 | 1.502 | 0.09 | 0.27 | 0.04 |
| Methionine | 43.00 | 41.43 | 6.629 | 46.25 | 53.71 | 55.00 | 46.62 | 4.864 | 0.86 | 0.86 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, L.G.; Morris, T.; Quilliam, C.; Rodrigues, L.A.; Loewen, M.E.; Weber, L.P. The Effects of Fermentation of Low or High Tannin Fava Bean-Based Diets on Glucose Response, Cardiovascular Function, and Fecal Bile Acid Excretion during a 28-Day Feeding Period in Dogs: Comparison with Commercial Diets with Normal vs. High Protein. Metabolites 2021, 11, 878. https://doi.org/10.3390/metabo11120878
Reis LG, Morris T, Quilliam C, Rodrigues LA, Loewen ME, Weber LP. The Effects of Fermentation of Low or High Tannin Fava Bean-Based Diets on Glucose Response, Cardiovascular Function, and Fecal Bile Acid Excretion during a 28-Day Feeding Period in Dogs: Comparison with Commercial Diets with Normal vs. High Protein. Metabolites. 2021; 11(12):878. https://doi.org/10.3390/metabo11120878
Chicago/Turabian StyleReis, Luciana G., Tressa Morris, Chloe Quilliam, Lucas A. Rodrigues, Matthew E. Loewen, and Lynn P. Weber. 2021. "The Effects of Fermentation of Low or High Tannin Fava Bean-Based Diets on Glucose Response, Cardiovascular Function, and Fecal Bile Acid Excretion during a 28-Day Feeding Period in Dogs: Comparison with Commercial Diets with Normal vs. High Protein" Metabolites 11, no. 12: 878. https://doi.org/10.3390/metabo11120878
APA StyleReis, L. G., Morris, T., Quilliam, C., Rodrigues, L. A., Loewen, M. E., & Weber, L. P. (2021). The Effects of Fermentation of Low or High Tannin Fava Bean-Based Diets on Glucose Response, Cardiovascular Function, and Fecal Bile Acid Excretion during a 28-Day Feeding Period in Dogs: Comparison with Commercial Diets with Normal vs. High Protein. Metabolites, 11(12), 878. https://doi.org/10.3390/metabo11120878